Published online Jan 18, 2020. doi: 10.5312/wjo.v11.i1.36
Peer-review started: March 26, 2019
First decision: June 11, 2019
Revised: October 18, 2019
Accepted: November 7, 2019
Article in press: November 7, 2019
Published online: January 18, 2020
Processing time: 290 Days and 9.5 Hours
The alpha-defensin lateral flow (ADLF) test is a new diagnostic tool for periprosthetic joint infection (PJI). Test accuracy for combined cohorts of hip and knee PJI has been reported to be good.
To assess the accuracy of the ADLF test for hip PJI, and to compare three different diagnostic criteria for PJI.
A cohort of 52 patients was identified, with a painful or poorly functioning total hip- or hemi-arthroplasty, that underwent aspiration and a subsequent ADLF test. PJI was diagnosed with Musculoskeletal Infection Society (MSIS) criteria, and sensitivity, specificity, overall accuracy, positive predictive value and negative predictive value were calculated. Furthermore, test specifics were compared with the European Bone and Joint Infection Society (EBJIS) and 2018 International Consensus Meeting (ICM) criteria for PJI.
Using MSIS criteria, sensitivity was 100% (CI: 54%-100%) and specificity was 89% (CI: 76%-96%). Six true positives and 5 false positives were found, including one case of metallosis. Using EBJIS criteria, more PJIs were found (11 vs 6), sensitivity was lower (71%, CI: 42%-92%) and specificity was higher (97%, CI: 86%-100%), with 4 false negatives and one false positive result. Using 2018 ICM criteria, sensitivity was 91% (62%-100%) and specificity 100% (91%-100%). The results in this cohort are comparable to previous studies.
Overall test accuracy of the ADLF test was good in this cohort, with a sensitivity of 100% and specificity of 89%. Using different PJI definition criteria, sensitivity and specificity changed slightly but overall accuracy remained around 90%. Using the ADLF test in metallosis cases can result in false positive results and should be performed with caution.
Core tip: The alpha-defensin lateral flow (ADLF) test is a new diagnostic tool for periprosthetic joint infection (PJI). We evaluated a cohort of 52 patients that underwent aspiration of hip arthroplasty to assess test accuracy. Using Musculoskeletal Infection Society criteria, sensitivity was 100% (CI: 54%-100%) and specificity was 89% (CI: 76%-96%). Using European Bone and Joint Infection Society criteria, sensitivity was lower (71%, CI: 42%-92%) and specificity was higher (97%, CI: 86%-100%), Using 2018 International Consensus Meeting criteria, sensitivity was 91% (62%-100%) and specificity 100% (91%-100%). The results in this cohort are comparable to previous studies.
- Citation: Kuiper JW, Pander P, Vos SJ. Good accuracy of the alpha-defensin lateral flow test for hip periprosthetic joint infection: A pilot study in a retrospective cohort of 52 patients. World J Orthop 2020; 11(1): 36-46
- URL: https://www.wjgnet.com/2218-5836/full/v11/i1/36.htm
- DOI: https://dx.doi.org/10.5312/wjo.v11.i1.36
Periprosthetic joint infection (PJI) is one of the most serious complications of total hip arthroplasty (THA). It generally requires one or more surgeries, weeks of hospitalization and long-term antibiotic treatment. Overall, it is a considerable financial and logistic burden to hospitals and rhe health care system in general[1,2]. The patients themselves, however, are the ones most afflicted by this complication. Treatment methods range from curative therapy with revision arthroplasty to months of living without a functioning hip articulation (Girdlestone procedure) or to life-long suppressive antibiotic therapy (for inoperable patients with a low grade PJI)[1].
Because treatment of PJI differs from other revision indications, it is important to accurately exclude PJI before revision surgery takes place. PJI can be challenging to diagnose and several definitions have been proposed in the past. The most recent (modified) definition by the Musculoskeletal Infection Society (MSIS) includes various laboratory values and aspiration results[3]. This definition has been used as the gold standard for PJI in the last years. However, in the last years, two new definitions have been suggested: The European Bone and Joint Infection Society (EBJIS) has recently proposed other diagnostic criteria which may have a lower threshold for the detection of PJI, and therefore possibly a higher specificity[4]; the 2018 International Consensus Meeting (ICM) criteria includes the most recent tests into a cumulative score, with substantially higher sensitivity and specificity[5].
