INTRODUCTION
The skeleton is the third most common site of metastasis, with breast, prostate and lung cancer accounting for 80% of bone metastases. The incidence of bone metastasis is uncertain, but post-mortem studies have reported incidences of 74% and 68% for breast and prostate cancer, respectively, and 28%-34% for lung, thyroid and kidney carcinomas[1,2]. Other neoplasms, such as gastrointestinal tract tumours are reported to rarely metastasize to the skeleton but there may be geographical differences[3]. In line with these post-mortem findings, the Scandinavian Sarcoma Group registry found that prostate, renal cell, lung cancer and myeloma accounted for 78% of surgically-treated bone tumours[4]. The most common site for bony metastases is the spine. In the appendicular skeleton, the femur is the most common site, followed by the humerus and then the tibia[3].
Symptomatic metastasis to bone is commonly referred to as metastatic bone disease (MBD). MBD can present with hypercalcaemia, bone pain, spinal cord or nerve root compression, impending or manifested fractures[1]. In cancer patients, bone pain is highly suggestive of bone metastases[4]. The exact number of patients living with MBD is unknown, but the number is expected to rise primarily due to the ageing population and the advancement in treatment of the most common cancer types[1,5]. MBD is painful and has a significant negative effect on quality of life, the negative effects can be improved with surgery[6]. Most patients with MBD are palliative. Median survival from diagnosis of bone metastasis ranges from 6 mo in lung cancer and melanoma, to 12-53 mo in prostate cancer and 19-48 mo in breast cancer[4]. Reflecting this, median postoperative survival is, depending on the primary cancer, 3-12 mo[7].
Despite the fact that MBD severely affects a large group of palliative patients and is a driver of overall oncology cost[8], research is scarce and mainly consists of retrospective case series which are heterogenous and difficult to draw conclusions from. Solid evidence is lacking in almost every field, from surgical management to adjuvant treatment[9-12].
As the main symptoms of metastatic bone disease are pain, loss of mechanical function due to instability, and neurological compromise, the goal of surgery is to provide pain relief, restore biomechanical stability and potential neurological compromise. Nonsurgical treatments such as radiotherapy, chemotherapy or local tumour ablation are almost never effective in relieving pain associated with biomechanical insufficiency and instability related to pathological fractures. Another important axiom is that treatment of pathological fractures cannot rely on bone healing as these fractures rarely ever heal.
Surgical reconstruction should be stable enough to last the often short lifetime of the patient and allow for immediate mobilization, anything less than that should be considered a surgical failure. As the point of best skeletal stability is achieved directly after surgery in most cases, orthoses, braces or other devices meant to support the surgical fixation should not be used.
INDICATION FOR SURGICAL TREATMENT
The decision to operate on a metastatic bone lesion, with or without a pathological fracture is not always easy. Complete pathological fractures in the long bones of the upper or lower extremity, especially the femur and the humerus, are almost always treated surgically as this is the only way to allow for patient mobilization and adequate pain relief. Non-surgical treatment in these cases is reserved for patients that are in a late terminal stage of their disease. On the other hand, complete pathological fractures in flat bones of the axial skeleton, such as the ribs, the innominate bones of the pelvis, the sternum and the scapula are almost never treated with surgery.
Pathological fractures of the spine are treated in the general context of metastatic spinal disease, the degree and characteristics of the neurological impairment together with the overall condition of the patient and the expected oncological outcome are the primary factors taken into account in the choice of treatment. Patients with severe, but not complete neurological deficits, those with recent onset of symptoms and those with good prognosis are the most likely to benefit from surgery. Clinically useful staging systems are available in order to select patients for surgical treatment[13-15]. There has been a considerable shift towards surgical treatment in the past decades, with evidence showing a clear benefit in the neurological outcome of patients treated with surgery which may also have an implication for survival[16].
Fractures of the small bones of the hand and foot are extremely rare and there are very few data regarding their treatment[17,18]. When treating patients with impending fractures the surgical indications are relative. A combination of prediction of future fracture risk, severity of clinical symptoms, location and extent of the lesion, expected patient survival and the potential harm of failed surgery should be carefully considered. As an exception, bone metastasis from haematological malignancy without any obvious pathological fracture should preferably be treated non-surgically. These neoplasms are usually highly radiosensitive and tend to respond quickly to chemotherapy. The indication for surgical treatment is a complex decision-making process and reflects the considerably high variation in practice among different clinics and regions[19].
