Published online Jun 18, 2019. doi: 10.5312/wjo.v10.i6.255
Peer-review started: February 11, 2019
First decision: April 15, 2019
Revised: May 7, 2019
Accepted: May 21, 2019
Article in press: May 22, 2019
Published online: June 18, 2019
Processing time: 137 Days and 19.9 Hours
Surgical site infections following anterior cruciate ligament (ACL) reconstruction are an uncommon but potentially devastating complication. In this study, we present an unusual case of recurrent infection of the knee after an ACL reconstruction, and discuss the importance of accurate diagnosis and appropriate management, including the issue of graft preservation versus removal.
A 33-year-old gentleman underwent ACL reconstruction using a hamstring tendon autograft with suspensory Endobutton fixation to the distal femur and an interference screw fixation to the proximal tibia. Four years after ACL reconstruction, he developed an abscess over the proximal tibia and underwent incision and drainage. Remnant suture material was found at the base of the abscess and was removed. Five years later, he re-presented with a lateral distal thigh abscess that encroached the femoral tunnel. He underwent incision and drainage of the abscess which was later complicated by a chronic discharging sinus. Repeated magnetic resonance imaging revealed a fistulous communication between the lateral thigh wound extending toward the femoral tunnel with suggestion of osteomyelitis. Decision was made for a second surgery and the patient was counselled about the need for graft removal should there be intra-articular involvement. Knee arthroscopy revealed the graft to be intact with no evidence of intra-articular involvement. As such, the decision was made to retain the ACL graft. Re-debridement, excision of the sinus tract and removal of Endobutton was also performed in the same setting. Joint fluid cultures did not grow bacteria. However, tissue cultures from the femoral tunnel abscess grew Enterobacter cloacae complex, similar to what grew in tissue cultures from the tibial abscess five years earlier. In view of the recurrent and indolent nature of the infection, antibiotic therapy was escalated from Clindamycin to Ertapenem. He completed a six-week course of intravenous antibiotics and has been well for six months since surgery, with excellent knee function and no evidence of any further infection.
Prompt and accurate diagnosis of surgical site infection following ACL reconstruction, including the exclusion of intra-articular involvement, is important for timely and appropriate treatment. Arthroscopic debridement and removal of implant with graft preservation, together with a course of antibiotics, is a suitable treatment option for extra-articular knee infections following ACL reconstruction.
Core tip: Chronic surgical site infections following anterior cruciate ligament (ACL) reconstructions are rare. Most infections occur in the acute or subacute post-operative periods. Astute clinic judgement and patient involvement are key when managing recurrent infections post-ACL reconstruction. Early intervention involving joint washout and debridement as well as commencing culture-directed antibiotic therapy are key. The decision for graft-sparing versus graft-sacrificing surgery remains controversial.
- Citation: Koh D, Tan SM, Tan AHC. Recurrent surgical site infection after anterior cruciate ligament reconstruction: A case report. World J Orthop 2019; 10(6): 255-261
- URL: https://www.wjgnet.com/2218-5836/full/v10/i6/255.htm
- DOI: https://dx.doi.org/10.5312/wjo.v10.i6.255
Surgical site infections following arthroscopic anterior cruciate ligament (ACL) reconstruction are uncommon. Current literature suggests the incidence rate to be around 1.7%[1-3] with almost all cases presenting in the acute or subacute post-operative periods[1,2,4-6]. Prompt diagnosis and treatment are necessary to avoid devastating consequences such as septic arthritis and its sequelae[7].
Various management protocols have been described in the literature with limited consensus on the best practice. Controversy remains with regards to the decision for graft-sparing versus graft-sacrificing surgery, especially for recurrent infections. Most authors, however, agree on the need for early irrigation and debridement as well as culture-directed antibiotic therapy[8].
While Gram-positive cocci are the most frequently encountered pathogens in surgical site infections, members of the Enterobacteriaceae family, namely En-terobacter cloacae complex (ECC), have also been reported[6,9]. ECC is a major cause of nosocomial and opportunistic infections, but its involvement in joint and graft infections is rare[6,9]. Its ability to form biofilms, produce cytotoxins and its intrinsic resistance to multiple beta-lactam antibiotics make this pathogen difficult to eradicate and treat effectively[10]. To date, there is a paucity of literature describing chronic or delayed surgical site infections after ACL reconstruction. We describe the first case of a delayed, recurrent infection involving both the tibial and femoral surgical sites which occurred at four and nine years respectively following an ACL reconstruction.