None of the criteria are ideal in terms of speed, ease of use, high sensitivity and high specificity. Therefore, new diagnostic tools are constantly being developed. One of the most studied new diagnostic markers of the last few years is the determination of alpha-defensin (AD), a protein released by white blood cells in synovial fluid. Two different versions to test this exist: The AD immunoassay test, which is a laboratory test with a readout within 24 h; and the Synovasure® lateral flow test (Synovasure®, Zimmer Biomet, Warsaw, Indiana). This point-of-care test can show directly whether an arthroplasty might be infected, but may have lower accuracy[6-8].
The aim of this pilot study was to identify a cohort of patients in whom the AD lateral flow (ADLF) test was already performed in the last two years in our hospital, and to assess the accuracy of the ADLF test for this cohort [sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV)] by comparing it to the current gold standard for the diagnosis of PJI. As a secondary aim, the more recently proposed EBJIS and 2018 ICM definitions were applied to the cohort as well, to investigate the differences between the definitions.
Since 2015, one of the orthopedic surgeons in our hospital, with a subspecialty in PJI, started using the ADLF test (Synovasure®, Zimmer Biomet, Warsaw, Indiana) for all aspirations of potential hip PJI. This cohort was identified by using our own database and cross-referencing with surgical records. Data were retrospectively collected and analyzed. Patients were included if an ADLF test was performed after aspiration of THA or hemi-arthroplasty (HA) in the study period. Exclusion criteria were: significantly incomplete medical record data (e.g., missing culture results, unavailable data on surgery performed elsewhere), aspiration of other arthroplasty than THA or HA, unavailability of ADLF test (not performed or missing data).
All patients underwent sterile aspiration of the hip joint as part of the diagnostic work-up for a painful or poorly functioning hip arthroplasty, between January 2015 and March 2018. This aspiration was performed in the operating room under sterile conditions with the help of fluoroscopy. After aspiration, the ADLF test was performed according to manufacturer guidelines if enough material was available (e.g., no dry tap). A white blood cell count (WBC) and polymorphonuclear neutrophil percentage (PMN%) were performed, and one or two samples were used for culturing in blood culture bottles (aerobic and anaerobic).
During revision surgery, at least 6 tissue cultures were collected, from joint capsule/synovium, acetabular and femoral interface. Sonication and histopathology were not standardly performed during the study period. Antibiotic treatment was guided by prior cultures results, or vancomycin (1000 milligrams twice daily) was administered until culture results were known, which could take up to 14 d.
After identification of all patients that underwent aspiration, the following data were collected: patient characteristics; arthroplasty details (time after initial surgery, hemi- or total hip arthroplasty, articulation, use of cement); C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), serum leukocyte count and presence of a sinus tract at presentation; aspiration characteristics (amount, aspect, ADLF test results, WBC and PMN%, number of cultures and culture results); follow-up data (revision performed, intra-operative histology and culture results, diagnosis, PJI criteria met).
Three recent definitions of PJI for calculation of test accuracy were used. The MSIS definition was used as the standard[3]. EBJIS and ICM definitions were also used for comparison[4,5]. See Table 1, Table 2, Table 3, Table 4 and Table 5.
| Two or more positive periprosthetic cultures with phenotypically identical organisms, or |
| A sinus tract communicating with the joint, or |
| Having at least three of the following minor criteria: |
| Elevated serum C-reactive protein > 10 mg/L AND erythrocyte sedimentation rate > 30 mm/h; |
| Elevated synovial fluid white blood cell count 3.000 cells/μL OR ++ result on leukocyte esterase test strip; |
| Elevated synovial fluid polymorphonuclear neutrophil percentage (PMN%) > 80%; |
| Positive histological analysis of periprosthetic tissue; |
| A single positive culture. |
| Sinus tract OR purulence around the prosthesis; |
| Acute inflammation on histopathology of periprosthetic tissue; |
| Elevated synovial fluid white blood cell count of more than 2.000 cells/μL OR elevated synovial fluid polymorphonuclear neutrophil percentage (PMN%) > 70%; |
| Microbial growth in synovial fluid OR > 2 tissue samples (for highly virulent microorganisms already one positive sample confirms infection) OR sonication fluid (≥ 50 CFU/mL). |
| Two positive cultures of the same organism | Infected |
| Sinus tract with evidence of communication to the joint or visualization of the prosthesis |
| Preoperative score: Minor criteria | Score | Decision |
| Serum: Elevated CRP or D-Dimer | 2 | ≥ 6: Infected |
| Serum: Elevated ESR | 1 | |
| Synovial: Elevated synovial WBC count or LE | 3 | 2-5: Possibly infected1 |
| Synovial: Positive alpha-defensin | 3 | |
| Synovial: Elevated synovial PMN (%) | 2 | 0-1: Not infected |
| Synovial: Elevated synovial CRP | 1 |
| Inconclusive preoperative score1or dry tap | Score | Decision |
| Preoperative score | - | ≥ 6: Infected; 4-5: Inconclusive; 0-3: Not infected |
| Positive histology | 3 | |
| Positive purulence | 3 | |
| Single positive culture | 2 |
The main aim of this study was to assess sensitivity and specificity with PPV and NPV of the ADLF test, using the MSIS criteria for PJI as mentioned above. The second aim was to compare these criteria with the EBJIS and 2018 ICM criteria.
