Published online Oct 18, 2021. doi: 10.5312/wjo.v12.i10.768
Peer-review started: April 30, 2021
First decision: June 16, 2021
Revised: June 30, 2021
Accepted: August 23, 2021
Article in press: August 23, 2021
Published online: October 18, 2021
Processing time: 166 Days and 18.7 Hours
Among the various complications associated with total hip arthroplasty (THA) periprosthetic osteolysis and wear phenomena due to the release of metal particles, are two of the most common and have been reported to be correlated because of inflammatory responses directed towards released particles that generally activate macrophagic osteolytic effects. Therein, new masses known as pseudotumors can appear in soft tissues around a prosthetic implant. To date, there is paucity of reliable data from studies investigating for any association between the above mentioned adverse events.
To investigate for the existence of any association between serum and urine concentrations of metal-ions released in THA and periprosthetic osteolysis for modular neck and monolithic implants.
Overall, 76 patients were divided into three groups according to the type of hip prosthesis implants: Monoblock, modular with metal head and modular with ceramic head. With an average f-up of 4 years, we conducted a radiological evaluation in order to detect any area of osteolysis around the prosthesis of both the femur and the acetabulum. Moreover, serum and urinary tests were performed to assess the values of Chromium and Cobalt released. Statistical analysis was performed to determine any association between the ion release and osteolysis.
For the 3 study groups, the monolithic, modular ceramic-headed and modular metal-headed implants had different incidences of osteolysis events, which were higher for the modular implants. Furthermore, the most serious of these (grade 3) were detected almost exclusively for the modular implants with metal heads. A mapping of the affected areas was performed revealing that the highest incidences of osteolysis were evidenced in the pertrochanteric region at the femur level, and in the supero-external region at the acetabular level. Regarding the evaluation of the release of metals-ions from wear processes, serum and urinary chromium and cobalt values were found to be higher in cases of modularity, and even more so for those with metal head. Statistical linear correlation test results suggested positive correlations between increasing metal concentrations and incidences areas of osteolysis. However, no cases of pseudo-tumor were detected.
Future studies are needed to identify risk factors that increase peri-prosthetic metal ion levels and whether these factors might be implicated in the triggering of local events, including osteolysis and aseptic loosening.
Core Tip: In this study a rigorous and statistically proven correlation was made between the release of periprosthetic metal ions in hip arthroplasty and the phenomenon of osteolysis, for severity and localization. A novel aspect of this study was that these evaluations were classified according to the types of prostheses: Monolithic, modular with ceramic head and modular with metal head. This was done so to conduct a contextual comparison between them. In fact, the results appeared quite clear, although further randomized trials and studies of higher scientific evidence will be needed.
- Citation: Manfreda F, Bufi E, Florio EF, Ceccarini P, Rinonapoli G, Caraffa A, Antinolfi P. Osteolysis in total hip arthroplasty in relation to metal ion release: Comparison between monolithic prostheses and different modularities. World J Orthop 2021; 12(10): 768-780
- URL: https://www.wjgnet.com/2218-5836/full/v12/i10/768.htm
- DOI: https://dx.doi.org/10.5312/wjo.v12.i10.768
The introduction of total hip arthroplasty (THA) was one of the most important achievements in medical landscape over the last century. It led to an improvement in the quality of life of patients by reducing pain and improving hip functionality. Additionally, surgeons and engineers sought to improve upon the surgical technique and the design of the prostheses, with the aim of reproducing the natural biomechanics of the hip, to improve functionality and longevity of the implant. Likewise, as the technology improved the lifespan of the implants, issues with debris from joint surfaces were encountered.
The advent of modularity in THA brought to orthopedic surgery a great potential gain for restoring biomechanical parameters. In fact, the use of the modular neck prosthesis has the advantage of restoring the offset, the length of the limbs and the neck version, compared to a mono-block prosthesis[1]. The major disadvantages associated with the modular neck prothesis have been reported to be corrosion, adverse reactions of tissue spaces and increased blood metal ions release[1,2], often leading to mechanical failure of the implant and a possible systemic toxicity. In addition, these adverse events derive from the higher contact-surfaces of implants. In fact, a larger number of elements in a modular system correspond to a larger amount of stress-forces of the implant surfaces.
The active corrosion process of metallic surfaces and the particles released due to wear are a source of soluble metal ions[3] As stated above, orthopaedic implants generate ions and metal particles, most of them of Cobalt and Chrome (CoCr), predominantly of nanometric dimensions[4]. CoCr particles are generally smaller than 50 nm[5], while larger particles can be formed from the smaller size particles agglomeration[6]. Within periprosthetic tissues, particles larger than 0.1 μm are degraded by macrophages, where they are subsequently eliminated from the joint[6]. However, nanometric particles are not able to stimulate phagocytosis by macrophages[7,8]. Ultra-fine particles are generally more inflammatory than fine particles of the same material[9]. The precise mechanism underlying this increased activity is currently unknown. However, it is thought to be the result of a complex series of biological reactions, which depend on particle size, shape, and chemistry and surface properties. Generally, the inflammatory response to the accumulation of particles and ions within periprosthetic tissues is considered one of the major causes of aseptic mobilization and implant failure[10]. In many cases, a thin fibrous membrane develops at the interface between the implant and the bone. This measures 0.1-0.3 mm in width at the femoral component, increasing with implant survival, and > 0.1 mm at the acetabular component. This may be considered a normal response to a foreign body and does not necessarily indicate a damaging inflammatory reaction. However, whenever excess debris is produced and/or macrophages are unable to phagocytize it all, there is a dramatic increase in particles in the periprosthetic tissue[7]. The accumulation of these debris particles tends to increase the infiltration of inflammatory cells (macrophages and mononuclear giant cells) into the tissue. These cells drive the subsequent inflammatory reaction by creating fibrin deposits at the interface. Activation of macrophages leads to the release of inflammatory mediators such as interleukin-6 and tumor necrosis factor-α. In some cases this histiocytic reaction may cause a progressive destruction of the surrounding bone, known as osteolysis, which is mediated by a complex series of biological interactions between activated macrophages, osteoclasts and osteoblasts[8,11]. Osteolysis may depend on the composition and shape of the debris particles. Experimental evidence suggests that osteolysis, that is caused by CoCr particles, may be due to a direct action of inflammatory cytokines, in contrast to osteolysis caused by titanium and polyethylene[12]. With regard to periprosthetic tissues, a significant amount of macrophage cells and a dramatic perivascular accumulation of T-cells and/or B-cells as well as plasma cells were found[13-16].