A 33-year-old gentleman who presents with a history of recurring ACL surgical site infections.
This patient underwent left knee ACL reconstruction, using a hamstring tendon autograft with Endobutton fixation to the distal femur and interference screw fixation to the proximal tibia. He had an uneventful recovery and was able to return to his pre-injury activity level. Four years after his index surgery, he developed a left proximal tibial abscess over the interference screw site. He underwent incision and drainage of the tibial abscess. Intraoperatively, a remnant non-absorbable Ethibond suture was found at the base of the abscess. This remnant suture was removed and there was no infective extension into the tibia tunnel or graft. Tissue cultures grew ECC. He completed a two-week course of culture-directed oral Ciprofloxacin and was subsequently noted to be well during outpatient follow-up.
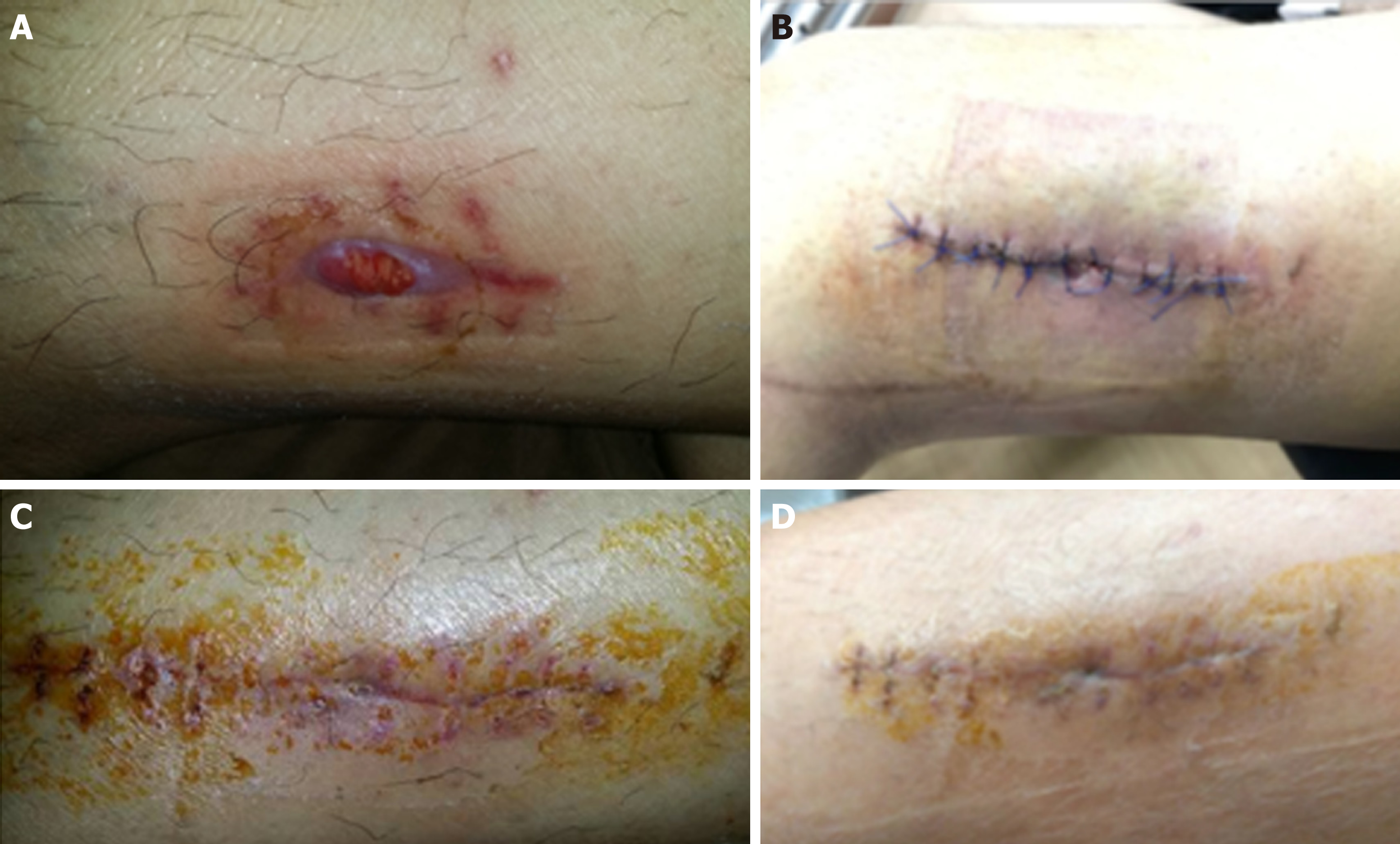
Nine years after the index surgery, this patient spontaneously developed left lateral thigh swelling (Figure 1). Magnetic resonance imaging (MRI) revealed a large rim-enhancing fluid collection form the femoral tunnel extending to the subcutaneous tissue with marrow enhancement adjacent to the femoral tunnel. An incision and drainage were performed and initial wound cultures yielded no bacterial growth. This was complicated by persistent discharge and the formation of a sinus tract over the lateral thigh wound. Patient subsequently presented to our clinic seeking a second opinion.