To assess the performance of the ADLF test, the sensitivity, specificity, PPV and NPV were calculated. Except for age, the scale variables were described using the median and the range regarding a non-normal distribution measured by means of the One-sample Kolmogorov Smirnov test. 95%CI were calculated and are described.
Between January 2015 and December 2017, 83 hip aspirations were conducted. 31 patients were excluded because the ADLF test was not performed. Therefore, a total of 52 patients (52 aspirations) were included in this pilot study, with a mean age of 72 years. See Table 6 for demographics and comparison with the excluded patients. The median time between primary surgery and aspiration was 35 mo (range 3-266 mo) and 46 (88%) patients had a THA. 31 of 46 THA patients had a metal on polyethylene articulation. The median CRP and ESR before aspiration were 6 mg/L (range 1-195 mg/L) and 13 mm/h (range 3-120 mm/h) respectively, and the median WBC in synovial fluid was 800 cells/μL (range 10-264.000 cells/μL).
| Cohort | Excluded | |
| Total (n) | 52 | 31 |
| Age [mean (SD), yr] | 72 (9.2) | 67.5 (9.5) |
| Gender (n) | ||
| Male | 24 | 12 |
| Female | 28 | 19 |
| Operated side (n) | ||
| Right | 28 | 12 |
| Left | 24 | 19 |
| Prosthesis (n) | ||
| Total hip arthroplasty | 46 | 26 |
| Hemi-arthroplasty | 6 | 5 |
| Articulation (n) | ||
| MoP | 31 | 18 |
| CoP | 8 | 4 |
| CoC | 4 | 3 |
| MoM | 3 | 1 |
| HA | 6 | 5 |
| Time to aspiration [Median (range), mo] | 35 (3-266) | 32.4 (3-191) |
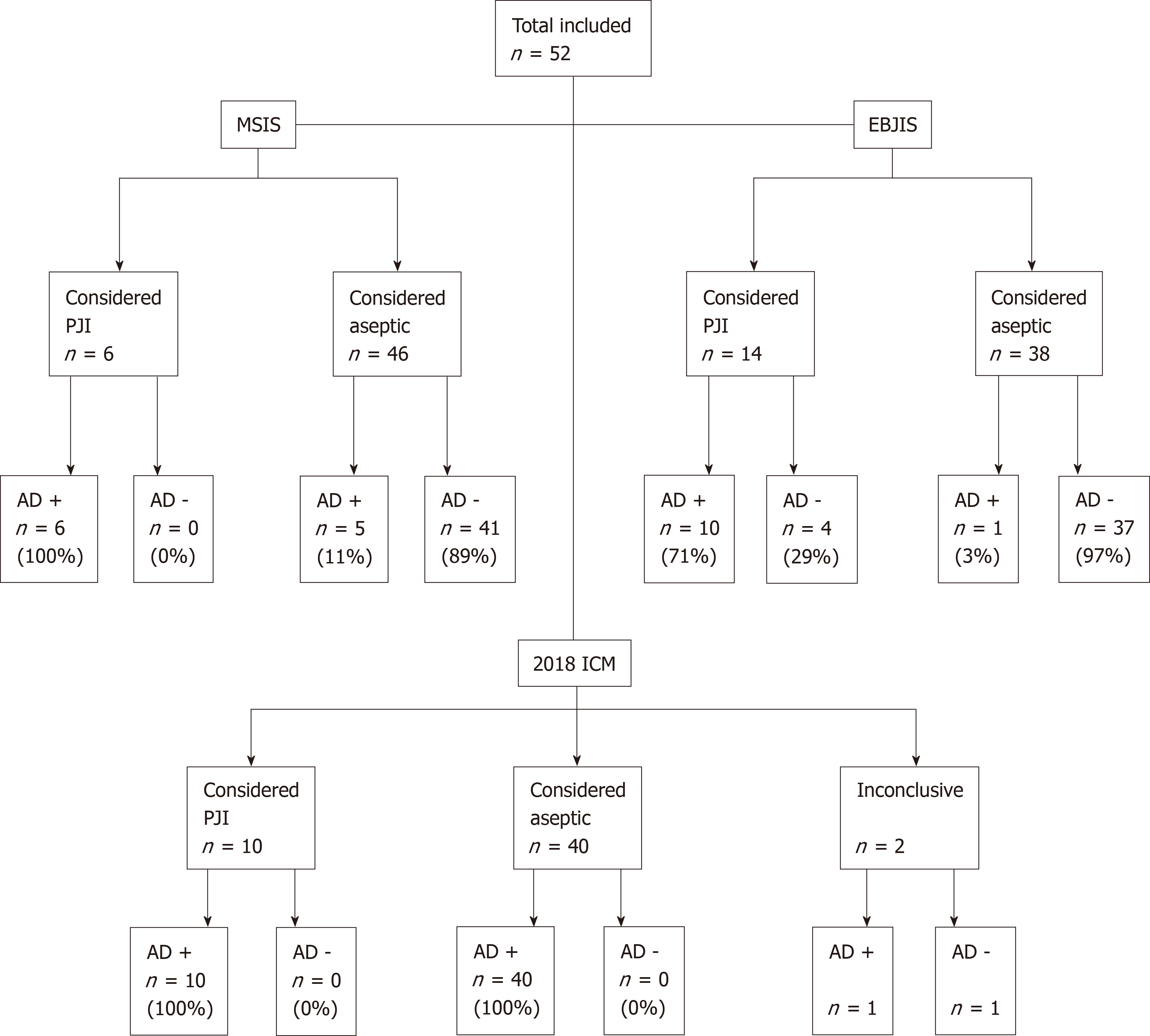
In 11 patients (21%) the ADLF test was positive. According to the MSIS criteria, 6 patients had a PJI. Using these criteria, sensitivity was 100% (CI: 54%-100%), specificity was 89% (CI: 76%-96%), PPV was 55% (CI: 34%-73%) and NPV was 100%. The overall accuracy was 90% (CI: 79%-97%). None of the ADLF test results were false negative and 5 were false positive (Figure 1). One of the false positive cases had a metal-on-metal (MoM) articulation. See Table 7 for details on all positive ADLF tests or inconclusive/positive criteria.
| Case | Criteria | A | B | C | D | E | F | G | H | I | PJI? | |||||||