In addition, a relation between the phenomenon of osteolysis and a pseudotumor has been well described in literature.
It would be worthwhile to investigate for an association among the amount of metal-ion release, the potential consequent osteolysis as a direct response, and the presence of pseudotumors.
To date, few studies have compared modular and monoblock implants clinically, radiographically and tribologically. In this paper, we sought to investigate for the presence of any association between serum and urine concentrations of metal-ions released in THA and periprosthetic osteolysis for modular neck and monolithic implants
Type of study: Comparative retrospective. Level III Evidence.
The inclusion and exclusion criteria we chose were the following.
Inclusion criteria: (1) Diagnosis: Primary hip OA; (2) Age under 86 years old; (3) First implant prostheses; (4) Use of implants: ABG II Stryker® Modular Neck metal or ceramic ones, and ABG II Stryker® with metal head; and (5) Polyethylene head-acetabulum interface
Exclusion criteria: (1) Patients not present at 2-year follow-up; (2) Septic loosening; (3) Contralateral hip or other prosthetic implants; and (4) Occupational Hazard for metals (Metallic industries; chemical/pharmaceutical industries; textile industries; glass processing; paint processing; photographic processing).
Because of the severe criteria, we could enroll only 81 patients, of which 5 were lost over follow-up (76): 23 monoblock prostheses with metal heads (Group A, Table 1), 21 modular prostheses with ceramic heads (Group B, Table 2), 32 modular prostheses with metal heads (Group C, Table 3). The hip surgical approach adopted was the same for all the patients: Kocher-Langebeck posterior approach. The mean age for Group A was 71.08 years, for Group B it was 71.2 years, and for Group C it was 70.9 years. The mean follow-up period for Group A was 46.10 mo; for Group B it was 39.19 mo; for Group C it was 47.05 mo.
| Group A | |||||
| Patient | Age | Cr serum (μg/L) | Cr urine (μg/L) | Co serum (μg/L) | Co urine (μg/L) |
| 1 | 58 | 0.53 | 0.69 | 3.9 | 6 |
| 2 | 63 | 0.14 | 0 | 0 | 0.76 |
| 3 | 69 | 0.92 | 2.71 | 3.22 | 14.53 |
| 4 | 76 | 0.8 | 2.14 | 11.1 | 34.9 |