Clinical examination revealed a discharging sinus over the left lateral thigh wound. He was afebrile, ambulated well, demonstrated good range of motion in the left knee and anterior drawer test was negative. There was no clinical evidence suggesting septic arthritis.
Laboratory investigations revealed elevated C-reactive protein (CRP) of 17.8 mg/L but normal erythrocyte sedimentation rate (ESR) of 1.0 mm/hr as well as total white cell count (TW) of 5.70 × 109/L.
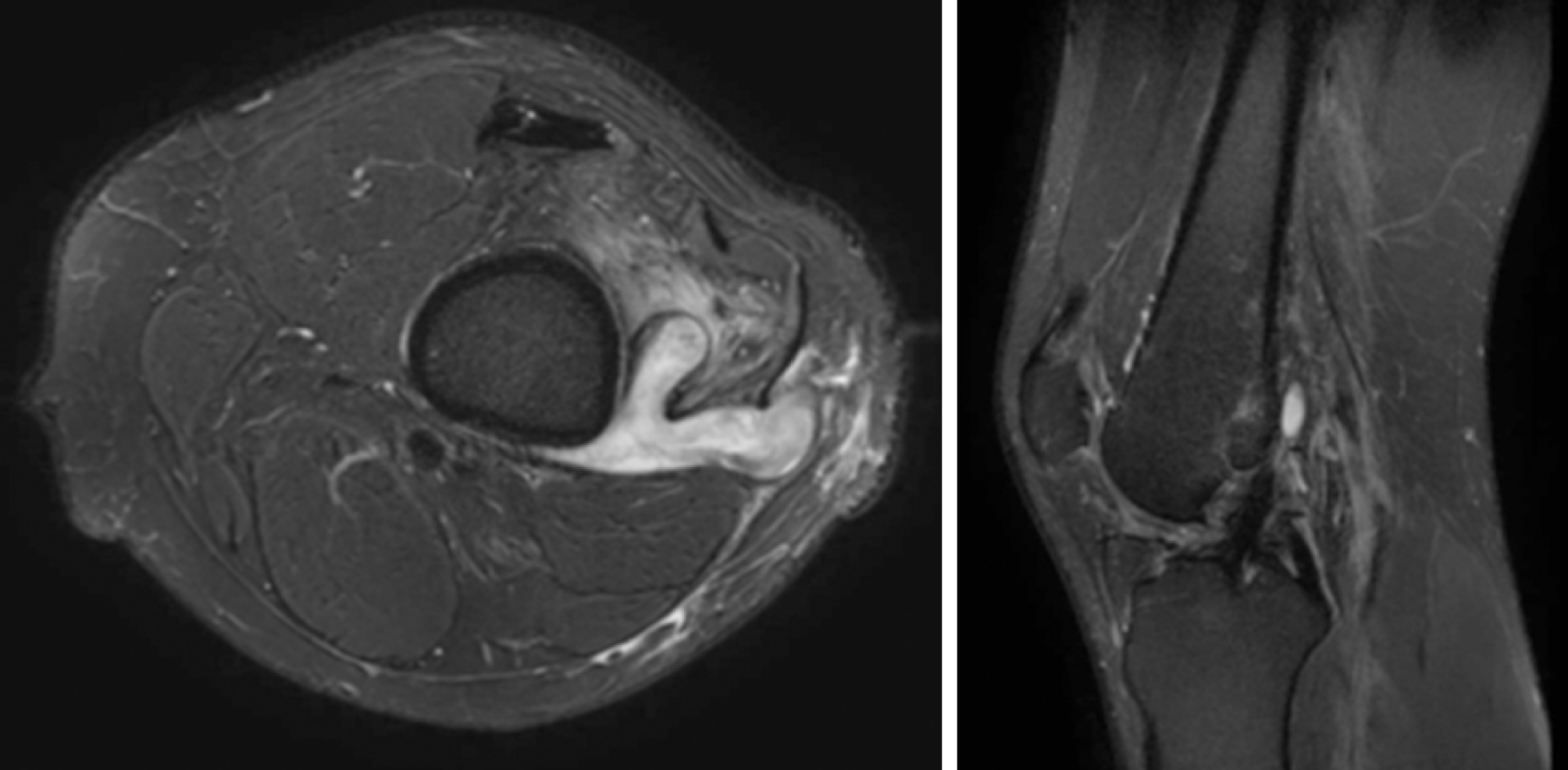
Repeat MRI showed a fistulous communication between the lateral thigh wound extending toward the femoral tunnel with early features of osteomyelitis with no evidence of graft infection (Figure 2).
Recurrent surgical site infection after ACL reconstruction.
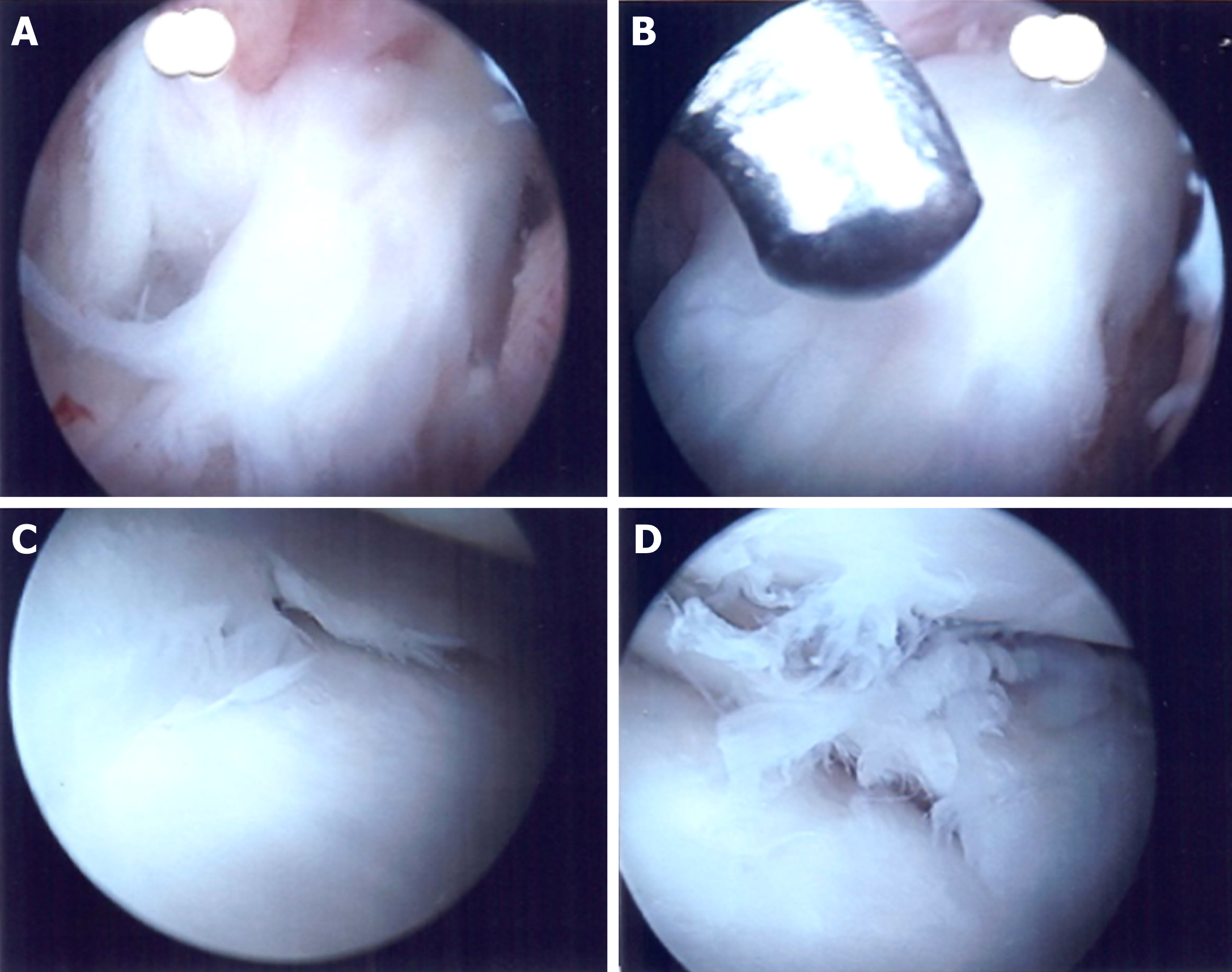
The patient was counselled extensively for further debridement and understood the potential need for graft removal should there be evidence of intra-articular involvement. Arthroscopic exploration and washout as well as lateral thigh wound re-debridement, wound exploration, excision of sinus tract and removal of Endobutton was performed. Intra-operatively, the graft was noted to be in tact with no evidence of infection within the knee (Figure 3). In addition, the sinus tract led to the Endobutton site. The tract was excised in its entirety and the Endobutton removed. Underlying bone was curetted and noted to be healthy.
Tissue cultures from the sinus tract grew ECC, demonstrating similar antibiotic sensitivities to the tibial abscess cultures done in 2012. Joint fluid cultures, however, did not grow any organisms. Antibiotic therapy was escalated to Ertapenem in view of the recurrent nature of the infection. He completed a six-week course of antibiotics. Six months after surgery, his wound had healed well (Figure 1) and the inflammatory markers had normalised (CRP 4.6 mg/L, ESR 2 mm/h and TW 5.51 × 109/L). He remains asymptomatic with excellent knee function and has returned to his normal activities.
Majority of post-ACL reconstruction surgical site infections occur acutely (< 2 wk after surgery) and sub-acutely (between 2 wk and 2 mos after surgery)[4,6,11]. Pathogens commonly identified are skin commensals associated with peri-operative inoculation. Graft contamination can occur at any point during its harvest, preparation and re-introduction. Prior to a graft’s re-incorporation, its lack of blood supply makes it a potential nidus for infection[4,12]. Another source of infection includes the surgical incisions or arthroscopic portals. The use of long instruments is also a potential source of intra-articular inoculation[4,12,13].
Mouzopoulos et al[4] postulated that late infections (> 2 mo) are often extra-articular in origin. The tibia and femoral tunnels are potential tracks for infections – bridging extra-articular infections with the intra-articular environment. The relatively superficial position of the interference screws and metallic endobutton, coupled with local tissue injury, predisposes to wound complications and infections. Judd et al[13] reported similar findings after noting eight out of eleven intra-articular infections having concomitant extra-articular wound complications - both of which having similar causative organisms on culture. McAllister et al[14] recommended the use of non-cannulated interference screws suggesting cannulated screws are a potential conduit for extra-articular pathogens.
In patients with extra-articular infection after ACL graft reconstruction, the risk of intra-articular involvement is ever present. Communication of the extra-articular with the intra-articular space via both tibial and femoral tunnels is a constant risk factor. The presence of foreign material such as sutures promote bacterial seeding and limits the effect of antibiotic clearance. In a recurrent case of infection, we suggest the removal of hardware with a view of graft removal should its integrity be com-promised or if there is evidence of intra-articular involvement.
In our patient, the first surgical site infection involved the tibial interference screw site and occurred four years after the index surgery, with a subsequent infection five years later on the femoral site, centred around the Endobutton. Reaction to the implants and suture material and seeding of bacteria around these implants may be the cause of the infection on both occasions. Some studies have demonstrated increased risk of infection with hamstring autograft use[2,5,13]. Kim et al[2] hypothesised that the relative short length of the harvested hamstring autograft results in additional suture and hardware within the bone tunnels[5]. The increased foreign body load – both graft and hardware, increases both infection risks and foreign body re-actions[2,5,15]. Even when sterile, foreign body reaction can stimulate inflammatory mediators, activating multinucleated giant cells. This produces a reaction of varying severity, ranging from oedema, sinus development to destruction to local structures[13,16].
In view of the chronic and indolent nature of the infection in our patient, incomplete eradication of the infection remained a concern. Our patient was counselled extensively pre-operatively and was agreeable for graft removal should there be a clinical indication.
The management of septic arthritis after ACL reconstruction remains controversial. Multiple management protocols have been proposed with limited consensus amongst authors. The main debate involves graft preservation versus graft removal in recurrent infections. Most authors to date opt for graft preservation treatment protocols involving long-term culture-directed antibiotics, arthroscopic irrigation and debridement as the first line of action. Should recurrent infections occur, this process is repeated until the complete eradication of infection. Proponents for graft pre-servation recommend such a protocol, boasting success rates of 85%[17]. This pathway is most agreeable amongst most authors and patients as it avoids morbidity from graft removal and the need for a staged reconstruction.
Opponents of graft preservation argue that the graft, if left in place, will remain a source of infection[1]. Kim et al[2] reported that an average of 1.9 procedures were needed for complete eradication of infection. Failure to eradicate the pathogen completely would result in repeated infections thereby requiring additional procedures. In patients with recurrent infections, risk of graft failure, loss of hyaline cartilage, damage to menisci and arthrofibrosis are complications that these group of authors strive to avoid[4,17,18]. Prompt removal of the infected graft and early reconstruction have also demonstrated good outcomes.
Intra-articular involvement can be ascertained through thorough history taking; detailed clinical examination and obtaining pertinent laboratory tests (e.g., CRP, ESR and TW). Arthroscopy can be performed to visualise the intra-articular structures and study the ACL graft. Joint fluid can also be collected for culture and to ascertain bacterial antibiotic sensitivities. In our patient, it was only after establishing that this was an extra-articular infection that the decision made intra-operatively to proceed with debridement, graft preservation and removal of implant.
Interestingly, ECC was cultured from affected tissue on both occasions. While Staphylococcus aureus and coagulase-negative Staphylococcus are the commonest skin flora accounting for up to 88% of post-arthroscopic infections, there are reports involving gram-negative bacteria as well[6]. However, the literature detailing gram-negative pathogens such as ECC is scarce, limiting our understanding of its true incidence and pathogenicity[10]. ECC comprises of six species showing genetic relatedness to E. cloacae – namely E. ludwigii, E. nimipressuralis, E. kobei, E. asburiae, E. cloacae and E. hormaechei[19]. ECC are commensals of the gut, but is known to cause up to 5% of hospital-acquired sepsis[5,6]. The gastrointestinal tract as well as our skin are common sites whereby ECC is contracted[1,19]. Other sources include medical devices as well as intravenous products. It is a pathogen of increasing interest, due to its ability to form biofilms, secrete various cytotoxins as well as its innate resistance to beta-lactams due to AmpC beta-lactamase production[10,20]. These traits make it a potentially difficult pathogen to treat, especially after it has colonised an implant. This may explain the insidious onset of symptoms and chronic nature of this case.
The attributes of E. cloacae make complete eradication challenging, especially in setting of implant involvement. Unlike common pathogens (e.g., Staphylococcus aureus and coagulase-negative Staphylococcus), E. cloacae presents as an indolent chronic infection presenting years after initial inoculation. The cause remains unclear. However, in the setting of prior infection, incomplete eradication is the most likely cause for recurrent surgical site infection. In addition, foreign body reaction played an important role in the initial swelling and effusion over the interference screw and Endobutton sites. Non-involvement of the graft, noted during arthroscopy, suggests that the graft did not serve as a conduit for infection. The authors have demonstrated that favourable outcomes can be achieved with graft preservation when the graft is not compromised, even with chronic recurrent surgical site infections.
This report illustrates an unusual case of recurrent infections involving both the tibial and femoral surgical sites years after an ACL reconstruction. It emphasizes the importance of prompt and accurate diagnosis – especially the exclusion of intra-articular infection. The authors have demonstrated that in cases without graft involvement, debridement with graft preservation, removal of implants and a course of antibiotics may be a suitable treatment option.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country of origin: Singapore
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elgafy H, Kennedy JG S-Editor: Ji FF L-Editor: A E-Editor: Ma YJ
| 1. | Hantes ME, Raoulis VA, Doxariotis N, Drakos A, Karachalios T, Malizos KN. Management of septic arthritis after arthroscopic anterior cruciate ligament reconstruction using a standard surgical protocol. Knee. 2017;24:588-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Kim SJ, Postigo R, Koo S, Kim JH. Infection after arthroscopic anterior cruciate ligament reconstruction. Orthopedics. 2014;37:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Van Tongel A, Stuyck J, Bellemans J, Vandenneucker H. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction: a retrospective analysis of incidence, management and outcome. Am J Sports Med. 2007;35:1059-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Mouzopoulos G, Fotopoulos VC, Tzurbakis M. Septic knee arthritis following ACL reconstruction: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2009;17:1033-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Binnet MS, Başarir K. Risk and outcome of infection after different arthroscopic anterior cruciate ligament reconstruction techniques. Arthroscopy. 2007;23:862-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Babcock HM, Matava MJ, Fraser V. Postarthroscopy surgical site infections: review of the literature. Clin Infect Dis. 2002;34:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Hogan CJ, Fang GD, Scheld WM, Linden J, Diduch DR. Inhibiting the inflammatory response in joint sepsis. Arthroscopy. 2001;17:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Matava MJ, Evans TA, Wright RW, Shively RA. Septic arthritis of the knee following anterior cruciate ligament reconstruction: results of a survey of sports medicine fellowship directors. Arthroscopy. 1998;14:717-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Morand PC, Billoet A, Rottman M, Sivadon-Tardy V, Eyrolle L, Jeanne L, Tazi A, Anract P, Courpied JP, Poyart C, Dumaine V. Specific distribution within the Enterobacter cloacae complex of strains isolated from infected orthopedic implants. J Clin Microbiol. 2009;47:2489-2495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Mezzatesta ML, Gona F, Stefani S. Enterobacter cloacae complex: clinical impact and emerging antibiotic resistance. Future Microbiol. 2012;7:887-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 345] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 11. | Fong SY, Tan JL. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction. Ann Acad Med Singapore. 2004;33:228-234. [PubMed] |
| 12. | Williams RJ, Laurencin CT, Warren RF, Speciale AC, Brause BD, O'Brien S. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction. Diagnosis and management. Am J Sports Med. 1997;25:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 149] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Judd D, Bottoni C, Kim D, Burke M, Hooker S. Infections following arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 2006;22:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | McAllister DR, Parker RD, Cooper AE, Recht MP, Abate J. Outcomes of postoperative septic arthritis after anterior cruciate ligament reconstruction. Am J Sports Med. 1999;27:562-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 140] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Kim TK, Jeong TW, Lee DH. Foreign body reaction after PLC reconstruction caused by a broken PLLA screw. Orthopedics. 2014;37:e1129-e1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Ambrose CG, Clanton TO. Bioabsorbable implants: review of clinical experience in orthopedic surgery. Ann Biomed Eng. 2004;32:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Saper M, Stephenson K, Heisey M. Arthroscopic irrigation and debridement in the treatment of septic arthritis after anterior cruciate ligament reconstruction. Arthroscopy. 2014;30:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Cadet ER, Makhni EC, Mehran N, Schulz BM. Management of septic arthritis following anterior cruciate ligament reconstruction: a review of current practices and recommendations. J Am Acad Orthop Surg. 2013;21:647-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Hoffmann H, Roggenkamp A. Population genetics of the nomenspecies Enterobacter cloacae. Appl Environ Microbiol. 2003;69:5306-5318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 214] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 20. | Davin-Regli A, Pagès JM. Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front Microbiol. 2015;6:392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 333] [Cited by in RCA: 337] [Article Influence: 33.7] [Reference Citation Analysis (0)] |