| Art | Sinus tract | Visible purulence | CRP (mg/ L) | ESR (mm/h) | WBC count (cells/μL) | PMN% | Culture synovial fluid (nr of cultures) | Culture tissue (nr of cultures) | AD | MSIS (+/-; criteria) | EBJIS (+/-; criteria) | 2018 ICM (+/-/i; criteria) | Revision surgery | 1- or 2-stage | ||||
| 1 | CoC | - | - | 6.5 | 21.0 | 19100 | 91 | neg (1) | neg (6) | + | - | E,F | + | E,F | + | E,F,I | Yes | 2-stage |
| 2 | MoP | Yes | - | 9.0 | 23.0 | - | - | pos (2) | pos (8/9) | + | + | A,G,H | + | A,G,H | + | A,G,H,I | Yes | 2-stage |
| 3 | MoP | - | - | 8.5 | 29.0 | 3000 | 4 | neg (2) | - | - | - | + | E | - | No | - | ||
| 4 | MoP | - | - | 9.8 | 16.0 | 6200 | 77 | neg (2) | pos (5/8) | + | + | E,H | + | E,F,H | + | E,H,I | Yes | 2-stage |
| 5 | CoC | - | - | 6.9 | 10.0 | 2300 | - | neg (1) | - | - | - | + | E | - | No | - | ||
| 6 | MoP | - | - | 12.0 | 26.0 | 10 | - | neg (2) | pos (1/2) | - | - | C,H | - | i | C,H | Yes | 1-stage | |
| 7 | MoP | - | - | - | - | 9350 | - | neg (1) | - | - | - | E | + | E | - | E | No | - |
| 8 | HA | - | - | - | - | - | - | neg (1) | pos (1/6) | + | - | H | - | i | H,I | Yes | 1-stage | |
| 9 | MoP | - | - | 144.0 | 64.0 | 37300 | 92 | pos (1/2) | pos (2/6) | + | + | C,D,E,F,G,H | + | E,F,G,H | + | C,D,E,F,H,I | Yes | DAIR |
| 10 | MoP | - | - | 61.0 | 72.0 | 7900 | 95 | pos (1/1) | pos (9/9) | + | + | C,D,E,F,G,H | + | E,F,G,H | + | C,D,E,F,H,I | Yes | 2-stage |
| 11 | MoP | - | - | 71.0 | 55.0 | 35300 | 97 | pos (2/2) | pos (7/7) | + | + | C,D,E,F,G,H | + | E,F,G,H | + | C,D,E,F,H,I | Yes | 2-stage |
| 12 | HA | - | - | 9.6 | - | 4900 | 81 | - | - | + | - | E,F | + | E,F | + | E,F,I | No | - |
| 13 | MoM | - | Yes | 195.0 | - | 264000 | 96 | neg (2) | - | + | + | B,C,E,F | + | B,E,F | + | B,C,E,F,I | No | - |
| 14 | MoP | - | - | 15.0 | 13.0 | 8000 | - | neg (1) | neg (6) | + | - | C,E | + | E | + | C,E,I | Yes | 2-stage |
| 15 | HA | - | - | 1.0 | 9.0 | 4000 | 15 | neg (2) | - | - | - | E | + | E | - | E | No | - |
| 16 | MoM | - | - | 5.7 | 8.0 | 5170 | 52 | neg (3) | - | + | - | E | + | E | + | E,I | No | - |
In total, 19 patients underwent revision surgery after aspiration. 10 of these had no PJI suspicion and underwent direct revision. In 8 patients, PJI was suspected because of aspiration results or symptoms, and a two-stage revision was performed. In one patient, debridement, antibiotics and implant retention (DAIR) was performed because PJI was classified as acute hematogenous (symptom duration of 8 d with a prior well-functioning THA).