| 5 | 77 | 0.07 | 0.11 | 0.1 | 0.1 |
| 6 | 65 | no | 1.48 | 5.03 | 14.9 |
| 7 | 71 | 0.1 | 0.4 | 0.82 | 0.2 |
| 8 | 74 | 0.08 | 0.61 | 0.73 | 2.1 |
| 9 | 75 | 0.5 | 1.13 | 4.3 | 9.6 |
| 10 | 78 | 0.77 | 2.94 | 10.65 | 27.5 |
| 11 | 79 | 2.05 | 4.36 | 7.92 | 17.4 |
| 12 | 75 | 0.7 | 1.7 | 4.6 | 9.4 |
| 13 | 65 | 0.37 | 2.3 | 7.84 | 26 |
| 14 | 75 | 1.14 | 3.86 | 6.38 | 24.2 |
| 15 | 70 | 0.1 | 0.1 | 0.1 | 0.1 |
| 16 | 69 | 0.3 | 1.25 | 1.77 | 7.1 |
| 17 | 72 | 0.34 | 0.04 | 0.1 | 0 |
| 18 | 71 | 0.76 | 1.07 | 2.06 | 9.3 |
| 19 | 74 | 0.1 | 0.09 | 0.67 | 0.1 |
| 20 | 65 | 0.1 | 0.22 | 1.34 | 3.9 |
| 21 | 64 | 0.1 | 0.52 | 0.34 | 0.2 |
| 22 | 72 | 1.87 | 3.69 | 7.25 | 12.38 |
| 23 | 78 | 0.1 | 1.02 | 2.41 | 10.7 |
| Mean | 71.08 | 0.54 | 1.41 | 3.59 | 10.06 |
| ST.DEV | 0.56 | 1.33 | 3.46 | 10.19 |
| Group B | |||||
| Patient | Age | Cr serum (μg/L) | Cr urine (μg/L) | Co serum (μg/L) | Co urine (μg/L) |
| 24 | 68 | 1.56 | 3.9 | 4.19 | 19.2 |
| 25 | 71 | 0.1 | 2.34 | 2.09 | 21.15 |
| 26 | 69 | 0.38 | 1.59 | 2.3 | 15.2 |
| 27 | 70 | 0.67 | 1.91 | 0.61 | 0.2 |
| 28 | 71 | 2.25 | 7.52 | 5.6 | 17.7 |
| 29 | 75 | 0.77 | 2.36 | 6.33 | 19.5 |
| 30 | 55 | 0.44 | 1.55 | 3.76 | 19.9 |
| 31 | 74 | 0.53 | 1.9 | 3.4 | 23.9 |
| 32 | 76 | 0.55 | 1.94 | 2.95 | 29.4 |
| 33 | 74 | 0.18 | 0.24 | 1.83 | 4.7 |
| 34 | 69 | 0.11 | 1.54 | 0.85 | 0.2 |
| 35 | 81 | 0.2 | 1.28 | 1.79 | 2.1 |
| 36 | 78 | 0.5 | 0.49 | 3.93 | 12.5 |
| 37 | 65 | 0.38 | 3.89 | 1.44 | 12.4 |
| 38 | 74 | 1.03 | 2.59 | 6.28 | 26.4 |
| 39 | 79 | 0.73 | 3.19 | 2.55 | 4.7 |
| 40 | 72 | 0.35 | 1.57 | 1.93 | 16 |
| 41 | 73 | 0.24 | 0.78 | 2.19 | 5.3 |
| 42 | 55 | 1.13 | 1.97 | 1.93 | 7.2 |
| 43 | 71 | 0.1 | 1.41 | 0.46 | 27.2 |
| 44 | 77 | 1.96 | 3.48 | 3.63 | 24.4 |
| Mean | 71.28 | 0.67 | 2.34 | 3.05 | 14.42 |
| ST.DEV | 0.60 | 1.66 | 1.76 | 9.18 |
| Group C | |||||
| Patient | Age | Cr serum (μg/L) | Cr urine (μg/L) | Co serum (μg/L) | Co urine (μg/L) |
| 45 | 84 | 1.78 | 4 | 7.02 | 27.7 |
| 46 | 73 | 0.9 | 4.89 | 9.8 | 28.9 |
| 47 | 67 | 2.19 | 8.4 | 5.1 | 19.1 |
| 48 | 73 | 0.27 | 2.56 | 4.43 | 24.9 |
| 49 | 69 | 1.26 | 5.04 | 8.56 | 40.1 |
| 50 | 68 | 0.26 | 0.8 | 3.01 | 15 |
| 51 | 73 | 0.32 | 3.18 | 3.2 | 18.2 |