To expedite decision-making, several algorithms have been developed, regarding both the characteristics of the lesion as well as the expected survival of the patient. One of the most commonly used algorithms is the classification introduced by Mirels for predicting fracture risk[20]. The original study is limited by a small study base, consisting of mainly breast cancer patients. It is still widely used but is rather obsolete[21,22]. For a more reliable prediction of fracture risk, computed tomography (CT) based measures of structural stability can be used[23]. If CT scans are not readily available, a newly developed model that requires nothing more than a scale for predicting impending fractures of the lower extremity through single stance weight bearing has been suggested by Howard et al. However, this method still needs external validation[24].
Another critical parameter in the decision to proceed to surgery is the expected patient survival. Certain metastatic lesions in the axial skeleton, for example spinal metastases, are probably not amenable to surgical treatment if estimated patient survival is poor, generally less than 3 mo. The development of algorithms to be used as tools in order to predict survival has been a significant achievement in this field, making predictions much more accurate, and considerably facilitates the task of the treating physician.
METHODS AND OUTCOME OF SURGICAL TREATMENT
The surgical method and the implant to be used should be chosen wisely. As a rule of thumb, the longer the expected survival of the patient, the more extensive the surgery.
In patients with a particularly good survival prognosis such as those with solitary metastases from breast cancer or renal cell carcinoma, en bloc resection and reconstruction with a tumour prosthesis is justified as these tumours have a lower risk of relapse. En bloc resection in these cases is also associated with improved survival[19,25].
Doctors are known to overestimate when predicting survival. As discussed in the previous paragraph, precision in estimation is critical in selecting patients who will benefit from surgery and is essential for preoperative planning[26,27]. Estimating survival and correlating choice of implant to survival estimates, prevents too extensive surgery and rehabilitation in patients with short survival and an unacceptably high implant failure rate in those who live longer. As externally validated survival models that are free to use exist, there are no reasons not to do so[28-31,32]. There are generally three treatment strategies: Osteosynthesis, prosthetic reconstruction or local excision with or without reconstruction (i.e., curettage with or without cementation, cementoplasty, excision arthroplasty, resection of a bone segment or amputation).
Surgical treatment of metastases in the appendicular skeleton
In the long bones, surgery is recommended even when estimated survival is as short as two-six weeks[33]. The whole bone should be examined radiologically preoperatively, and this can be performed with plain radiographs or a CT-scan. In the long bones, the femur is the commonest site of MBD, and the proximal femur is the most common site for pathological fractures in the femur. Unlike in native fractures of the proximal femur, delayed time to surgery > 48 h does not seem to affect postoperative complication rate, further strengthening the hypothesis that careful preoperative planning should be undertaken in these patients[34]. The implant of choice when performing surgeries of the femoral neck is an endoprosthesis. Only if the patient is deemed unfit for prosthesis-surgery, a percutaneous screw fixation may be considered, occasionally with cement reinforcement.
In the trochanteric region, intramedullary nails (IM-nails), conventional prostheses or tumour prostheses can be used. As complications related to non-union and tumour growth such as implant breakage and loosening tend to occur 6-12 mo postoperatively, in patients with a shorter estimated survival (3-6 mo), IM-nailing is adequate[10,12,35,36]. However, IM-nails should not be used if there is tumour mass affecting the neck of the femur, even if the fracture itself is in the intertrochanteric region. A large nail diameter adds mechanical stability and reduces the risk of implant breakage, providing the patient with a better chance of early pain reduction. Because of this, we believe that proper reaming should always be done despite the added cardiovascular risk.
In the proximal femur, prosthesis surgery is associated with better functional outcomes and a lower risk of revision surgery[33]. However, it is associated with a higher risk of systemic complications in the postoperative period due to surgical trauma and generally requires a longer period of rehabilitation. Due to this, prosthesis surgery is generally reserved for patients with a longer estimated survival. When there are no clinical signs of osteoarthritis a hemiarthroplasty is often sufficient. Total hip replacement poses a higher risk of dislocation and is associated with longer operating times and thus perioperative risks[37]. However, the only study published so far comparing morbidity and mortality between hemiarthroplasty and total hip replacement in MBD patients found no difference in short-term morbidity and mortality[33].