Of 6 patients with PJI, 5 patients (83%) underwent revision surgery. One patient was treated with suppressive antibiotics because of extensive co-morbidity, and died 5 mo later, unrelated to PJI. Seven cases (of 19 revisions) had positive cultures (Table 8). In 4 of these 7 patients, the micro-organism found intraoperatively corresponded to aspiration culture results. The other 3 patients had negative preoperative synovial cultures. Two of these three did not meet PJI criteria (both MSIS and EBJIS), as they had only one positive intraoperative culture with a low virulence micro-organism (Cutibacterium acnes and Staphylococcus capitis). Both patients underwent direct revision, without macroscopic suspicion of PJI, and were free of symptoms at the last follow-up. One patient was treated with DAIR, as described above. The other 4 patients with positive cultures underwent two-stage revision. See Table 8 for microbiology characteristics.
| Case | Culture after aspiration (positive cultures/nr of total cultures) | Culture after revision (positive cultures/nr of total cultures) |
| 2 | Propionibacterium acnes (2/2) | Cutibacterium acnes (8/9) |
| Staphylococcus aureus (1/2) | ||
| 4 | Negative (2/2) | Staphylococcus capitis (5/8) |
| Staphyloccus hominis (1/8) | ||
| Staphylococcus epidermidis (1/8) | ||
| Propionibacterium acnes (1/8) | ||
| 7 | Negative (2/2) | Propionibacterium acnes (1/2) |
| 8 | Negative (1/1) | Staphylococcus capitis (1/6) |
| 9 | Staphylococcus aureus (1/2) | Staphylococcus aureus (2/6) |
| 10 | Pseudomonas aeruginosa (1/1) | Pseudomonas aeruginosa (9/9) |
| Staphylococcus epidermidis (1/9) | ||
| 13 | Staphylococcus lugdunensis (2/2) | Staphylococcus lugdunensis (7/7) |
Three patients (6%) had a MoM hip articulation. One patient was not suspected of PJI. The 2 other patients had a positive ADLF test (67%): 1 patient did not meet MSIS PJI criteria, but did have PJI according to EBJIS criteria (elevated WBC count of 5170 cells/μL). The last patient had PJI, according to both MSIS and EBJIS criteria. Due to severe comorbidity, this patient was considered inoperable, as a result of which intraoperative cultures were never obtained.
When adhering to the criteria by the EBJIS, PJI was found in 14 patients and the ADLF test had a sensitivity of 71% (CI: 42%-92%), specificity of 97% (CI: 86%-100%), PPV of 91% (CI: 58%-99%) and NPV of 90% (CI: 80%-96%). The overall accuracy was 90% (CI: 79%-97%). Using the EBJIS criteria, 4 ADLF test results were false negative and one was false positive (Figure 1).
Ten patients had PJI according to these criteria, and 40 patients had no PJI. Two cases were inconclusive, 1 with positive and 1 with negative ADLF test. Excluding these cases, no false positives and false negatives were found, and the ADLF test had 100% sensitivity (69%-100%) and specificity (91%-100%). When classifying these cases as infected, as they are likely to be treated as infected cases, sensitivity was 91% (62%-100%), specificity 100% (91%-100%). PPV, NPV and accuracy were 100%, 98% (86%-100%) and 98% (90%-100%), respectively. See Figure 1.
In this study the accuracy of the ADLF test was assessed in 52 patients with a suspicion of hip PJI.
The measured sensitivity and specificity of the ADLF test were 100% and 89% respectively, with an overall accuracy of 90%. In comparison, when applying the EBJIS criteria, sensitivity and specificity were 71 and 97% respectively. Overall accuracy was the same, 90%. When using the new 2018 ICM criteria, a sensitivity of 91% and a specificity of 100% were found, with an accuracy of 98%.
The existing literature describes a large range in sensitivity and specificity. This can partly be explained by study-related factors. The results for sensitivity range from 67.0% to 97.1% and for specificity from 82.4% to 100%[4,6,8-11]. The results of the current study are comparable to these studies.
Renz et al[4] also reported results of the ADLF test when using EBJIS criteria, and found a sensitivity of 54.4%, specificity of 99.3%, PPV of 97.7%, and NPV of 78.6% (for MSIS criteria these numbers were 84.4%, 96.4%, 86.4% and 95.8%, respectively). In a cohort of 212 patients, 45 patients had PJI according to MSIS criteria, and 79 with the use of EBJIS criteria. With this lower threshold, the prevalence of PJI is higher and the number of false positives is lower. This is similar for the current study. The only study on the new 2018 ICM criteria[5] is the one in which the definition is proposed, and they described no accuracy of the AD test alone. Since the AD test is used in the criteria, one may argue that it is not a good gold standard to assess the accuracy of the AD test itself.
In previous studies, metallosis was often excluded due to false positive results[6,8,12-14]. It is known that patients with a MoM articulation may develop adverse local tissue reactions (ALTR) due to metal wear debris. Even with other articulations, metal debris can be found (e.g., with taper-cup impingement or other taper related problems)[15]. Differentiating between PJI and ALTR can be challenging as patients may have elevated inflammatory parameters, peri-articular purulent appearance, falsely elevated WBC and a false positive ADLF test[8,15]. Of 5 false-positive ADLF tests, 1 was a case of metallosis. Other studies found even higher rates of metallosis among the false-positive cases, although the numbers are small: 1/3, 2/4 and 3/5[11,16,17]. One study excluded 3 false positive cases because of metallosis[8]. A recent Dutch study described 1 case of metallosis, with a negative ADLF test in a cohort of 37 patients[18]. Okroj et al[15] studied the results of AD testing in 26 cases of metallosis. They found 1 true positive, and 8 false positive results (31%). Therefore, the value of the ADLF test in metallosis cases should be interpreted critically and with caution.
In this cohort, 6 patients with painful or non-functioning hemiarthroplasty were included. In 2 of 6 cases the ADLF test was positive. According to MSIS criteria, both were false positive. Using EBJIS criteria, 1 true positive, 1 false positive and 1 false negative were found. With the 2018 ICM criteria, 1 true positive and 1 positive in an inconclusive case were found. No other studies described AD testing of hip hemiarthroplasties. Further studies are needed to provide guidance on AD testing for painful hip hemiarthroplasties.
Within the scope of this study, its limitations are acknowledged. The number of patients was relatively small, and due to the retrospective design, not all measurements needed for the PJI criteria were performed. Furthermore, ADLF test was not performed in all patients that underwent aspiration, mostly due to insufficient amount of aspiration fluid or bloody fluid aspiration. Therefore, selection bias may have occurred. Although several statistical methods exist to address missing data, we believe these are more useful for big data trials than for this retrospective study[19].
Because only hip arthroplasty patients were included in this cohort, comparison with previous studies is more difficult, as most other studies described results of both hip and knee PJI.
Further research is crucial, considering the variety in sensitivity and specificity in different studies. A prospective follow-up study has already been started to evaluate the ADLF test in a larger, prospective cohort, in which a comparison to the leukocyte-esterase test will also be made.
In conclusion, in a cohort of 52 patients that underwent aspiration for a painful or poor-functioning hip arthroplasty, the ADLF test had a sensitivity of 100% and specificity of 89% and an overall accuracy of 90%. Other definition criteria showed slightly different test specifics but overall accuracy was high for the EBJIS and 2018 ICM criteria as well. The ADLF test is an easy-to-use point of care test, which requires little material and can provide a quick perioperative result. This can be useful during revision surgery or when aspiration yields almost no synovial fluid. Nevertheless, caution is advised when interpreting the results, in particular when metallosis is present or possible.
Periprosthetic joint infection (PJI) is one of the most severe complications after hip arthroplasty. Therefore, it is important to diagnose this problem accurately. Several diagnostic criteria are currently used for PJI diagnosis.
One of the newest additions to the diagnostic options is the determination of alpha-defensin, a protein released by white blood cells in synovial fluid. Two tests are available, the lateral flow test being the fastest (point of care) test. This test of aspirated joint fluid has not yet been extensively studied.
The objective was to identify a cohort of patients after hip arthroplasty in whom the alpha-defensin lateral flow (ADLF) test was already performed in the last two years in our hospital, and to assess the accuracy of the ADLF test for this cohort.
The database of patients after total hip arthroplasty who underwent aspiration and ADLF testing was checked and data were retrospectively collected and analyzed. All patients underwent sterile aspiration of the hip joint as part of the diagnostic work-up for painful or poor functioning hip arthroplasty, between January 2015 and March 2018. Three recent definitions of PJI for calculation of test accuracy were used. The Musculoskeletal Infection Society (MSIS) definition was used as the standard. European Bone and Joint Infection Society (EBJIS) and International Consensus Meeting (ICM) definitions were also used for comparison.
A total of 52 patients (52 aspirations) were included in this pilot study. In 11 patients (21%) the ADLF test was positive. According to the MSIS criteria, 6 patients had a PJI. Using these criteria, sensitivity was 100% (CI: 54%-100%), specificity was 89% (CI: 76%-96%), positive predictive value (PPV) was 55% (CI: 34%-73%) and negative predictive value (NPV) was 100%. When adhering to the criteria by the EBJIS, PJI was found in 14 patients and the ADLF test had a sensitivity of 71% (CI: 42%-92%), specificity of 97% (CI: 86%-100%), PPV of 91% (CI: 58%-99%) and NPV of 90% (CI: 80%-96%).
When using the 2018 ICM criteria and classifying inconclusive cases as infected, sensitivity was 91% (62%-100%), specificity 100% (91%-100%). PPV, NPV and accuracy were 100%, 98% (86%-100%) and 98% (90%-100%), respectively.
The diagnostic accuracy of the ADLF test for hip arthroplasty was high: Sensitivity was 100%, specificity 89%. Using different PJI diagnosis criteria, overall accuracy remained 90% or higher. These results are comparable to prior findings by other authors. Caution should be taken when when interpreting the results of the ADLF test in metallosis cases, as metallosis may cause false positive results.
This study confirms the good clinical test results for the ADLF test found by others. However, the use of the ADLF test in cases of suspected PJI in hemi-arthroplasty or metal-on-metal has not been studied well, and one should be careful not to rely solely on the ADLF test, especially in those cases.
Many thanks to Hayko Heres Diddens for his help in editing the manuscript and his continuous support.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country of origin: Netherlands
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pongcharoen B, Zhang ZH S-Editor: Dou Y L-Editor: A E-Editor: Liu MY
| 1. | Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR; Infectious Diseases Society of America. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:e1-e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1397] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 2. | Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am. 2005;87:1746-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 240] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 3. | Zmistowski B, Della Valle C, Bauer TW, Malizos KN, Alavi A, Bedair H, Booth RE, Choong P, Deirmengian C, Ehrlich GD, Gambir A, Huang R, Kissin Y, Kobayashi H, Kobayashi N, Krenn V, Lorenzo D, Marston SB, Meermans G, Perez J, Ploegmakers JJ, Rosenberg A, C Simpfendorfer, Thomas P, Tohtz S, Villafuerte JA, Wahl P, Wagenaar FC, Witzo E. Diagnosis of periprosthetic joint infection. J Orthop Res. 2014;32 Suppl 1:S98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Renz N, Yermak K, Perka C, Trampuz A. Alpha Defensin Lateral Flow Test for Diagnosis of Periprosthetic Joint Infection: Not a Screening but a Confirmatory Test. J Bone Joint Surg Am. 2018;100:742-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 5. | Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, Shohat N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J Arthroplasty. 2018;33:1309-1314.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 885] [Cited by in RCA: 1394] [Article Influence: 199.1] [Reference Citation Analysis (0)] |
| 6. | Gehrke T, Lausmann C, Citak M, Bonanzinga T, Frommelt L, Zahar A. The Accuracy of the Alpha Defensin Lateral Flow Device for Diagnosis of Periprosthetic Joint Infection: Comparison with a Gold Standard. J Bone Joint Surg Am. 2018;100:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Eriksson HK, Nordström J, Gabrysch K, Hailer NP, Lazarinis S. Does the Alpha-defensin Immunoassay or the Lateral Flow Test Have Better Diagnostic Value for Periprosthetic Joint Infection? A Meta-analysis. Clin Orthop Relat Res. 2018;476:1065-1072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Kasparek MF, Kasparek M, Boettner F, Faschingbauer M, Hahne J, Dominkus M. Intraoperative Diagnosis of Periprosthetic Joint Infection Using a Novel Alpha-Defensin Lateral Flow Assay. J Arthroplasty. 2016;31:2871-2874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Sigmund IK, Holinka J, Gamper J, Staats K, Böhler C, Kubista B, Windhager R. Qualitative α-defensin test (Synovasure) for the diagnosis of periprosthetic infection in revision total joint arthroplasty. Bone Joint J. 2017;99-B:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 10. | Suda AJ, Tinelli M, Beisemann ND, Weil Y, Khoury A, Bischel OE. Diagnosis of periprosthetic joint infection using alpha-defensin test or multiplex-PCR: ideal diagnostic test still not found. Int Orthop. 2017;41:1307-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Berger P, Van Cauter M, Driesen R, Neyt J, Cornu O, Bellemans J. Diagnosis of prosthetic joint infection with alpha-defensin using a lateral flow device: a multicentre study. Bone Joint J. 2017;99-B:1176-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472:3254-3262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 277] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 13. | Frangiamore SJ, Saleh A, Kovac MF, Grosso MJ, Zhang X, Bauer TW, Daly TM, Ricchetti ET, Iannotti JP. Synovial fluid interleukin-6 as a predictor of periprosthetic shoulder infection. J Bone Joint Surg Am. 2015;97:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Shahi A, Parvizi J, Kazarian GS, Higuera C, Frangiamore S, Bingham J, Beauchamp C, Valle CD, Deirmengian C. The Alpha-defensin Test for Periprosthetic Joint Infections Is Not Affected by Prior Antibiotic Administration. Clin Orthop Relat Res. 2016;474:1610-1615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Okroj KT, Calkins TE, Kayupov E, Kheir MM, Bingham JS, Beauchamp CP, Parvizi J, Della Valle CJ. The Alpha-Defensin Test for Diagnosing Periprosthetic Joint Infection in the Setting of an Adverse Local Tissue Reaction Secondary to a Failed Metal-on-Metal Bearing or Corrosion at the Head-Neck Junction. J Arthroplasty. 2018;33:1896-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-Defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96:1439-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 235] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 17. | Bonanzinga T, Zahar A, Dütsch M, Lausmann C, Kendoff D, Gehrke T. How Reliable Is the Alpha-defensin Immunoassay Test for Diagnosing Periprosthetic Joint Infection? A Prospective Study. Clin Orthop Relat Res. 2017;475:408-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 18. | Scholten R, Visser J, Van Susante JLC, Van Loon CJM. Low sensitivity of a-defensin (Synovasure) test for intra-operative exclusion of prosthetic joint infection. Acta Orthop. 2018;89:357-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Zhang Z. Missing data imputation: focusing on single imputation. Ann Transl Med. 2016;4:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 107] [Reference Citation Analysis (0)] |