| 52 | 74 | 0.1 | 1.96 | 2.7 | 3.5 |
| 53 | 62 | 0.84 | 2.96 | 3.5 | 12.3 |
| 54 | 79 | 0.81 | 3.27 | 4.45 | 19.7 |
| 55 | 71 | 0.55 | 2.72 | 3.86 | 18.6 |
| 56 | 78 | 2.5 | 1.9 | 5.22 | 14.8 |
| 57 | 73 | 0.84 | 1.99 | 0.46 | 7.5 |
| 58 | 59 | 1.31 | 3.8 | 4.77 | 25.3 |
| 59 | 75 | 0.19 | 2.99 | 4.03 | 22.5 |
| 60 | 59 | 0.39 | 0.88 | 0.76 | 7.89 |
| 61 | 74 | 0.9 | 3.5 | 4.56 | 24.7 |
| 62 | 52 | 0.55 | 0.98 | 5.55 | 19.8 |
| 63 | 64 | 0.7 | 2.21 | 4 | 14.5 |
| 64 | 85 | 0.59 | 7.19 | 5.72 | 41.6 |
| 65 | 72 | 1.94 | 4.69 | 7.14 | 41.9 |
| 66 | 77 | 0.15 | 1.94 | 2.17 | 16.1 |
| 67 | 71 | 0.1 | 1.58 | 1.33 | 7.9 |
| 68 | 72 | 1.4 | 3.57 | 8.84 | 35.4 |
| 69 | 85 | 1.24 | 2.07 | 13.78 | 60.2 |
| 70 | 32 | 2.65 | 1.77 | 7.03 | 16.7 |
| 71 | 79 | 1.27 | 3.28 | 2.09 | 18.2 |
| 72 | 79 | 0.84 | 1.7 | 4.52 | 17 |
| 73 | 74 | 0.96 | 3.89 | 15.47 | 36.8 |
| 74 | 60 | 0.14 | 1.05 | 4.94 | 11.16 |
| 75 | 79 | 0.49 | 2.41 | 1 | 6 |
| 76 | 78 | 0.75 | 1.5 | 7.22 | 18 |
| Mean | 70.93 | 0.91 | 2.95 | 5.29 | 21.73 |
| ST.DEV | 0.69 | 1.75 | 3.41 | 12.64 |
Although we did not conduct any invasive procedures, all patients included in the study signed an informed consensus.
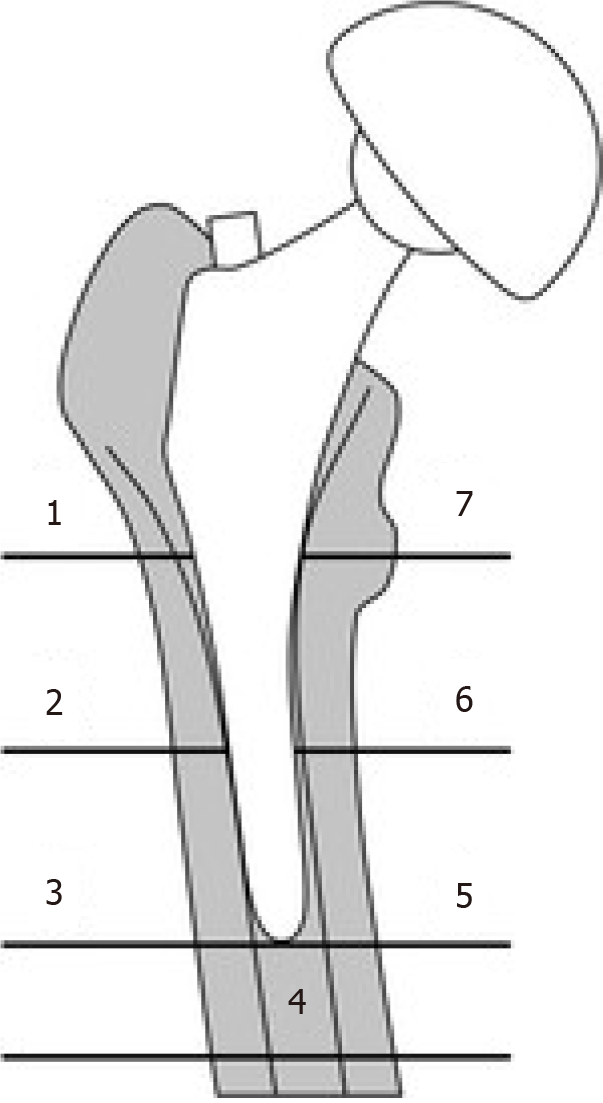
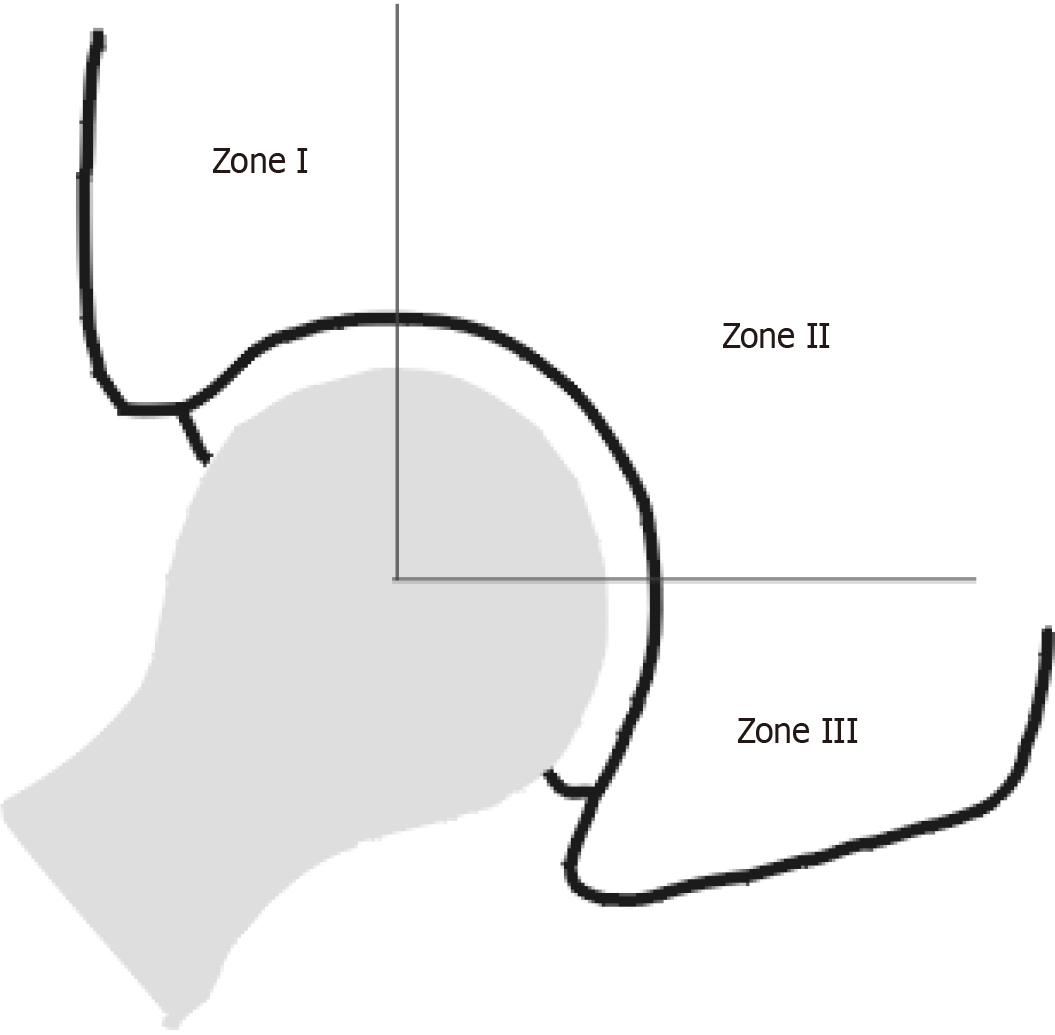
For each patient, we conducted anterior-posterior weight-bearing and axial radiographs on the operated hip. Both of the x-ray-projections were evaluated by the Authors, who looked for possible osteolytic processes and periprosthetic aseptic detachment, and whenever localized, both the site and degree of osteolysis were recorded. The assessment of osteolysis was conducted by revealing the presence of radiolucent areas or lines around the implant in all the zones described by Gruen for the femur and DeLee for the acetabulum[17] (Figures 1 and 2).
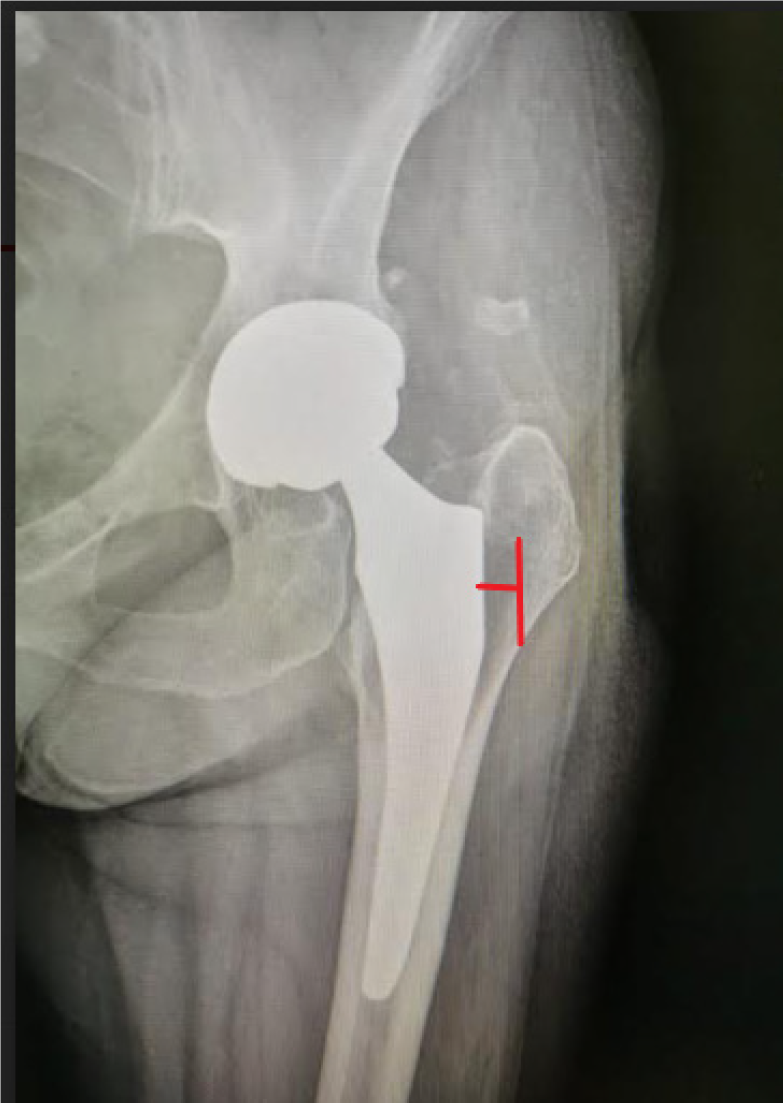
As suggested in literature, we quantified grades of osteolysis by measuring radiolucent signs in mm with the software for viewing the radiographs (Figure 3).
The degree of osteolysis was established as follows: Grade 0 no osteolysis; grade 1 osteolysis of 1-2 mm; grade 2 osteolysis between 2.1-3 mm; grade 3 osteolysis > 3 mm[17].