In the femoral diaphysis, IM-nails are preferred, provided there is sufficient bone stock. Osteosynthesis with long plates are also used. There is seldom the need for more advanced reconstructions, such as intercalary prostheses which are reserved for patients with excellent prognosis only. MBD of the distal third of the femur can be managed with osteosynthesis, and adjuvant bone cement is often needed due to poor bone quality in this region. Other options are conventional cemented knee arthroplasty for lesions restricted to the subchondral area or tumour prosthesis for larger lesions[38].
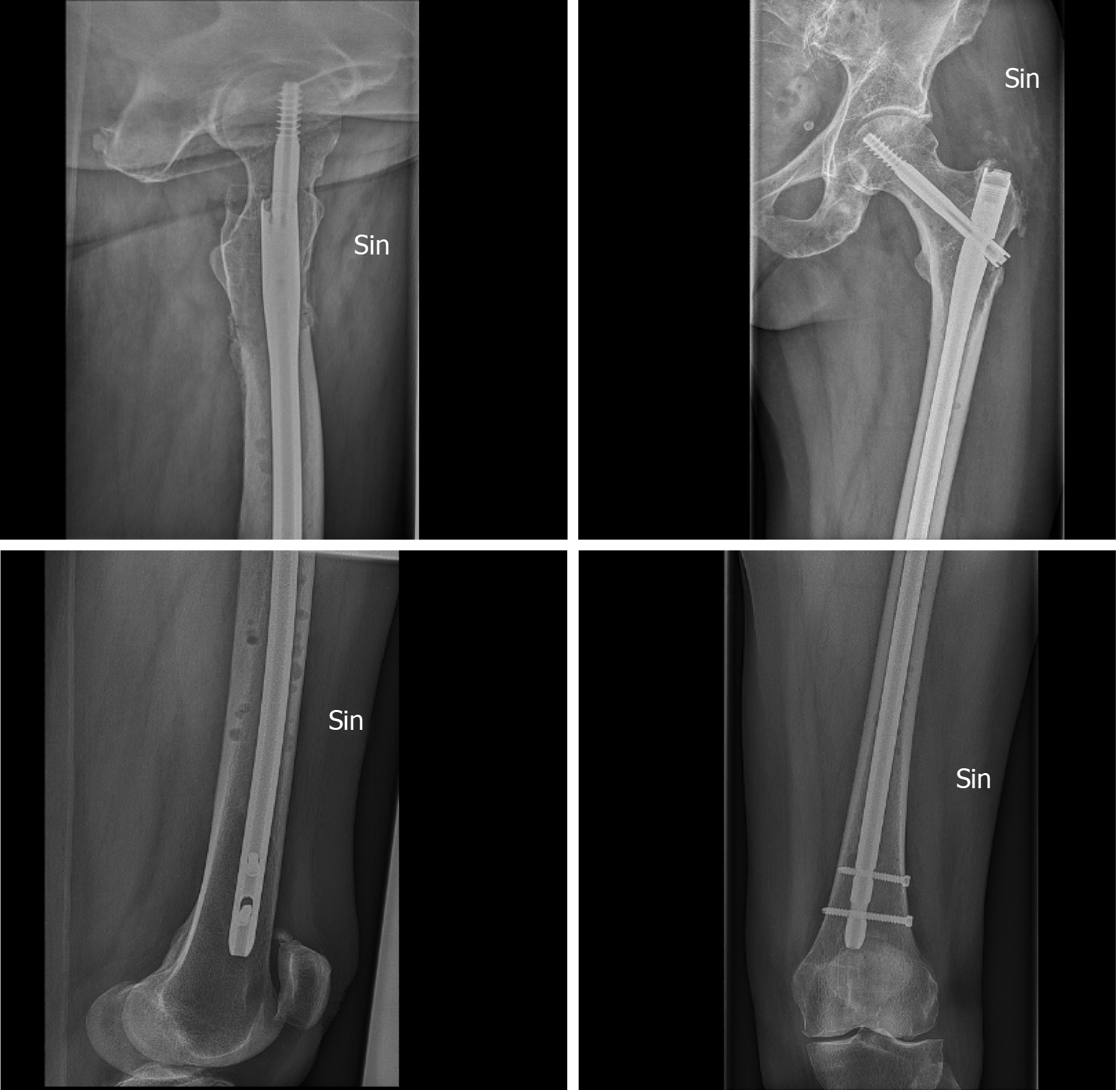
Despite the fact that that prosthesis surgery is the preferred surgical method for patients with a life expectancy of more than 6 mo, treatment of a lesion in the upper metaphyseal region and the life expectancy of MBD patients is possibly increasing. Varady et al[39] found a trend towards the increased use of IM-nails in patients operated between 2009-2017, indicating a possible change in general treatment strategies (Figures 1 and 2).
Figure 1 Multiple bone metastases in the femur of a patient with an estimated survival of 4 mo.
Intramedullary nailing with a long intramedullary nail. The patient was able to mobilize immediately and there were no re-operations.
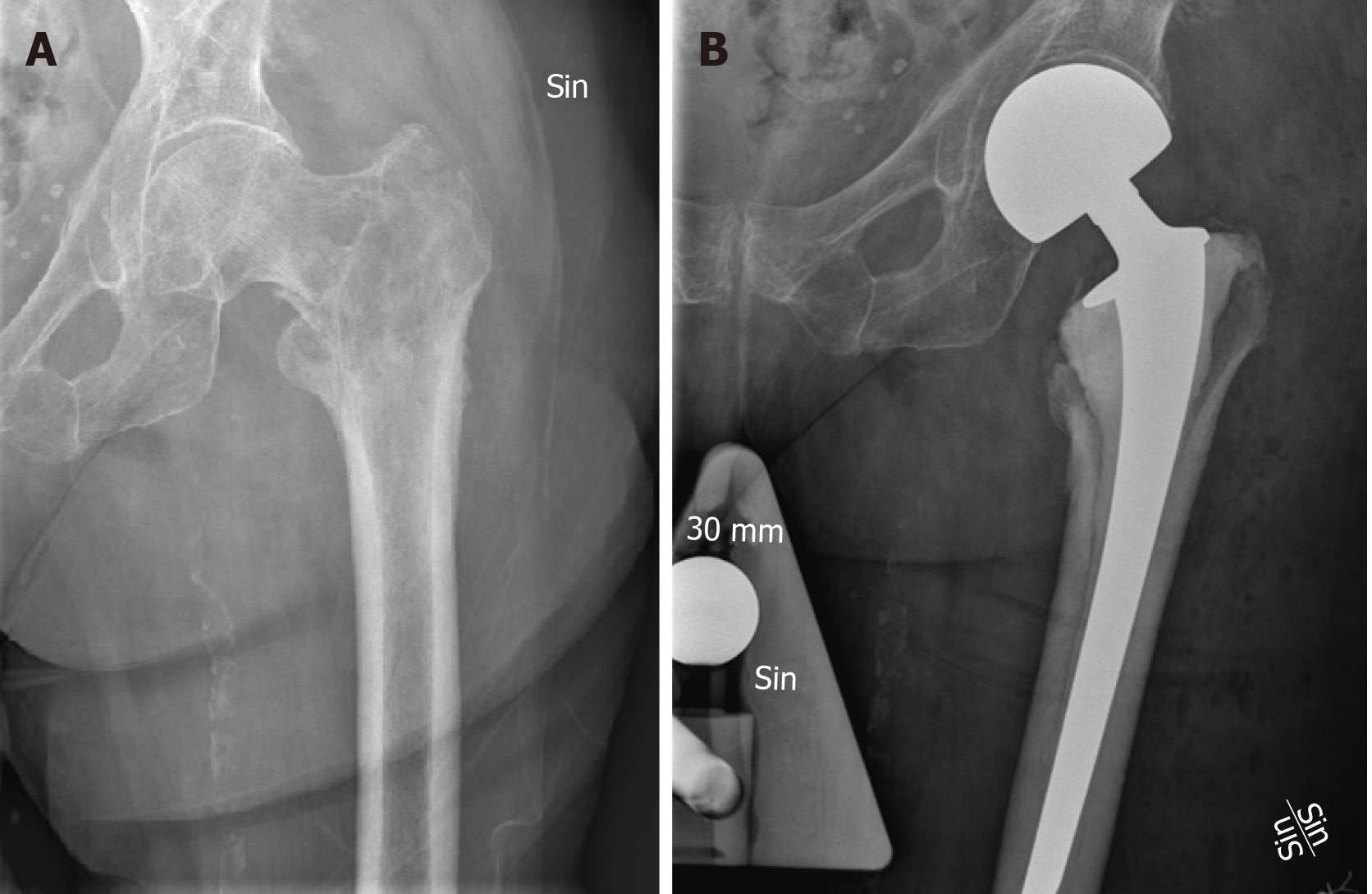
Figure 2 Solitary osteolytic lung cancer metastasis.
A: Solitary osteolytic lung cancer metastasis of the trochanteric region of the left femur in a 63-year-old female, with expected survival of approximately 1 year; B: Curettage of the lesion and reconstruction with a cemented hemiarthroplasty.
The humerus is the second most common site for MBD of the long bones[9]. Standard surgical options are the same as for the femur. As the humerus is not a weight bearing bone, the primary surgical indication should be residual pain after radiotherapy or a complete fracture with significant symptoms. In the upper metaphysis of the humerus, plates and screws with cement augmentation as needed or prosthesis surgery can be used, with prosthesis surgery being preferred for patients with large bone destruction or a longer life expectancy. In the meta-diaphysis or diaphysis region of the humerus, the most common implants of choice are IM-nails or plate and screw fixation, with cement if needed. In the humerus, pathological fractures of the diaphysis are the most common reason for surgery. As in the femur, IM nailing and prosthesis surgery have the lowest reported reoperation rates and most reoperations are due to non-union, with an increasing complication rate over time[37,39]. The distal third of the humerus accounts for the highest complication rates[37]. Tertiary centres should preferably be consulted in these cases and patients transferred as decisions regarding surgical options and adjuvant treatment are best handled by experienced surgeons.
Surgical treatment of metastases in the axial skeleton
Regarding pathological fractures of the axial skeleton, surgery is mainly indicated in cases of acetabular involvement or spinal metastasis. When the acetabulum is affected by MBD there is usually significant pain at ambulation. When protrusion of the femoral head occurs, the joint may be locked prohibiting even sitting in a wheelchair. Non-surgical treatment is not efficient in relieving such biomechanical symptoms. Surgery may entail a simple excision arthroplasty in patients with very poor general condition or terminal disease. Cementoplasty may be considered in constrained, relatively small lytic periacetabular lesions without any displaced pathological fracture[40,41]. In relatively small defects that are not contained, and extend to the hip joint, curettage and a cemented total hip arthroplasty are sufficient. When there is a displaced fracture and the general condition of the patient allows, more advanced reconstruction may be necessary. The general principle is the transfer of the biomechanical load from the proximal femur to the intact pelvic bone. This can be achieved using an anti-protrusion acetabular cage with screws placed in an antegrade or retrograde mode[42,43]. When there is extensive bone loss prohibiting the aforementioned technique, the skeletal defect may be bridged using a pelvic prosthesis (usually an ice-coned device) that is docked to the remaining intact pelvic bone, a technique which usually requires adequate bone stock around the posterior iliac bone[44]. In extreme cases of bone loss, the implant may be inserted in the sacrum. The outcome of surgical treatment of acetabular lesions is generally good, with most patients regaining ambulatory capacity and experiencing pain relief. However, the complication rate in this area is also significant, with dislocations of the prostheses being common, probably due to the insufficient bone stock which prevents optimal placing of the implants. Furthermore, infections are also frequent, due both to the microbial flora of the area and the bulky implants used.
In metastatic spinal disease, the most common method of surgical treatment is posterior decompression (laminectomy), with or without posterior fixation with pedicle screws and rods[45]. Although metastatic spinal disease generally involves the spinal body rather than the posterior elements, and anterior decompression should theoretically be advantageous, posterior-only approaches have been shown to be equally effective. When there is no pathological fracture of the involved vertebra, decompression is generally sufficient. In cases of pathological fracture with biomechanical instability, posterior fixation is recommended, especially when the expected survival is longer than 6 mo. The functional outcome of surgery for metastatic spinal disease has been shown to be superior to treatment with radiotherapy only, and the majority of patients experience improvement of neurological function and regain the ability to ambulate[16,46]. There is also evidence that these patients have longer survival which may be attributed to rapid mobilization. Common complications in this area are infections, since there is generally poor soft-tissue coverage especially in the thoracic spine, which is the most common area for metastatic spinal disease in cancer patients.
ADJUVANT TREATMENT OF METASTATIC BONE DISEASE
Single therapy low dose radiotherapy (RT) provides good pain relief in most MBD patients and is often the first line of treatment[47]. However, preoperative RT has been associated with higher complication rates[19,37]. Postoperative RT is widely used with the intention to decrease the risk of metastatic growth and subsequent implant loosening, loss of function and pain. As pointed out in a review article on the subject by Willeumier et al[11,48], this practice is possibly harmful as postoperative RT theoretically could inhibit soft tissue healing and evidence of its beneficial effects are scarce and mainly based on one retrospective cohort study.
Inhibition of osteoclast activity is a well-established method to prevent skeletal events, such as pathological fractures, in patients with MBD. Bisphosphonates have been the standard of care since the late 1990s, with zoledronate showing the most potent effect. Besides their direct inhibition of osteoclast activity, other mechanisms such as stimulation of innate immune cells have been proposed to explain their observed effect on patient survival. Their use is best documented in breast and hormone-resistant prostate cancer[48]. A significant achievement during the past decade has been the introduction of denosumab, a monoclonal antibody against RANKL which directly blocks osteoclast activity and is less nephrotoxic than bisphosphonates[49]. Current guidelines describe the indication for the use of these agents in order to prevent skeletal-related events such as pathological fractures. However, their effect on the outcome of surgical treatment of these fractures has to our knowledge not been investigated[48]. Theoretically, they may prevent progression of osteolytic lesions after surgical treatment, or even promote consolidation of the lesion, analogous to the effect denosumab has on giant cell tumours of the bone.
Percutaneous image-guided interventions, such as embolization and thermal ablation of bone metastases are also available. Embolization of feeding vessels may considerably facilitate surgery of a metastatic bone lesion, especially in central locations such as the pelvis and spine[50]. Certain primary tumours such as renal cancers are well-known for their propensity to bleed profusely. Pre-operative embolization can reduce the associated morbidity and allow for a better surgical outcome. Thermal ablation, most often radiofrequency ablation or cryoablation, is used when open surgical treatment is contraindicated. It relies on a direct thermal effect destroying the malignant cells[51,52].
Bone cement has also been shown to have a thermal effect on tumour cells due to the exothermal polymerization reaction. Its use is common in surgery for MBD, both in cases of osteosynthesis as well as during endoprosthetic reconstruction. In the former case, cement provides structural support after curettage of the bone lesion. Furthermore, it can be used separately to mechanically reinforce the bone in cases of constrained lytic metastatic lesions (cementoplasty), commonly in the spine or acetabulum. Despite its vast use in surgery for MBD, whether the use of bone cement contributes to a decreased risk of surgical failure has not been established.
CONCLUSION
MBD is still indicative of end-stage cancer and for most patients also bone pain and possibly fractures. Orthopaedic surgery is effective in reducing pain and restoring ambulatory capacity in these palliative patients but must be carefully planned to avoid causing unnecessary harm. Patients with complicated fractures, especially of the distal ends of the long bones should be referred to tertiary centres, as should patients with single or oligometastases and a very long life expectancy in whom en bloc resection of the tumour might be beneficial. High quality research is still lacking, probably due to the practical difficulties of performing prospectively randomized studies in this palliative study group.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country/Territory of origin: Sweden
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xu GY S-Editor: Wang J L-Editor: Webster JR E-Editor: Wang LL