We also measured serum and urinary levels of Cr and Co for each patient, as shown in Tables 1-3. Patients underwent blood and urinary analyses for the study of Cr and Co values at the laboratory of Occupational Diseases of the University Clinic of Perugia. Presently, normal ranges in serum for Cr and Co are defined as 0.1-0.5 μg/L and 0.05-0.1 μg/L, respectively. While normal ranges in urine for Cr and Co are 0.05-0.35 μg/L and 0.1-1.5 μg/L[18].
We investigated for graphical and statistical correlations between levels of Cr and Co in blood and urinary exams, in order to estimate the hypothetical direct effect of higher metallic levels on the processes of osteolysis. For this, we used the statistical test of linear regression.
Finally, in all the cases of either high levels of metallic ions or painful-symptomatic, we recommended an magnetic resonance imaging (MRI), so to exclude for the presence of a pseudo-tumor around the prosthesis or other tissues-lesions.
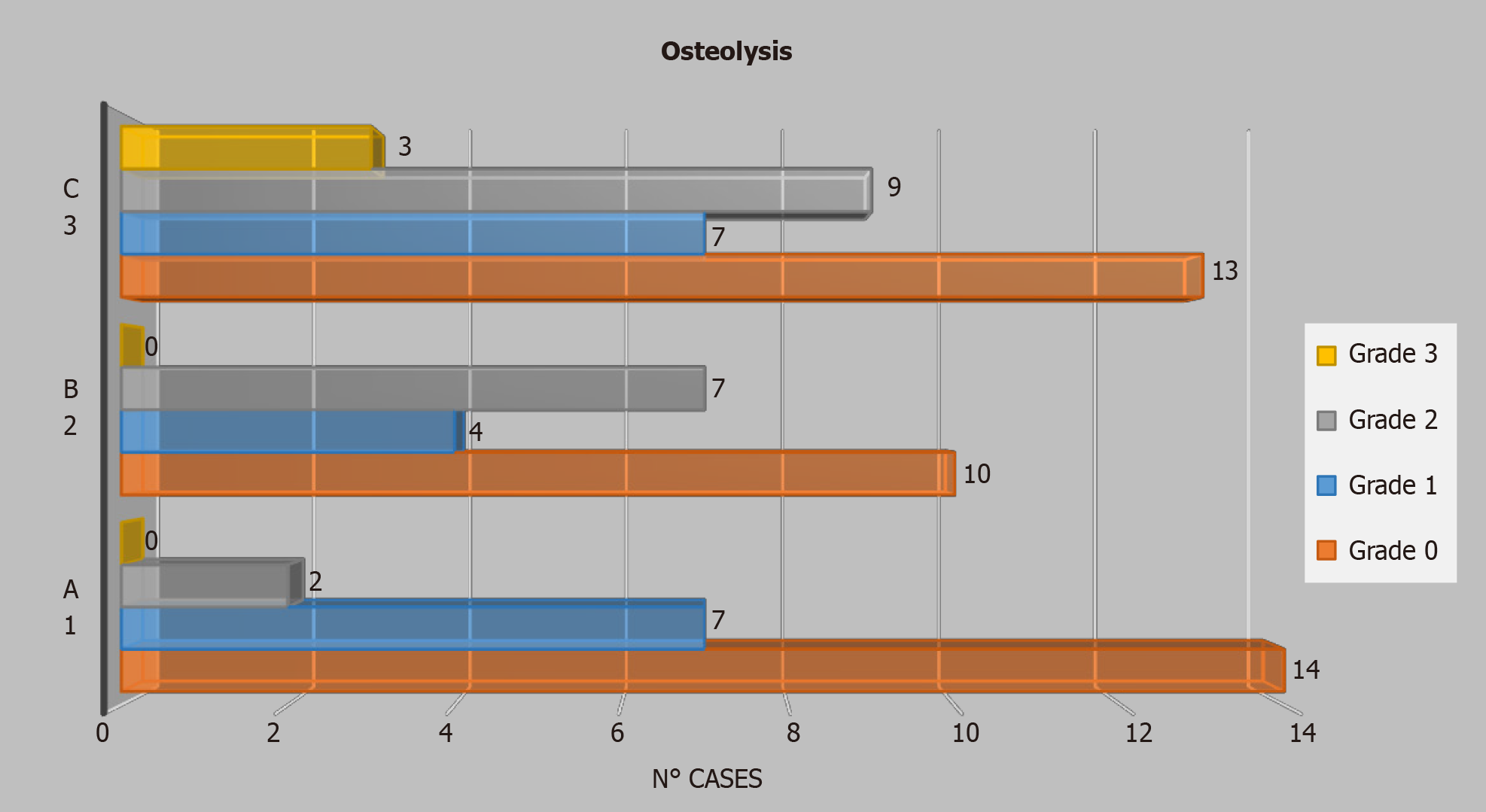
As stated above, in the radiological evaluations we determined the site and grade of the osteolysis, finding that only Group C presented a grade 3 osteolysis (Figure 4) while grade 2 was prevalent in THAs with a modular neck (16 cases), compared to monoblock THAs (2 cases).
In Figure 4, we present the final report on the observed processes of osteolysis for each group, while in Table 4 we report the localizations of the osteolysis in the peri-prosthetic field along with relative severity (Figure 4 and Table 4).
| Group A | Group B | Group C | |
| Site of osteolysis | |||
| Gruen zone 7 | 2 | 2 | 4 |
| Gruen zone 1 | 2 | 6 | 12 |
| DeLee zone 1 and 2 | 5 | 3 | 3 |
We calculated the mean values for each group: Serum Cr was 0. 54 (SD 0.56) μg/L (normal range 0. 1-0.2 μg/L) for Group A, 0.67 (SD 0.60) μg/L for Group B and 0.91 (SD 0.69) μg/L for Group C; the mean value of serum Co was 3.59 (SD 3.46) μg/L (normal range 0.05-0.3 μg/L) for Group A, 3.05 (SD 1.76) μg/L for Group B and 5.29 (3.46) μg/L for group C; the mean value of urine Cr was 1.41 (1.33) μg/L (normal range 0.05-0.35 μg/L) for Group A, 2.34 (SD 1.66) μg/L for Group B and. 95 (SD 1.75) μg/L for Group C; the mean value of urine Co was 10.06 μg/L (SD 10.19) (normal range 0.1-1.5 μg/L) for Group A, 14.42 (SD 9.18) μg/L for Group B and 21.73 (SD 12.64) μg/L for Group C (Tables 1-3).
We observed that in the modular THAs groups (B and C) there were higher serum and urinary Cr and Co levels and higher prevalence of osteolysis.
Table 5 reports on the correspondence between mean values of metal-concentration and cases of osteolysis.
| Cr Serum (μg/L) | Cr urine (μg/L) | Co serum (μg/L) | Co urine (μg/L) | Cases of Gr. 2 osteolysis | Cases of Gr. 3 osteolysis | |
| Group 1 | 0.54 | 1.41 | 3.59 | 10.06 | 2 | 0 |
| Group 2 | 0.67 | 2.24 | 3.05 | 14.42 | 7 | 0 |
| Group 3 | 0.91 | 2.95 | 5.29 | 21.73 | 9 | 3 |
Linear regressions conducted in order to quantify a direct relation between ion-release and osteolysis revealed a positive result (where positivity corresponds to P > 0 at linear regression) for every test; though there were varying degrees of significance for each test.
Cr levels from blood exams showed a coefficient of linear regression equal to 0. 048 for grade 2 of osteolysis and 0.101 for grade 3 osteolysis.
Coefficients for Cr in urine resulted being 0.21 for grade 2 osteolysis and 0.37 for grade 3; resulting in a stronger correlation, compared to the Cr in the blood.
Coefficients for Co in the blood resulted being 0.17 for grade 2 of osteolysis and 0.66 for grade 3 (both stronger than Cr). Whereas, coefficients for Co in the urine resulted being 1.51 for grade 2 of osteolysis and 3.16 for grade 3.
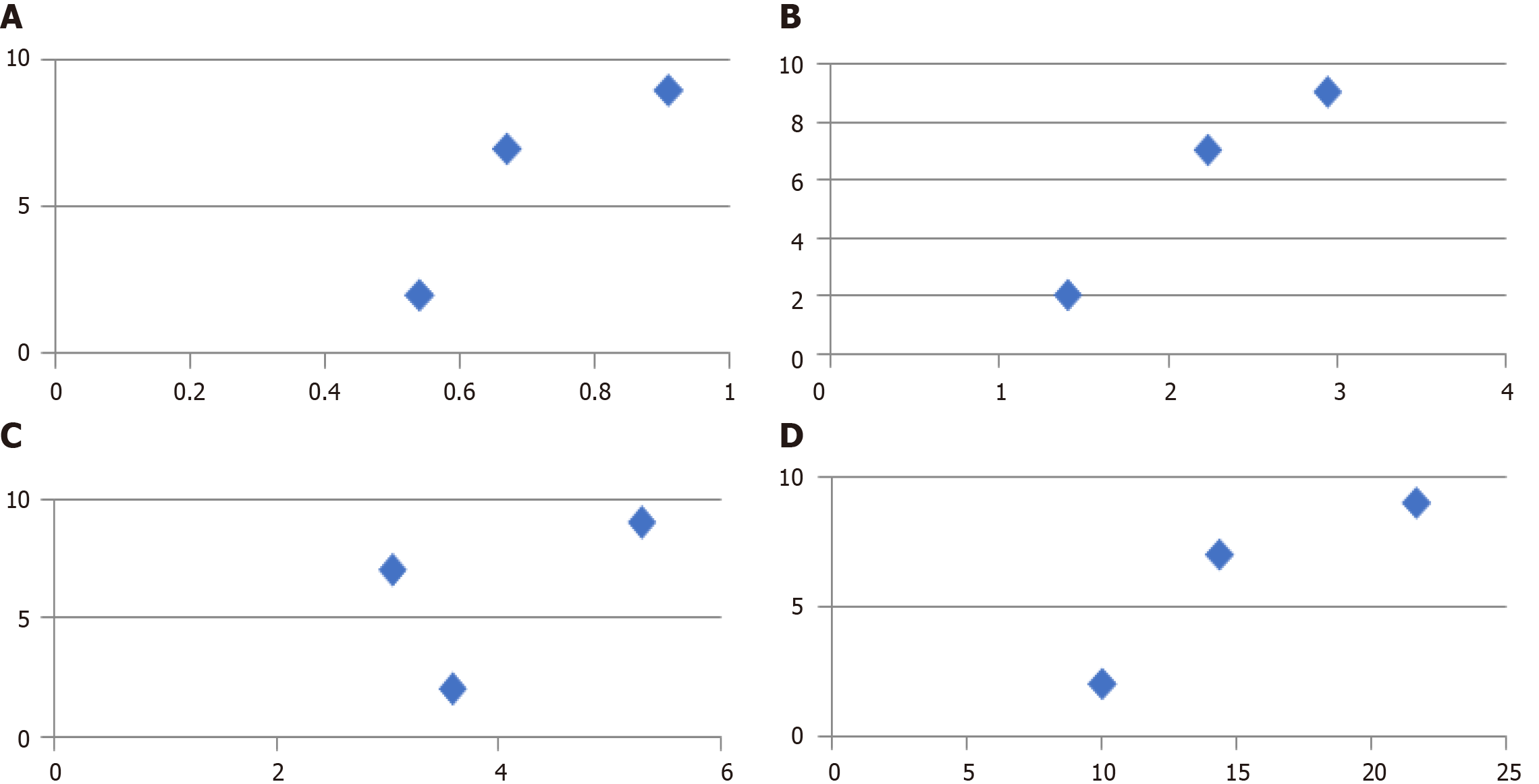
Graphs below (Dispersion graphs) show that, although there having been a variability in coefficients of linear regression for the different exams, every test result exhibited a regular tract of linear correlation, except for the serum cobalt values (Figure 5).
As for pseudotumors, of the few MRI tests we were able to carry out, there were no diagnosed cases. Additionally, all patients with loosening of the THA met exclusion criteria, so we could not conduct a correlation between the levels of metals and loosening of THA.
Corrosion at the stem-neck junction was first described in 1980[19]. Neck-to-stem wear is more significant than the head-to-neck junction, due to the higher mechanical stresses and an increased lever arm in the former[20].
Our study suggester this condition, highlighting a greater degree of osteolysis for the proximal portion of the femur (zones 1 and 7).
The choice of materials have been reported to affect the durability and survival of the implant. In current-day hip prostheses, the physical properties of CoCr provide an ideal surface for supporting the load and movement with minimal degradation over a long period. The CoCr Mo neck has increased rigidity and wear resistance compared to Ti6Al4V[21]. One possible cause of corrosion is the failure of the stem, due to a loss of tension of soft tissues, that creates micromovements of the neck; also inappropriate neck orientation on the stem creates a stress concentration on the neckline; impingement between the femoral neck and the acetabular cup, osteolysis and fretting are other possible causes of dissociation[21,22]. In addition, the formation of pseudotumors is a known complication of modular neck prostheses, even though there is no widely held consensus regarding a possible correlation with urinary and blood metal ions levels[23]. The pathophysiology of these lesions is not known, although it is assumed to be a consequence of local chronic inflammation, due to the release of metal particles causing necrosis and cell cytotoxicity[22-26]. Some authors have reported that patients with pseudotumors have higher chromium and cobalt serum levels, compared to patients without pseudotumors[27]. Furthermore, over the last decade, physicians have described additional adverse effects beyond osteolysis, including pseudotumors and loosening caused by metallosis. In fact, several studies have described how Metal-on–Metal prostheses may be a potential cause of ALVAL lesions[28]. ALVAL is short for “Aseptic Lymphocyte-Dominant Vasculitis-Associated Lesions” a histological entity denoting a chronic inflammatory response to metal particles, as a T-lymphocyte-mediated type IV hypersensitivity reaction. Specifically, the particles activate cytotoxic T-lymphocytes and macrophages, which in turn leads to tissue damage[29].
Joint prostheses in CoCr are not subject to standard biological monitoring and the acceptable levels of CoCr in the blood and urine have yet to be established. To date, it is uncommon practice to measure CoCr serum and urinary levels in patients who had undergone prosthetic implant surgery. There is no standardized assessment for maximum levels, either for either blood or urine levels, which might assist surgeons in patient management; instead surgeons can rely upon only clinical symptoms and/or adverse reactions. Therefore, although there is no currently held agreement on what the possible decision limits could be for increases in blood and urine metal levels, any elevation should always be considered relevant and worthy of attention.
While there have been studies that have analyzed these events from a pathophysiological perspective, it is evident that in literature there have been few studies that have been designed with a clinical, radiological and tribological approach for patients with modular and monoblock prostheses, regardless of the coupling of metals and non-metallic material (ceramic, polyethylene) used in their modularity.
For the most part, most studies have statistically evaluated ion elevations relating to the type of prostheses, whereas few have correlated osteolytic events and/or conducted clinical follow-up. Another limitation of past studies concerns their short follow-ups, with only a few studies planning periods longer than 40 mo.
In 2007, Daniel et al[30] conducted a four-year follow-up after THAs in young and active patients. Like in our study, the authors reported significant increases in the levels of metal ions at 1-year follow-up, compared to pre-operative times. At 4-year follow-up, the same Authors observed a progressive reduction in these levels; statistically significant for Cr but not for Co[30].
In 2020, Pozzuoli et al[31], were able to perform a 7-year follow-up, from which they reported a revision-rate for MOM prostheses where they conducted clinical and radiographic evaluations in relation to metal-ion release[31].
For our study results, we saw how the release of metal ions could, either directly or indirectly, set off the activity of the osteoclasts, and might, therefore, have been responsible for periprosthetic bone resorption, and in some cases for aseptic loosening of the implant itself. From our radiogram findings, it is evident (Graph I) that the cases of marked osteolysis were only in Group C, which was also the group with the most cases of grade-two osteolysis. We can therefore affirm that, in our study, a greater release of metal ions corresponded to a greater number of osteolysis cases.
The statistical tests of the linear regression used, generally showed positive correlations between detected increases in the values of metal ions and the severity of osteolysis.
However, these results need to be challenged by further investigations. Our sample of patients underwent investigations that could not be complete, including biomechanical tests and/or longer follow-up. Unfortunately, MRI was not performed on all the patients in this series, and when done so, it could not be performed in a standardized manner. This made those few examinations, neither clinically nor statistically comparable. So, it was not possible to hypothesize on a relationship between either the phenomenon of inflammatory lesions and the degree of metal release or the severity of periprosthetic osteolysis.
Periprosthetic osteolysis in total hip replacement is one of the most significant mid- and long-term adverse events that has been described over the years. The cause of this event has been widely debated, with a wide consensus on a macrophage inflammatory response due to the presence of metal ions released from the implants caused by wear mechanisms. Our study results indicate a direct quantitative, as well as qualitative relationship, between the release of the most common periprosthetic metal ions in THAs (Cr and Co) and the presence of periprosthetic osteolysis. Furthermore, our results showed how modularity in THAs has irrefutable biomechanical advantages but is, however, associated with both a higher degree of metal ion release and greater prevalence of osteolysis events. These increases were even greater when metals rather than ceramic components were detected in the modularity.
To obtain a robust level of evidence, future randomized and controlled trials should be designed to identify further risk factors that could affect the levels of metal ions, which could also be associated either systemic adverse effects or local events, such as osteolysis, aseptic loosening and/or tissues-lesions.
Osteolysis is one of the most common and important adverse reactions to total hip arthroplasty (THA). Therefore it’s important to define if there are conditions that facilitate its occurrence.
There is a lack of works studying the correlation between metal ions levels and osteolysis and its different prevalence between modular THA and monolithic prostheses.
Studies analyzing these topics would help the surgeons in the choice of the implants and in the in a correct patients’ follow-up. So that we designed this work aiming to have a comprehensive vision of a complication, such as the osteolysis, in THA.
We enrolled 76 patients who underwent an operation of first implant of THA, with no other prosthesis and no Cobalt and Chrome (CoCr) work exposure. We divided them in three groups: Patients with monoblock prosthesis with metal head (Group A,), patients with modular prosthesis with ceramic head (Group B), patients with modular prosthesis with metal head (Group C). We analyzed the presence, if any, of osteolysis, its localization and the serum and urinary metal ions levels (Cr and Co).
We found out a direct correlation between the release of periprosthetic metal ions and osteolysis, also this study highlights that modularity is related to a higher metal ion release and osteolysis events.
Our study reveals that there is a correlation between metal ions levels and presence and severity of osteolysis and that this is more evident in modular THA, due to higher corrosion.
Obviously there is a need for more studies to obtain a good level of evidence and confirm these findings.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oommen AT S-Editor: Fan JR L-Editor: A P-Editor: Wang LYT
| 1. | Atwood SA, Patten EW, Bozic KJ, Pruitt LA, Ries MD. Corrosion-induced fracture of a double-modular hip prosthesis: a case report. J Bone Joint Surg Am. 2010;92:1522-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Dangles CJ, Altstetter CJ. Failure of the modular neck in a total hip arthroplasty. J Arthroplasty. 2010;25:1169.e5-1169.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Jacobs JJ, Gilbert JL, Urban RM. Corrosion of metal orthopaedic implants. J Bone Joint Surg Am. 1998;80:268-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 474] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 4. | Keegan GM, Learmonth ID, Case CP. A systematic comparison of the actual, potential, and theoretical health effects of cobalt and chromium exposures from industry and surgical implants. Crit Rev Toxicol. 2008;38:645-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Brown C, Williams S, Tipper JL, Fisher J, Ingham E. Characterisation of wear particles produced by metal on metal and ceramic on metal hip prostheses under standard and microseparation simulation. J Mater Sci Mater Med. 2007;18:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Brown C, Fisher J, Ingham E. Biological effects of clinically relevant wear particles from metal-on-metal hip prostheses. Proc Inst Mech Eng H. 2006;220:355-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 397] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 8. | Revell PA, al-Saffar N, Kobayashi A. Biological reaction to debris in relation to joint prostheses. Proc Inst Mech Eng H. 1997;211:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 124] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Oberdörster G. Pulmonary effects of inhaled ultrafine particles. Int Arch Occup Environ Health. 2001;74:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 592] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 10. | Jones LC, Frondoza C, Hungerford DS. Immunohistochemical evaluation of interface membranes from failed cemented and uncemented acetabular components. J Biomed Mater Res. 1999;48:889-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Purdue PE, Koulouvaris P, Potter HG, Nestor BJ, Sculco TP. The cellular and molecular biology of periprosthetic osteolysis. Clin Orthop Relat Res. 2007;454:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 268] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 12. | Masui T, Sakano S, Hasegawa Y, Warashina H, Ishiguro N. Expression of inflammatory cytokines, RANKL and OPG induced by titanium, cobalt-chromium and polyethylene particles. Biomaterials. 2005;26:1695-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Davies AP, Willert HG, Campbell PA, Learmonth ID, Case CP. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Joint Surg Am. 2005;87:18-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 307] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 14. | Korovessis P, Petsinis G, Repanti M, Repantis T. Metallosis after contemporary metal-on-metal total hip arthroplasty. Five to nine-year follow-up. J Bone Joint Surg Am. 2006;88:1183-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 209] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Milosev I, Trebse R, Kovac S, Cör A, Pisot V. Survivorship and retrieval analysis of Sikomet metal-on-metal total hip replacements at a mean of seven years. J Bone Joint Surg Am. 2006;88:1173-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Köster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 681] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 17. | Molloy DO, Munir S, Jack CM, Cross MB, Walter WL, Walter WK Sr. Fretting and corrosion in modular-neck total hip arthroplasty femoral stems. J Bone Joint Surg Am. 2014;96:488-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Cornelis R, Heinzow B, Herber RF, Christensen JM, Poulsen OM, Sabbioni E, Templeton DM, Thomassen Y, Vahter M, Vesterberg O. Sample collection guidelines for trace elements in blood and urine. IUPAC Commission of Toxicology. J Trace Elem Med Biol. 1996;10:103-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 88] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Lucas LC, Buchanan RA, Lemons JE. Investigations on the galvanic corrosion of multialloy total hip prostheses. J Biomed Mater Res. 1981;15:731-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 64] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Kretzer JP, Jakubowitz E, Krachler M, Thomsen M, Heisel C. Metal release and corrosion effects of modular neck total hip arthroplasty. Int Orthop. 2009;33:1531-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Nganbe M, Khan U, Louati H, Speirs A, Beaulé PE. In vitro assessment of strength, fatigue durability, and disassembly of Ti6Al4V and CoCrMo necks in modular total hip replacements. J Biomed Mater Res B Appl Biomater. 2011;97:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Meftah M, Haleem AM, Burn MB, Smith KM, Incavo SJ. Early corrosion-related failure of the rejuvenate modular total hip replacement. J Bone Joint Surg Am. 2014;96:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23:943-944. [PubMed] |
| 24. | Watters TS, Eward WC, Hallows RK, Dodd LG, Wellman SS, Bolognesi MP. Pseudotumor with superimposed periprosthetic infection following metal-on-metal total hip arthroplasty: a case report. J Bone Joint Surg Am. 2010;92:1666-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468:2321-2327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 406] [Cited by in RCA: 388] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 26. | Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90:847-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 716] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 27. | Kwon YM, Glyn-Jones S, Simpson DJ, Kamali A, McLardy-Smith P, Gill HS, Murray DW. Analysis of wear of retrieved metal-on-metal hip resurfacing implants revised due to pseudotumours. J Bone Joint Surg Br. 2010;92:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 28. | Wiley KF, Ding K, Stoner JA, Teague DC, Yousuf KM. Incidence of pseudotumor and acute lymphocytic vasculitis associated lesion (ALVAL) reactions in metal-on-metal hip articulations: a meta-analysis. J Arthroplasty. 2013;28:1238-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Watters TS, Cardona DM, Menon KS, Vinson EN, Bolognesi MP, Dodd LG. Aseptic lymphocyte-dominated vasculitis-associated lesion: a clinicopathologic review of an underrecognized cause of prosthetic failure. Am J Clin Pathol. 2010;134:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Daniel J, Ziaee H, Pradhan C, Pynsent PB, McMinn DJ. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty: four-year results of a prospective longitudinal study. J Bone Joint Surg Br. 2007;89:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Pozzuoli A, Berizzi A, Crimì A, Belluzzi E, Frigo AC, Conti G, Nicolli A, Trevisan A, Biz C, Ruggieri P. Metal Ion Release, Clinical and Radiological Outcomes in Large Diameter Metal-on-Metal Total Hip Arthroplasty at Long-Term Follow-Up. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |