Published online Mar 18, 2019. doi: 10.5312/wjo.v10.i3.145
Peer-review started: November 2, 2018
First decision: November 29, 2018
Revised: December 6, 2018
Accepted: January 10, 2019
Article in press: January 10, 2019
Published online: March 18, 2019
Processing time: 126 Days and 16.9 Hours
Legg-Calvé-Perthes disease (LCPD) is a clinical condition affecting the femoral head of children during their growth. Its prevalence is set to be between 0.4/100000 to 29.0/100000 children less than 15 years of age with a peak of incidence in children aged from 4 years to 8 years. LCPD aetiology has been widely studied, but it is still poorly understood.
To analyse the available literature to document the up-to-date evidence on LCPD aetiology.
A systematic review of the literature was performed regarding LCPD aetiology, using the following inclusion criteria: studies of any level of evidence, reporting clinical or preclinical results and dealing with the aetiology or pathogenesis of LCPD. Two reviewers searched the PubMed and Science Direct databases from their date of inception to the 20th of May 2018 in accordance with the Preferred Reporting Items for Systemic Reviews and Meta-Analyses guidelines. To achieve the maximum sensitivity of the search strategy, we combined the terms: ‘‘Perthes disease OR LCPD OR children avascular femoral head necrosis” with “pathology OR aetiology OR biomechanics OR genetics” as either key words or MeSH terms.
We include 64 articles in this review. The available evidence on LCPD aetiology is still debated. Several hypotheses have been researched, but none of them was found decisive. While emerging evidence showed the role of environmental risk factors and evidence from twin studies did not support a major role for genetic factors, a congenital or acquired predisposition cannot be excluded in disease pathogenesis. One of the most supported theories involved mechanical induced ischemia that evolved into avascular necrosis of the femoral head in sensible patients.
The literature available on the aetiology of LCPD presents major limitations in terms of great heterogeneity and a lack of high-profile studies. Although a lot of studies focused on the genetic, biomechanical and radiological background of the disease, there is a lack of consensus on one or multiple major actors of the etiopathogenesis. More studies are needed to understand the complex and multifactorial genesis of the avascular necrosis characterizing the disease.
Core tip: Legg-Calvé-Perthes disease is a complex disease affecting the epiphysis of the femoral head in the paediatric population. Historically considered an osteochondrosis, it is now being referred to as an idiopathic avascular necrosis of the femoral head in the paediatric population. Despite the aetiology of the disease having been widely researched, it is still not fully understood. The major hypothesis relies on a multifactorial genesis involving mechanical, genetic and systemic conditions. Further studies are necessary to understand the complex and multifactorial genesis of the avascular necrosis characterizing the disease.
- Citation: Pavone V, Chisari E, Vescio A, Lizzio C, Sessa G, Testa G. Aetiology of Legg-Calvé-Perthes disease: A systematic review. World J Orthop 2019; 10(3): 145-165
- URL: https://www.wjgnet.com/2218-5836/full/v10/i3/145.htm
- DOI: https://dx.doi.org/10.5312/wjo.v10.i3.145
Legg-Calvé-Perthes disease (LCPD) is a complex disease affecting the epiphysis of the femoral head in the paediatric population. Historically considered an osteochondrosis, it is now being referred to as an idiopathic avascular necrosis of the femoral head in the paediatric population. Among its prevalence there is no general agreement. It is set to be between 0.4/100000 to 29.0/100000 children < 15 years of age with a peak of incidence in children aged from 4 years to 8 years and a male/female ratio of 5:1[1,2]. A high profile epidemiological study held in 2017[3] involving 2.1 million individuals attempted to report a more accurate prevalence of this disease. An overall prevalence of 9.3 per 100000 subjects was found. The male/female ratio was 3.1:1. Even though the study was conducted in Sweden from 1973 to 1993, it is one of the most up-to-date sources of evidence of LCPD epidemiology.
Despite the aetiology of the disease having been widely researched, it is still not fully understood. While the major hypothesis relies on a multifactorial genesis, several hypotheses involving mechanical, genetic and systemic conditions have been proposed to explain the pathogenesis of the femoral head osteonecrosis. The best-supported theory involves interference with normal blood supply to epiphysis due to repetitive mechanical stress[4,5]. There is no consensus for the optimum treatment. The aim of treatment is to maintain the sphericity of the femoral head and the congruency of the femur-acetabulum relationship to prevent secondary degenerative arthritis, which eventually leads to total hip arthroplasty in 5% of cases[6]. Early diagnosis and management can help prevent the collapse of the femoral head, progressive femoral head deformity and impingement[7].
Children who have a skeletal age of 6.0 years or less at the onset of the disease do well without treatment[8]. Operative treatment should be considered in children who are 6 years old or older and have over 50% femoral head necrosis when the diagnosis is made[9].
We conducted this systematic review according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)[10]. A systematic review of two medical electronic databases (PubMed and Science Direct) was performed by two independent authors from their date of inception to May 20, 2018. To achieve the maximum sensitivity of the search strategy, we combined the terms: ‘‘Perthes disease OR LCPD OR children avascular femoral head necrosis” with “pathology OR aetiology OR biomechanics OR genetics” as either key words or MeSH terms. The reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies and assessed using the inclusion and exclusion criteria.
Eligible studies for the present systematic review included those dealing with the aetiology of LCPD. The initial title and abstract screening was made using the following inclusion criteria: studies of any level of evidence, written in English, reporting clinical or preclinical results, published in peer review journals and dealing with the aetiology of LCPD. Exclusion criteria were articles written in other languages or studies with a focus on secondary/LCPD-like diseases caused by systemic conditions such as sickle-cell disease, inflammatory disease, the effects of chemotherapy, radiation or prolonged steroid use. We also excluded all the remaining duplicates, articles dealing with other topics, those with poor scientific methodology or without an accessible abstract. Reference lists were also hand-searched for further relevant studies. All publications were limited to in vivo, in vitro, animal and human studies in the English language. Abstracts, case reports, conference presentations, editorials and expert opinions were excluded.
All data were extracted from article texts, tables and figures. Two investigators independently reviewed each article. Discrepancies between the two reviewers were resolved by discussion and consensus. The final results and any remaining controversy on the reviewed article were reviewed and discussed with senior investigators.
In this systematic review, risk of bias assessment of the in vitro studies was not performed as there is no accepted grading scale for such studies. Risk of bias assessment of all in vivo selected full-text articles was performed according to the Regional Online Brownfields Information Network I for non-randomized studies[11]. The Regional Online Brownfields Information Network I (ROBINS-I) tool consists of three stage assessment of the studies included. First stage regards the planning of the systematic review, the second stage is the assessment of the common bias possibly found in these studies and the latter is about the overall risk of bias (Table 1).
| Ref. | Subjects | Association/molecule studied | Results |
| Perry et al[12] (2017) | A hospital case-control study (n = 149/146) | Tobacco smoke exposure during pregnancy | The odds of Perthes' disease significantly increased with reported in utero exposure after adjustment for socioeconomic deprivation (maternal smoking OR = 2.06, 95%CI: 1.17-3.63; paternal smoking OR = 2.09, 95%CI: 1.26-3.46). |
| Daniel et al[13] (2012) | 128 children with LCPD and 384 children attending the hospital for other orthopaedic complaints | environmental tobacco smoke, firewood smoke and socioeconomic status and the risk of LCPD | The main risk factors for LCPD were indoor use of a wood stove (adjusted OR, 2.56) and having a family member who smoked indoors (adjusted OR, 2.07). |
| García Mata et al[15] (2000) | 90 patients with LCPD and 183 normal children, as controls, selected at random to determine whether the condition of passive smoking is related to the disease | LCPD and passive smoking | The association between LCPD and passive smoking, after controlling for age and gender, became significant (p = 0.0000). Thus the risk of LCPD in passive smoking children is more than five times higher than in children who are not exposed to smoke. |
| Bahmanyar et al[17] (2008) | The Swedish Inpatient Register identified 852 individuals with a diagnosis of LCPD from 1983 to 2005, individually matched by year of birth, age, sex and region of residence with 4432 randomly selected control subjects. | Maternal smoking pregnancy and LCPD | Maternal smoking during pregnancy was associated with an increased LCPD risk, and heavy smoking was associated with a risk increase of almost 100%. Very low birth weight and caesarean section were independently associated with approximately 240% and 36% increases in the risk of LCPD, respectively. |
| Wiig et al[29] (2006) | 402 patients with a matched control group of non-affected children (n = 1025952) from the Norwegian Medical Birth Registry | Epidemiology and possible aetiology of LCPD | Applying Sartwell's log-normal model of incubation periods to the distribution of age at onset of Perthes' disease showed a good fit to the log-normal curve. Our findings point toward a single cause, either genetic or environmental, acting prenatally in the aetiology of Perthes' disease. |
| Perry et al[32] (2013) | 146 cases of LCPD and 142 hospital controls, frequency matched by age and sex | LCPD and hyperactivity | Significant associations (P < 0.05) existed with the majority of the psychological domains captured by the Strength and Difficulties Questionnaire [OR for "high" level of difficulties-Emotion OR 3.2, Conduct OR 2.1, Inattention-Hyperactivity OR 2.7, Prosocial behaviour OR 1.9]. Hyperactivity was especially marked among individuals within 2 years of diagnosis (OR = 8.6; P < 0.001), but not so among individuals over 4 years from diagnosis. |
| Berman et al[34] (2016) | 16 children with LCPD (age 9.1 ± 3.3, 75% males) were compared with their closest-aged siblings (age 9.3 ± 2.6, 30% males). | LCPD and ADHD | Our findings in a small cohort of children with LCPD and their comparably aged siblings do not support an association between LCPD and ADHD |
| Hailer et al[35] (2012) | 2579 patients with LCPD in Sweden during the period 1964-2005. 13748 individuals without LCPD were randomly selected from the Swedish general population | LCPD and risk of injury | Patients with LCPD are vulnerable to injuries that could be interpreted as a marker of hyperactive behaviour. |
| Hailer et al[36] (2014) | 4057 individuals with LCPD in Sweden during the period 1964-2011. 40570 individuals without LCPD were randomly selected from the Swedish general population | LCPD and ADHD | Compared to the control group, individuals with LCPD had a raised HR of 1.5 (95%CI: 1.2-1.9) for ADHD. |
| Türkmen et al[37] (2014) | The study included 3 groups of patients: Perthes patients, trauma patients and orthopaedic patients without Perthes disease or history of trauma. Each group was comprised of 56 males and 4 females. | LCPD and ADHD | ADHD was diagnosed in 7 patients in the Perthes group. The findings are not significant |
| Lee et al[39] (2013) | 38 male and 3 female patients with LCPD, and an equal number of age (range was 4-12) and sex-matched control patients with healthy fractures. | LCPD and leptin | Leptin, disease severity and treatment outcomes were associated. This correlation suggests that leptin might play an important role in LCPD pathogenesis. |
| Srzentić et al[51] (2014) | 37 patients with Perthes disease and 50 healthy controls | LCPD and IL-6 | Our study revealed that heterozygote subjects for the IL-6 G-174C/G-597A polymorphisms were significantly overrepresented in the control group than in the Perthes patient group. |
| Kamiya et al[52] (2015) | 28 patients with matched controls | LCPD and IL-6 | In the synovial fluid of the affected hips, IL-6 protein levels were significantly increased (LCPD: 509 ± 519 pg/mL, non-LCPD: 19 ± 22 pg/mL; P = 0.0005) on the multi-cytokine assay. |
| Perry et al[76] (2012) | 149 cases and 146 controls | Vascular abnormalities in LCPD patients | Children with Perthes disease exhibit small artery calibre and reduced function, which is independent of body composition. These data imply that that Perthes disease may reflect a wider vascular phenomenon that could have long-term implications for the vascular health of affected individuals. |
| Kitoh et al[78] (2003) | 125 children (105 boys, 20 girls) with unilateral LCPD | Delayed ossification in LCPD | Our findings support the hypothesis that a delay in endochondral ossification in the proximal capital femoral epiphysis may be associated with the onset of Perthes' disease. |
| Kocjančič et al[79] (2014) | 135 adult hips of patients who had been treated for Perthes disease in childhood with matched controls | Hip stress distribution in LCPD | No differences were found in resultant hip force and in peak contact hip stress between the hips that were in childhood subject to Perthes disease and the control population, but a considerable (148%) and significant (P < 0.001) difference was found in the contact hip stress gradient index, expressing an unfavourable, steep decrease of contact stress at the lateral acetabular rim. |
| Neidel et al[83] (1992) | 59 consecutive children with Perthes' disease and 59 matched controls | IGF-1 and LCPD | Our data may reflect an impaired synthesis or release of IGF I relative to age in Perthes' disease or changes in the affinity or metabolism of IGF binding proteins. The observed changes seem to be of a temporary nature. |
| Kim et al[82] (2009) | 56 immature pigs | HIF-1α and LCPD | Acute ischemic injury to the immature femoral head induced severe hypoxia and cell death in the bony epiphysis and the deep layer of the epiphyseal cartilage. Viable chondrocytes in the superficial layer of the epiphyseal cartilage showed HIF-1α activation and VEGF upregulation with subsequent revascularization occurring in the cartilage. |
| Matsumoto et al[84] (1998) | 27 children with Perthes' disease and 10 age-matched control subjects | IGF binding protein-3 and LCPD | The bone age was delayed 2 years or more compared with the chronological age in 7 of 18 patients, and all of them, except 1, showed decreased levels of IGFBP-3 on WLB. |
| Graseman et al[85] (1996) | 23 children with unilateral LCPD and in 23 sex and age matched controls | IGF binding protein-3 and LCPD | Data confirm that most children with LCPD are skeletally immature. However, IGF-I measured with IGF-II-blocked IGFBP binding sites, and IGFBP-3 serum concentrations analysed with respect to bone age showed no evidence for a disturbance of the hypothalamo-pituitary-somatomedin axis in these children. |
| Neidel et al[86] (1993) | 55 children with Perthes' disease and 55 age- and sex-matched controls | IGF and LCPD | Our findings indicate that low levels of circulating IGF I in Perthes' disease, as we have reported previously, are caused neither by altered concentrations of the principal IGF-binding protein, IGFBP-3, nor by an underlying growth hormone deficiency. |
The assessments were performed by three authors independently. Any discrepancy was discussed with the senior investigator for the final decision. All the raters agreed on the final result of every stage of the assessment.
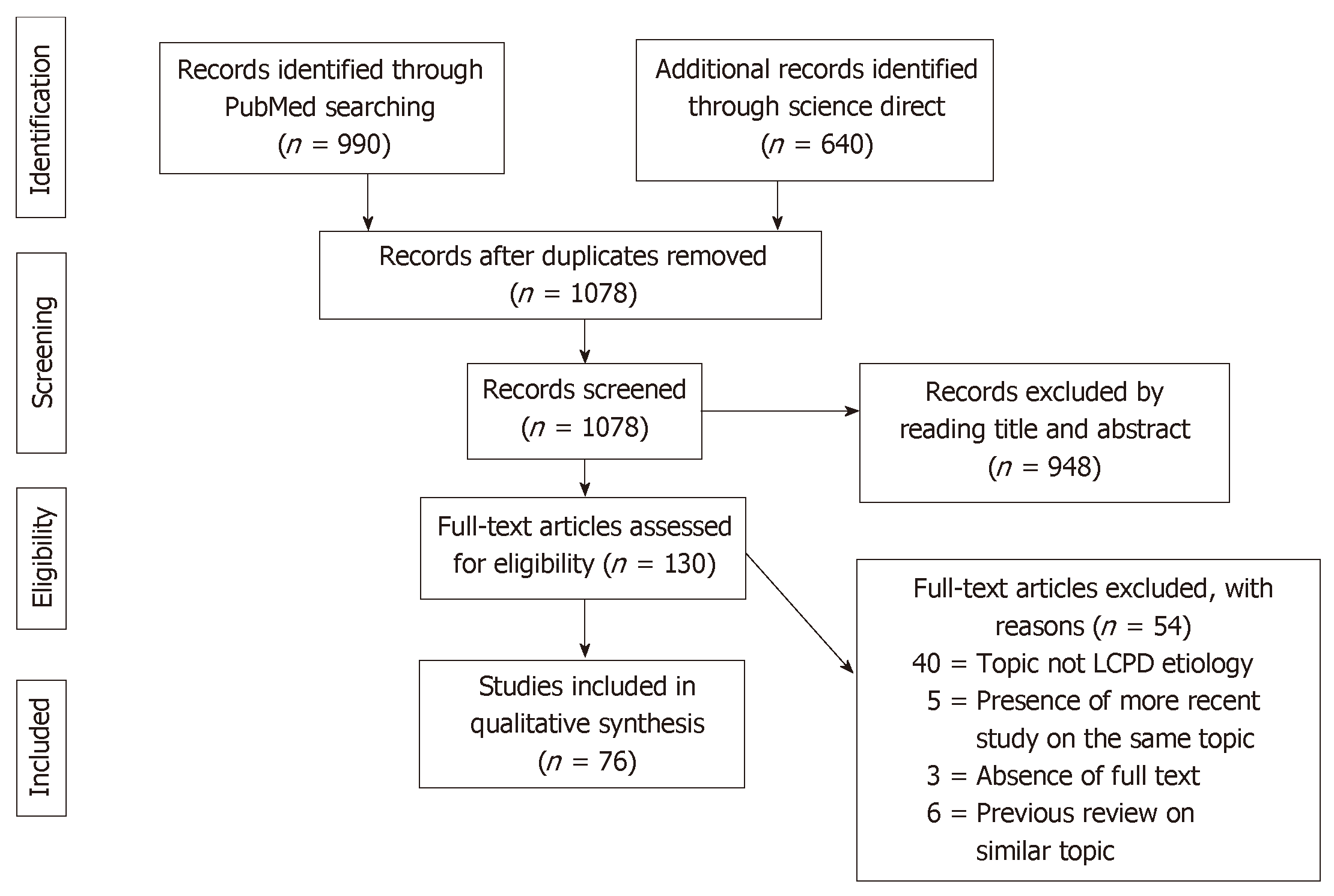
A total of 1630 articles were found. After the exclusion of duplicates, 1078 articles were selected. At the end of the first screening, following the previously described selection criteria, we selected 130 articles eligible for full text reading. Ultimately, after full text reading and reference list check, we selected n = 64 articles following previous written criteria. A PRISMA[10] flowchart of the method of selection and screening is provided (Figure 1). The included articles[11-85] mainly focus on genetic research, epidemiological studies, magnetic resonance imaging analysis and histological histochemical analysis. The main findings of the included articles are summarized (Tables 1-4).
| Ref. | Subjects | Association/molecule studied | Results |
| Gordon et al[14] (2004) | 60 patients with LCPD | Smoking and socio-economic status and the severity of LCPD | A significant association was noted between living with a smoker and LCPD as well as between increasing smoke exposure and increased risk of developing LCPD. No significant association was noted between lower income and LCPD. There was no association between increased smoke exposure and increased severity of LCPD as measured by the lateral pillar classification. |
| Glueck et al[16] (1998) | 39 children with Legg-Perthes disease | Second-hand smoke exposure | Second-hand smoke exposure had no significant effects on other measures of coagulation. Second-hand smoke exposure while in utero and during childhood appears to lower stimulated tissue plasminogen activator activity and additionally may depress heritable low stimulated tissue plasminogen activator activity, leading to hypofibrinolysis. Hypofibrinolysis may facilitate thrombotic venous occlusion in the head of the femur, leading to venous hypertension and hypoxic bone death, Legg-Perthes disease. |
| Sharma et al[18] (2005) | 240 children (263 hips) who presented with Perthes' disease in Greater Glasgow | Socio economic deprivation and LCPD | There was no significant evidence of a preponderance of Perthes' disease in the most deprived groups. |
| Pillai et al[19] (2005) | 40 LCPD patients and the Southwest Scotland registry | The incidence of LCPD in Southwest Scotland | The incidence of LCPD increases with deprivation and poor living standards. |
| Kealey et al[20] (2000) | 313 children with LCPD and Northern Ireland registry | Socio economic deprivation and LCPD | While the incidence of Perthes' disease was found to be associated with indicators of the level of deprivation for areas, there was no evidence to suggest that there was an increased risk in urban areas; the highest rate was found in the most deprived rural category |
| Perry et al[21] (2012) | The General Practice Research database was analysed to identify incident cases between 1990 and 2008 in children aged 0-14 years | LCPD incidence in United Kingdom | The incidence was declining in the study period. The declining incidence, along with the geographic variation, suggests that a major etiologic determinant in LCPD is environmental and closely linked to childhood deprivation. |
| Perry et al[22] (2012) | Scottish Morbidity Record, based in Scotland, United Kingdom using data from 2000-2010. A total of 443 LCPD patients | Socio economic deprivation and LCPD | The occurrence of Perthes' disease within urban environments is high, yet this appears to be a reflection of higher socioeconomic deprivation exposure. Disease rates appear equivalent in similarly deprived urban and non-urban areas, suggesting that the determinant is not a consequence of the urban environment. |
| Perry et al[23] (2011) | 1082 children with Perthes' disease (682 from a geographically defined area). Regional disease register in Merseyside, United Kingdom, 1976-2009 | Social deprivation and the declining incidence of LCPD | There was a marked decline in disease incidence over the study period, particularly in more deprived areas. The magnitude of the association with deprivation, and the changing incidence, strongly suggest that environmental factor(s) are a major aetiological determinant in Perthes' disease. |
| Hall and Barker[24] (1989) | Yorkshire region registry | Perthes incidence over the region | There were large geographical differences in incidence that could not be explained by urban-rural or social class differences. |
| Hall et al[25] (1983) | Case registry in Liverpool and adjacent parts of Knowsley and Sefton during 1976-81 | Incidence of LCPD in the region | The inner city of Liverpool, which has been shown to be underprivileged, had the highest yearly incidence of the disease ever reported: 21.1 cases/100000 children aged 14 years and under. The associations with poverty support the hypothesis that undernutrition is a causative factor in the disease. |
| Margetts et al[26] (2001) | Registry of Liverpool (1982-1995) | Incidence and distribution of LCPD in Liverpool | We suggest that environmental influences may come into play some years before a child presents with pain in the hip. There may be a genetic predisposition to the disease. |
| Metcalfe et al[27] (2016) | All twin pairs from the Danish Twin Registry (DTR) in which at least 1 individual had LCPD (81 twin pair) | Twin study of LCPD | This study found evidence of familial clustering in LCPD but did not show a genetic component. The absolute risk that a co-twin of an affected individual will develop LCPD is low, even in the case of monozygotic twin pairs. |
| Lappin et al[28] (2003) | 320 patients on the Northern Ireland Perthes' database | Birthweight and LCPD | We observed that the low birthweight twin in each case was the affected child. It is proposed that environmental factors associated with low birthweight are involved in the aetiology of Perthes' disease. |
| Loder et al[30] (1993) | 24 LCPD patients | LCPD and ADHD | One third (33%) of the children had abnormally high scores in profiles associated with ADHD (impulsive, hyperactive and psychosomatic categories), much higher than the 3%-5% incidence of ADHD in the general population. |
| Perry et al[31] (2012) | General Practise Research database in United Kingdom | LCPD comorbidities | The risk of Perthes' disease was significantly increased with the presence of congenital anomalies of the genitourinary and inguinal region, such as hypospadias (OR = 4.04, 95%CI: 1.41-11.58), undescended testis (OR = 1.83, 95%CI: 1.12-3.00) and inguinal herniae (OR = 1.79, 95%CI: 1.02-3.16). Attention deficit hyperactivity disorder was not associated with Perthes' disease (OR = 1.01, 95%CI: 0.48-2.12), although a generalised behavioural disorder was (OR = 1.55, 95%CI: 1.10-2.17). Asthma significantly increased the risk of Perthes' disease (OR = 1.44, 95%CI: 1.17-1.76), which remained after adjusting for oral/parenteral steroid use. |
| Podeszwa et al[33] (2015) | 11 LCPD patients | Psychological finding in patients undergoing surgery | A significant presence of depression and anxiety symptoms was reported. |
| Neal et al[38] (2016) | 150 patients (172 hips) with LCPD | LCPD and obesity | Obesity is common in patients with LCPD and is associated with a later stage of disease presentation. |
| Srzentić et al[48] (2015) | 37 LCPD patients | Markers of coagulation, inflammation and apoptosis in LCPD | The results presented indicate that apoptosis could be one of the factors contributing to the lack of balanced bone remodelling process in Perthes patients. |
| Calver et al[56] (1981) | 50 children with “irritable hip” | Radionuclide scanning in LCPD | Five of the 50 children seen during the one year had areas of ischemia in the capital femoral epiphysis demonstrated on the scan. All five developed radiological signs of Perthes' disease within 6 mo. The remaining 45 had radiographically normal hips at one year. |
| Royle and Galasko[60] (1992) | 192 patients with a typical transient synovitis syndrome | Scintigraphy in LCPD patients | Fifteen patients had evidence of ischemia of the femoral head, but only four patients went on to develop the typical radiographic features of Perthes' disease. The other 11 patients are thought to represent a minor, radiographically silent form of Perthes' disease. |
| Lamer et al[61] (2002) | 26 DGS MRI and bone scintigraphies of 25 hips in 23 children | Blood supply in LCPD | DGS MRI allows early detection of epiphyseal ischemia and accurate analysis of the different revascularisation patterns. These changes are directly related to the prognosis of LCPD |
| Atsumi et al[62] (2000) | 28 hips in 25 patients with LCPD | Blood supply in LCPD | We suggest that in Perthes' disease the blood supply of the LEAs is impaired at their origin and that revascularisation occurs from this site by ingrowth of small vessels into the femoral epiphysis. This process may be the result of recurrent ischemic episodes. |
| de Camargo et al[63] (1984) | 30 patients, including 26 aortographies and 6 selective angiographies | Blood supply in LCPD | The major angiographic alterations were: general decrease of blood flow in the affected hip, lack of a patent medial circumflex artery, an atrophic medial circumflex artery or obstruction of its branches, distended vessels in subluxations of the hip joint and almost complete absence of the obturator artery |
| Theron et al[64] (1980) | 11 cases of LCPD | Blood supply in LCPD | The balance between the respective vascular territories of the dilated superior and inferior capsular arteries is variable and seems to affect the position of the sequestrum and the centering of the femoral head. |
| Kitoh et al[78] (2003) | 125 children (105 boys, 20 girls) with unilateral LCPD | Delayed ossification in LCPD | Our findings support the hypothesis that a delay in endochondral ossification in the proximal capital femoral epiphysis may be associated with the onset of Perthes' disease. |
| Ref. | Subjects | Association/molecule studied | Results |
| Suehiro et al[49] (2005) | Mouse model | Osteonecrosis in rat model | Repetitive mechanical stress on the femoral heads from 5 wk to 9 wk of age played an important role in the aetiology of osteonecrosis |
| Gershuni et al[59] (1983) | Hip of the immature pig | Joint tamponade in LCPD animal model | The data from this experiment do not support the theory that tamponade of the femoral capital epiphysis is the cause of osteonecrosis in Legg-Calvé-Perthes syndrome |
| Cheon et al[66] (2015) | 10 piglets | Quantitative MRI in piglet model of LCPD | The epiphyseal ADC values of the ischemic hip decreased immediately (1 hour) after embolization. However, they increased rapidly at 1 wk after embolization and remained elevated until 4 wk after embolization. Perfusion MRI of ischemic hips showed decreased epiphyseal perfusion with decreased Kep immediately after embolization. |
| Li et al[67] (2006) | 20 femoral heads of 10 piglets | MRI in piglet model of LCPD | Gadolinium-enhanced MRI can identify early ischemia and its reversal of the capital femoral epiphysis induced by hip hyper-abduction |
| Babyn et al[68] (1998) | Piglet model | MRI in piglet model of LCPD | High resolution MRI can demonstrate changes in the CE associated with ischemic injury and may have a role in the assessment of the CE and its development after ischemic injury. |
| Li et al[69] (2008) | 25 piglets models | Diffusive MRI in a model of LCPD | Histological study revealed necrosis of chondrocytes and osteocytes and abnormal thickening of the epiphyseal cartilage in the ischemic femoral head. |
| Levin et al[70] (1999) | Rat model | Epiphysis studies in a rat model | Thickening and condensation of the subchondral bone, leading to increased stiffness of the subchondral zone, result in the osteoarthritis-like disorder. Mimicking the well-known phases of human osteonecrosis, the model readily allows for preclinical studies of therapeutic regimens. |
| Kandzierski et al[71] (2004) | Calf femurs | Calf femur experimental study | The author concludes that impaired blood flow within the growth layers additionally weakens the immature bone tissue of the femoral head and neck, which may lead to mechanical damage of the bone tissue itself, as well as to the epiphyseal blood vessels entering bony epiphysis. |
| Suehiro et al[72] (2000) | Twenty femora from 10 Wistar Kyoto rats | Standing and induction of OA | Repetitive mechanical stress on the femoral heads from 5 wk to 9 wk of age played an important role in the aetiology of osteonecrosis |
| Naito et al[74] (1992) | Canine femoral head | Acute effect of traction, compression, and hip joint tamponade on blood flow of the femoral head | These experimental data may have important implications for the pathogenesis of iatrogenic avascular necrosis in the treatment of congenitally dislocated hip, Legg-Perthes disease and avascular necrosis following nondisplaced femoral neck fracture |
| Kim et al[77] (2013) | 56 immature pigs | MRI in the initial stage of LCPD | Acute ischemic injury to the immature femoral head induced severe hypoxia and cell death in the bony epiphysis and the deep layer of the epiphyseal cartilage. Viable chondrocytes in the superficial layer of the epiphyseal cartilage showed HIF-1α activation and VEGF upregulation with subsequent revascularization occurring in the cartilage. |
| Zhang et al[81] (2015) | 6-wk-old Sprague Dawley rats | HIF-1α and LCPD | Hypoxia might be an etiological factor for femoral head necrosis. HIF-1α, VEGF as well as apoptotic genes participated in the pathophysiological process of ischemic osteonecrosis. |
| Kim et al[83] (2009) | 56 immature pigs | HIF-1α and LCPD | Acute ischemic injury to the immature femoral head induced severe hypoxia and cell death in the bony epiphysis and the deep layer of the epiphyseal cartilage. Viable chondrocytes in the superficial layer of the epiphyseal cartilage showed HIF-1α activation and VEGF upregulation with subsequent revascularization occurring in the cartilage. |
| Ref. | Subjects | Association/molecule studied | Results |
| O’Sullivan et al[40] (1985) | A family in which Legg-Calvé-Perthes disease (LCPD) occurred in four members | Genetic factors and LCPD | This unusually high incidence in one family raises questions about the genetic versus the environmental factors in the aetiology of LCPD. |
| Livesey et al[41] (1998) | Case report of three family with three female first-degree relatives affected by LCPD | Genetic factors and LCPD | First case of three first-degree relative affected |
| Miyamoto et al[42] (2007) | A Japanese family with an autosomal dominant hip disorder manifesting as LCPD | LCPD and COL2A1 | This is the first report of a mutation in hereditary LCPD. COL2A1 mutations may be more common in LCPD patients than currently thought, particularly in familial and/or bilateral cases. |
| Al-Omran and Sadat-Ali[43] (2013) | 2 generations of 4 male family members with LCPD-like features and mutation of the COL2A1 gene of the 12q13 chromosome | LCPD and COL2A1 | If LCPD occurs in any family member, we recommend genetic analysis and counselling as well as early radiological screening of related children. |
| Kannu et al[44] (2011) | Two children who presented with abnormal development of both hips and in whom novel mutations in the COL2A1 gene were found | LCPD and COL2A1 | The purpose of our report is to alert clinicians to the possibility that children who present with bilateral Perthes-like disease of the hip might have an underlying mutation in the gene encoding type II collagen. |
| Su et al[45] (2008) | Forty-two members of a 5-generation family | LCPD and COL2A1 | The p.Gly1170Ser mutation of COL2A1 in the family described is responsible for pathology confined to the hip joint, which presents as isolated precocious hip OA, AVN of the femoral head, or Legg-Calvé-Perthes disease. |
| Li et al[46] (2014) | Forty-five members of a four-generation family | LCPD and COL2A1 | In our research, we identify a heterozygous mutation (c.1888 G>A, p. Gly630Ser) in exon 29 of COL2A1 in the Gly-X-Y domain, in a Chinese family affected by LCPD and ANFH. |
| Woratanarat et al[47] (2014) | Twelve case–control studies met inclusion criteria and had sufficient data for extraction | Hypercoagulability and LCPD | The factor V Leiden mutation is significantly related to Perthes disease, and its screening in at‐risk children might be useful in the future. |
| Srzentić et al[48] (2015) | 37 LCPD patients | Markers of coagulation, inflammation and apoptosis in LCPD | The results presented indicate that apoptosis could be one of the factors contributing to the lack of balanced bone remodelling process in Perthes patients. |
| Liu et al[50] (2015) | Age- and sex-matched serum samples from 10 control subjects and 10 patients with LCPD were compared using the isobaric tags for relative and absolute quantification (iTRAQ) technique. | Serum proteomes in LCPD | The complement and coagulation cascades, and abnormal lipid metabolism may be involved in the pathogenesis of LCPD. |
| Srzentić et al[51] (2014) | 37 patients with Perthes disease and 50 healthy controls | LCPD and IL-6 | Our study revealed that heterozygote subjects for the IL-6 G-174C/G-597A polymorphisms were significantly overrepresented in the control group than in the Perthes patient group. |
| Kamiya et al[52] (2015) | 28 patients with matched controls | LCPD and IL-6 | In the synovial fluid of the affected hips, IL-6 protein levels were significantly increased (LCPD: 509 pg/mL ± 519 pg/mL, non-LCPD: 19 pg/mL ± 22 pg/mL; P = 0.0005) on the multi-cytokine assay. |
| Su et al[55] (2010) | a five-generation family with 42 members with a new type II collagenopathy | LCPD and COL2A1 | Our study demonstrated that the p.Gly1170Ser mutation of COL2A1 caused significant structural alterations in articular cartilage, which are responsible for the new type II collagenopathy. |
| Matsumoto et al[84] (1998) | 27 children with Perthes' disease and 10 age-matched control subjects | IGF binding protein-3 and LCPD | The bone age was delayed, 2 years or more compared with the chronological age in 7 of 18 patients, and all of them, except 1, showed decreased levels of IGFBP-3 on WLB. |
| Graseman et al[85] (1996) | 23 children with unilateral LCPD and in 23 sex and age matched controls | IGF binding protein-3 and LCPD | Our data confirm that most children with LCPD are skeletally immature. However, IGF-I measured with IGF-II-blocked IGFBP binding sites, and IGFBP-3 serum concentrations analysed with respect to bone age show no evidence for a disturbance of the hypothalamo-pituitary-somatomedin axis in these children. |
| Neidel et al[86] (1993) | 55 children with Perthes' disease and 55 age- and sex-matched controls | IGF and LCPD | Our findings indicate that low levels of circulating IGF I in Perthes' disease, as we have reported previously, are caused neither by altered concentrations of the principal IGF-binding protein, IGFBP-3, nor by an underlying growth hormone deficiency. |
While other environmental factors may be present, smoking seems to one of the most reported risk factor for developing LCPD[11-16]. In particular, Perry et al[12] recently showed how maternal smoking can affect the risk of developing the disease in a case control study. In addition to this, another four studies[13-16] report evidence of the association between environmental smoke and LCPD, both during maternal pregnancy and the childhood of the patient. Lastly, a study involving 852 patient showed how the smoking habit augmented the risk for LCPD by 100% in the examination sample[17].
Three different studies[14,18,19] involving an overall 340 LCPD patients explored the link between the disease and socioeconomic deprivation without revealing a significant association. On the contrary, Kealey et al[20], investigating 311 patients, found a higher prevalence among the most deprived rural category. In 2012, another study[21] analysed the incidence of LCPD between 1990 and 2008 in children between 0 years and 14 years in the United Kingdom. They reported how the incidence was coherently higher within the quintile with the highest degree of deprivation (risk ration = 1.49, 95% confidence interval (CI): 1.10–2.04) (P < 0.01). Five more studies[22-26] held in United Kingdom by the same research group reported similar results.
Metcalfe et al[27] reported an increased presence of low birth weight in children affected by LCPD. Similar to the results provided by Lappin et al[28], Sharma et al[18] reported an association between low birth weight and LCPD, whereas the weight of the children at the moment of the diagnosis and follow up did not show significant alteration. This was further supported by other studies with similar results[26]. On the contrary, Bahmanyar et al[17] reported a possible association between low birth weight and LCPD, but that was shown to be insignificant after evaluating other risk factors like maternal smoking. Only really low birth weight (< 1500 g) seemed to be associated independently with LCPD. Another nationwide study[29] held in Norway and involving 425 patients reported similar results. Their results supported the presence of environmental or genetic factors but not low birthweight. Similar results were reported by other epidemiological studies[12-16].
Loder et al[30] in 1993 studied the association between ADHD and LCPD in 24 patients. He reported the presence of one third (33%) of the children with abnormally high scores in profiles associated with ADHD. Perry et al[31,32] investigated both general practise registry of comorbidities in LCPD patients and the incidence of behavioural disturbance. ADHD was not associated with Perthes' disease (OR = 1.01, 95%CI: 0.48-2.12) in the first study[31]; while in the second one[32], a case control study involving 146 cases of LCPD and 142 hospital controls, the presence of behavioural disturbance was reported.
In 2015, a prospective study[33] evaluated in 58 adolescents patients (11 with LCPD) undergoing hip preservation surgery the presence of psychological disturbances. A significant presence of depression and anxiety symptoms was reported. Bergman et al[34] investigated ADHD in LCPD patients and in siblings of the child affected. A similar incidence was reported in both the groups. Hailer et al investigated the prevalence of the disease in two high profile studies held in 2012[35] and in 2014[36]. The first one involved 2579 LCPD patients and 13748 controls. It investigated the risk of injury in both groups and reported a higher percentage in the LCPD group. The second one involved 4057 individuals with LCPD in Sweden during the period 1964-2011 and 40570 individuals without LCPD randomly selected from the Swedish general population and matched by year of birth, sex and region. They reported that individuals with LCPD also had a raised hazard ratio (HR) of 1.5 (95%CI: 1.2-1.9) for ADHD.
Türkmen et al[37], instead, did not find a significant difference in ADHD prevalence between LCPD group and control groups.
A study held in 2016[38] reported a positive association between LCPD and obesity. Obesity was associated with a more severe clinical presentation and femoral head deformity. Lee et al[39] have investigated the levels of leptin in LCPD patients compared to control subjects. A significantly higher value concordant with the severity of the disease was reported.
Over the years, several cases of LCPD in the same family have been reported[40,41]. This evidence suggested a genetic role in the development of the disease. In particular, Miyamoto et al[42] were the first to report a case of familiar LCPD associated with a mutation of the Collagen type II gene (COL2A1). Thus, COL2A1 genes were proposed as potential pathogenic trigger of LCPD. Several case reports also found evidence of this association in LCPD patients[43,44]. Further studies investigated the relationship between this gene mutation and LCPD. Su et al[45] recruited 42 members of a five-generation family and found in 16 patients a p.Gly1170Ser mutation of COL2A1 cosegregated with LCPD, precocious hip osteoarthritis or avascular femoral head necrosis not linked with LCPD. Li et al[46] in 2014 held a study, including a four-generation family, reporting the presence of the mutation in six affected family members.
Metcalfe et al[27] investigated the presence of genetic factors using the information derived from the Danish Twin Registry. After studying concordance with LCPD in 81 twin pairs (10 monozygotic, 51 dizygotic and 20 unclassified), they concluded that the absolute risk that a co-twin of an affected individual will develop LCPD is low, even in the case of monozygotic twin pairs. While Metcalfe et al[27] did not find an association between LCPD and birth weight, another twin study[28] and a case control study[17] involving an overall of 320 twin patients and 852 patients, respectively, concluded that low birth weight may play a role in developing the disease.
A meta-analysis[47] held in 2012 investigated factor V Leiden, prothrombin II and methylenetetrahydrofolate reductase (MTHFR) polymorphism as sources of possible genetic aetiology of LCPD. They comprised 12 case-control studies, including 824 children in the Perthes group and 2033 children in the control group. Factor V Leiden polymorphism (carrying the minor A allele instead of the G allele) was associated with a three times higher incidence of the disease. Prothrombin II polymorphism (A allele instead of the G allele) reported giving a 1.5-fold increase risk of disease. There was no association between MTHFR polymorphism and Perthes disease. In 2015, Srzentic et al[48] investigated gene expression and variants by quantitative reverse-transcriptase polymerase chain reaction and reported no difference between LCPD patients and controls for Factor V Leiden, Factor II, MTHFR and Plasminogen activator inhibitor-1.
Also, a prospective study held in 2002 did not suggest that thrombotic diatheses due to deficiency of protein C, protein S or antithrombin III or due to factor-V Leiden mutation are major causes of Legg-Perthes disease[49].
Liu et al[50] analysed age- and sex-matched serum samples from 10 control subjects and 10 patients with LCPD. They reported a higher presence of proteins and factors linked to complement and coagulation cascade. Increased activity of these factors may contribute to LCPD aetiology. In 2014 Srzentić et al[51] studied the association of frequencies of genetic variants of immune response genes with LCPD. They found significantly over-represented heterozygous subjects with an interleukin-6 (IL-6) polymorphism (G-174C/G-597A) in the LCPD group. In 2015, another studied reported a significantly increased IL-6 protein level in the synovial fluid of the hips affected by LCPD[52].
Srzentić et al[48] investigated the expression of apoptosis genes by the quantitative reverse-transcriptase polymerase chain reaction technique in 37 patients. They reported a higher presence of proapoptotic factor Bcl-2-associated X protein (Bax) along with a significantly higher Bax/Bcl-2 ratio in the patient group. Zhang et al[49] found in a rat model similar results, with a higher expression of the apoptotic genes Casp3, Casp8 and Casp9 in chondrocytes after hypoxia.
Mechanical stress and ischemia damage: Ischemia damage was reported in the first research documents we have on LCPD and its pathology insight[53,54]. In 1976, one of the first studies[55] about the aetiology of LCPD reported the presence of areas of infarction in 51% of hips histopathologically examined in 57 cases of femoral head biopsy. Calver et al[56] examined radiologically the ischemia areas in the hips of 50 children. The five with evidence of ischemia areas developed LCPD within 6 mo. The other 45 were healthy at 1 year follow up. Catteral et al[57] and Ponseti et al[58], respectively, in two different historical studies reported an area of ischemia in two children’s biopsies and morpho-structural alteration possibly associated with ischemic damage. In addition, several animal studies were conducted. A study held in 1983[59] investigated how joint tamponade in pigs would provoke ischemia of the epiphyseal plate. The necessity of high and prolonged pressure, which is difficult to achieve in normal conditions, was reported. Several research studies reported evidence of ischemia damage using radiological techniques[60-64]. This provides evidence of its role in the enteropathogenesis of the LCPD. Recently, Pinheiro et al[65] proposed a biomechanical model that further supports a combined role of both ischemic condition, skeletal immaturity and altered biomechanics. Also, studies on hip osteonecrosis in piglets confirmed this aetiology[66-69].
Mechanical stress-induced ischemia was found to be a possible aetiology of LCPD also using several animal models[70-72] and experimental analysis models[65,73,74]. One particular study performed by Suehiro et al[49] involving Wistar Kyoto rats reported how repetitive mechanical stress on the femoral heads from 5 wk to 9 wk of age played an important role in the aetiology of osteonecrosis. This theory is historically and currently one of the most supported[54,61,75-77].
Delayed epiphysial growth and hip geometry alterations: In 2003, Kitoh et al[78] investigated the epiphyseal height (EH) and width (EW) of the unaffected hip in 125 children affected by LCPD. A positive linear correlation (R = 0.87) was observed in the EH: EW ratio in these patients. A smaller EH than expected for EW in our series indicated epiphyseal flattening of the femoral head in LCPD cases. This finding supports the hypothesis that a delay in endochondral ossification in the proximal capital femoral epiphysis may be associated with the onset of Perthes' disease.
In 2014, Kocjančič et al[79] investigated the resultant hip force and contact hip stress distribution in a population of 135 adult hips of patients who had been treated for LCPD. Contra-lateral hips with no record of disease were taken as control. The contact hip stress gradient index was 148% higher (P < 0.001); expressing an unfavourable, steep decrease of contact stress at the lateral acetabular rim. On the contrary, Pinheiro et al[80] reported how the anatomical variations appear to have only a limited effect on the stress distribution in the femoral epiphysis, even during high impact activities and in the presence of a skeletally immature epiphysis. However, the same group recently published a study[65] on a new biomechanical model and reported a possible involvement of vascular obstruction to the epiphysis that may arise when there is delayed ossification and when articular cartilage has reduced stiffness under compression.
Vascular endothelial growth factor (VEGF) and hypoxia-inducible factor (HIF-1): In 2004, an animal study[49] involving piglets with avascular necrosis reported increased VEGF protein and mRNA expression in the epiphyseal cartilage of the infarcted heads compared with the contralateral normal heads. Therefore, VEGF upregulation in the proliferative zone after ischemic damage was proposed as a possible stimulator of vascular invasion and granulation tissue formation. Zhang et al[81] reported also a higher expression of VEGF and HIF-1 in rat model chondrocytes.
VEGF was found to be low in LCPD patients in an Indian population-based study[49]. In 2014, 28 LCPD cases (mean age: 8 ± 3.8) and 25 healthy age-matched control subjects were investigated, and VEGF, endothelial progenitor cell and immunoglobulins were not significantly different between the groups (P = 0.354). The endothelial progenitor cell count was inversely correlated with serum immunoglobulin G levels in the LCPD group (r = 0.403, P = 0.03). The absolute endothelial progenitor cell count was also significantly higher in the fragmentation stage than in the healing stage, and they were greater in bilaterally affected cases than in unilaterally affected patients. Similar results were found in a pig model[82] and Sprague-Dawley rats[81]. In the rat model, however, the interruption of vascularization in the proximal femoral growth plate was not followed by diffuse damage.
Insulin growth factor 1 (IGF-1): The role of IGF-1 role in LCPD aetiology was investigated. In particular, a study in spontaneously hypertensive rat (SHR) demonstrated altered IGF-I expression during early postnatal life and suggested that the altered IGF-I expression may cause the mechanical vulnerability of the femoral epiphysis. Low levels of serum IGF-1 and IGF-1 binding protein 3 have been reported in patients with LCPD[83,84]. However, these results conflict with another two studies that reported normal IGF-1 binding protein levels[85,86].
The aetiology of LCPD is still mainly unknown. The heterogeneous prevalence reported opens the discussion for the examination of possible environmental and social factors involved in the aetiology of the disease[1-3,29]. In support of this, three studies[21,26,87] reported a possible association between the decrease in the incidence and lifestyle changes over recent years, exposure to environmental risk factors like smoking, delayed epiphyseal ossification, low birthweight, child deprivation and obesity. However, the results are not definitive.
Several studies reported strong evidence supporting the role of social and economic deprivation in the incidence of LCPD in children[14,17,28,18-22,24-26]. This was proposed as a possible consequence of two factors: low birth weight and smoking habits. In addition, maternal smoking is associated with a low birthweight[88,89]. Thus, low birthweight could be often falsely associated with LCPD because of the smoking habit typically present in these families, which is the more probable cause of the higher incidence of LCPD. For these reasons, the role of low birth weight lacks strong evidence[12-17,29], and the smoking habit, being more common in social-economical deprived families, may play a more important role in the etiopathogenesis of LCPD. This was further confirmed by studies investigating the association between smoking and LCPD, which reported a significant augmentation of the risk in smokers and smoke-exposed family. The mechanism proposed is the smoke-dependent damage of vessel endothelium, which could ultimately lead to epiphyseal infarction[17].
In addition, recent evidence supports obesity as a major risk factor[38]. Leptin is a hormone that is expressed in adipose tissue and, at lower levels, in gastric epithelium and placenta associated with both obesity and bone metabolism[90,91]. It has been reported that both leptin and obesity are positively associated with the severity of LCPD[38,39]. Most obese humans have very high plasma leptin concentrations, suggesting they are resistant to its anorectic and metabolic effects[92]. Based on these findings, Bartell et al[93] conducted a study involving intracerebroventricular and subcutaneous administration of leptin in leptin-deficient ob/ob mice, investigating its effect on bone and muscular tissues. In both experiments, leptin had a key role in the decrease of body weight, food intake and body fat and in the increase of muscle mass, bone mineral density, bone mineral content, bone area, marrow adipocyte number and mineral apposition rate. Thus, leptin administration seemed to be really effective in obese patient bone metabolism. Following this rationale, Zhou et al[94] in 2015 tested these effects in a LCPD obese rat model. Six weeks after surgery induced avascular necrosis of the femoral head, radiologic and histomorphometric assessments were performed. Radiographs showed better preservation of the femoral head architecture in the leptin-treated group. Histology and immunohistochemistry revealed that the leptin group had significantly increased osteoblastic proliferation and vascularity in infarcted femoral heads compared with control groups. The mechanism proposed is linked to both a direct action of leptin on bone metabolism and an indirect action through the upregulation of VEGF[95]. In particular, leptin acts physiologically through MAPK/ERK 1/2 and PI-3K/AKT1 pathways and a series of transcription factors such as HIF-1α[92,96]. Emerging evidence supports how leptin and obesity may play a role in LCPD etiopathogenesis. We strongly encourage further studies on leptin and obesity, associated with their effects of bone metabolism, in LCPD patients. These studies should be conducted both as a therapeutic option and as a possible actor in the aetiology of the disease.
The dissimilarity in incidence between male and female subjects was initially thought to rely on the different etiopathogenesis of the disease. In particular, pioneer studies on these differences reported a more severe presentation and prognosis in female patients than in male subjects[97]. However, recent high profile epidemiological studies reported no significant differences in clinical presentation, outcome and prognosis between boys and girls[98,99]. Thus, the LCPD male/female ratio appears not to be associated with different clinical presentation and should not be part of the clinical treatment algorithm.
While the reviewed literature provided evidence of cases of LCPD running in families[41-46], and epidemiological studies suggested a genetic role[29], twin studies[27,28] reported no significant concordance. This was further investigated by another recent study[48] involving 37 patients and 100 controls that reported no difference in genotype variants and expression of coagulation and inflammatory factors. However, Zheng et al[100] investigated the relationship between global DNA methylation involving 82 children with LCPD and 120 matched controls. They reported significant differences in the global methylation of peripheral blood DNA between patients with LCPD and matched controls. Further high-profile epigenetic studies in key tissues should be conducted providing new protagonists of the LCPD aetiology. COL2A1 alterations were also investigated. Several case reports and studies were reported in our review[42-46]. Their association with the disease was found to be weak in a case study published by Kenet et al[101] involving 119 LCPD affected children and 276 controls, further supported by a review of the literature. In the same study, the absence of significant association with Gaucher's disease and Factor V Leiden alterations was also reported. On the contrary, further histological and ultrastructural studies found how p.Gly1170Ser mutation of COL2A1 is involved in the pathogenesis of a type II collagenopathy[55]. This alteration leads to an amino acid change that perturbs a Gly-X-Y triple-helix repeat, which is a fundamental structure in type II collagen function[102]. Thus, COL2A1 alterations should be further studied to clarify their role in the pathogenesis and aetiology of the LCPD.
Besides factor V Leiden and Prothrombin II polymorphisms being associated with a three times higher likelihood of disease and a 1.5-fold increase of risk, the results were not statistically significant[47]. Thus, even though the topic was extensively studied, recent evidence of hypercoagulability is inconclusive. Baltzer et al[103] reported a case of a child affected by Kienbock’s disease and factor V thrombophilia who developed LCPD. The patient’s hypercoagulability state may link with the bilateral manifestation of Perthes disease. After a further review of the literature, the authors also found two other cases of multifocal osteonecrosis and hypercoagulable disorder in adult patients. We encourage additional molecular and clinical studies on hypercoagulative states and LCPD.
Among the studied molecules, inflammatory cytokines involved in immune responses provide a crucial prospective of research. In particular, a study[51] found how a polymorphism of IL-6, a proinflammatory cytokine involved in chronic inflammation and immune response[104], was found to be associated with LCPD. In addition, another study[52] reported a high synovial level of IL-6 in LCPD patients. This suggests that IL-6 is a possible future protagonist of new therapeutic approaches and translational research. We strongly encourage further studies on the IL-6 pathway. Another molecule studied is SIRT1, the mammalian ortholog of the yeast SIR2 (Silencing Information Regulator) and a member of the Sirtuin family. It was reported that it can inhibit nuclear factor kappa B (NF-kB) transactivational activity by deacetylating Lys310 of the RelA/p65 subunits. Thus, SIRT1 inhibits both its transcriptional activity and the release of inflammatory cytokines mediated by NF-kB[105]. What emerged in a recent research project held in 2017 is that treatment with interferon-β increased SIRT1 expression and inhibited secretion of IL-6 in avascular femoral head necrosis mouse model. Interferon-β activated SIRT1 in the RAW 264.7 cell and bone marrow-derived osteoclasts and decreased IL-6 secretion. What we can assume from these papers is that IL-6, NF-kB, SIRT1 and other molecules involved in the autoimmune response could be linked with LCPD aetiology and should be further investigated both as a possible cause of the disease and as a new potential therapeutic target.
A study of 2003[78] found how retarded epiphyseal ossification could be linked to LCPD. However, there is lack of consensus on the role of these anatomical alterations[65,79,80]. During development, the epiphyseal blood supply is almost exclusively provided by the deep branch of the medial femoral circumflex artery[75]. Both imaging and histological studies have shown a partial or complete loss of blood flow[61,77] and the development of ischemic necrosis[54] of the femoral head in LCPD. Mechanical-induced ischemia is one of the most supported hypotheses for the aetiology of LCPD[54,61,75-77]. Emerging evidence is supporting a role of repetitive mechanical stress to the epiphysis and de subsequent ischemia, as stated in different experimental models[65-69,73,74]. In addition, the association with ADHD[30-34,36] and hyperactive modification of behaviour could be easily explained with repetitive activity-induced mechanical stress. This was further explained with an higher risk of injury[35] in LCPD patients. The recent evidence strongly supports the role of mechanical stress in the aetiology of LCPD. We encourage high profile clinical studies to investigate more this etiopathogenesis.
Our study has some strengths. We extensively searched and identified all relevant genetic association studies, the most common comorbid and the possible etiologic hypothesis. Therefore, risk of bias assessment showed moderate overall risk that could influence our analysis.
The literature available on the aetiology of LCPD presents major limitations in terms of great heterogeneity and lack of high-profile studies. Although a lot of studies focused on the genetic, biomechanical and radiological background of the disease, there is lack of consensus on one or multiple major actors of the etiopathogenesis. While obesity, smoking exposure and child deprivation seems to be associated with LCPD aetiology, more studies are needed to understand the complex and multifactorial genesis of the avascular necrosis characterizing the disease.
Legg-Calvé-Perthes disease (LCPD) is a complex disease with a multifactorial aetiology. The etiopathogenesis of the disease was widely investigated in the last 20 years, but it is still unknown.
Numerous studies tried to explain the major actors in LCPD aetiology, but there is a lack of synthesis of the evidence.
The purpose of the study was to summarize the current evidence on the aetiology of LCPD.
Two databases (PubMed and Science Direct) were systematically searched for relevant articles by two independent reviewers from their date of inception to the 20th of May 2018. Every step of the review was done according to PRISMA guidelines. Due to article heterogeneity and the topic after data analysis, a descriptive (synthesis) analysis was performed.
Sixty-four articles were included in this systematic review after applying our inclusion and exclusion criteria. Available evidence on LCPD aetiology is still inconclusive. Several hypotheses have been researched but none of them was found decisive.
After our systematic review of the available evidence we conclude that LCPD aetiology relies on a multifactorial basis where environment in genetically predisposed patients participates in the pathogenesis of the disease.
Further clinical and preclinical studies are strongly encouraged to understand better the mechanical and vascular basis of the etiopathogenesis of the disease. Interesting perspectives from studies on Leptin, obesity, and mechanical trauma were found and should be further investigated.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Elgafy H, Slomiany BL S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Wu YXJ
| 1. | Loder RT, Skopelja EN. The epidemiology and demographics of legg-calvé-perthes' disease. ISRN Orthop. 2011;2011:504393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 2. | Perry DC, Hall AJ. The epidemiology and etiology of Perthes disease. Orthop Clin North Am. 2011;42:279-283, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Johansson T, Lindblad M, Bladh M, Josefsson A, Sydsjö G. Incidence of Perthes' disease in children born between 1973 and 1993. Acta Orthop. 2017;88:96-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Guerado E, Caso E. The physiopathology of avascular necrosis of the femoral head: an update. Injury. 2016;47 Suppl 6:S16-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Douglas G, Rang M. The role of trauma in the pathogenesis of the osteochondroses. Clin Orthop Relat Res. 1981;28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Larson AN, Sucato DJ, Herring JA, Adolfsen SE, Kelly DM, Martus JE, Lovejoy JF, Browne R, Delarocha A. A prospective multicenter study of Legg-Calvé-Perthes disease: functional and radiographic outcomes of nonoperative treatment at a mean follow-up of twenty years. J Bone Joint Surg Am. 2012;94:584-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Shah H. Perthes disease: evaluation and management. Orthop Clin North Am. 2014;45:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Herring JA, Kim HT, Browne R. Legg-Calve-Perthes disease. Part II: Prospective multicenter study of the effect of treatment on outcome. J Bone Joint Surg Am. 2004;86-A:2121-2134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Wiig O, Terjesen T, Svenningsen S. Prognostic factors and outcome of treatment in Perthes' disease: a prospective study of 368 patients with five-year follow-up. J Bone Joint Surg Br. 2008;90:1364-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47203] [Article Influence: 2950.2] [Reference Citation Analysis (0)] |
| 11. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 10888] [Article Influence: 1209.8] [Reference Citation Analysis (2)] |
| 12. | Perry DC, Thomson C, Pope D, Bruce CE, Platt MJ. A case control study to determine the association between Perthes' disease and the recalled use of tobacco during pregnancy, and biological markers of current tobacco smoke exposure. Bone Joint J. 2017;99-B:1102-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Daniel AB, Shah H, Kamath A, Guddettu V, Joseph B. Environmental tobacco and wood smoke increase the risk of Legg-Calvé-Perthes disease. Clin Orthop Relat Res. 2012;470:2369-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 14. | Gordon JE, Schoenecker PL, Osland JD, Dobbs MB, Szymanski DA, Luhmann SJ. Smoking and socio-economic status in the etiology and severity of Legg-Calvé-Perthes' disease. J Pediatr Orthop B. 2004;13:367-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | García Mata S, Ardanaz Aicua E, Hidalgo Ovejero A, Martinez Grande M. Legg-Calvé-Perthes disease and passive smoking. J Pediatr Orthop. 2000;20:326-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Glueck CJ, Freiberg RA, Crawford A, Gruppo R, Roy D, Tracy T, Sieve-Smith L, Wang P. Secondhand smoke, hypofibrinolysis, and Legg-Perthes disease. Clin Orthop Relat Res. 1998;159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Bahmanyar S, Montgomery SM, Weiss RJ, Ekbom A. Maternal smoking during pregnancy, other prenatal and perinatal factors, and the risk of Legg-Calvé-Perthes disease. Pediatrics. 2008;122:e459-e464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Sharma S, Sibinski M, Sherlock DA. A profile of Perthes' disease in Greater Glasgow: is there an association with deprivation? J Bone Joint Surg Br. 2005;87:1536-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 19. | Pillai A, Atiya S, Costigan PS. The incidence of Perthes' disease in Southwest Scotland. J Bone Joint Surg Br. 2005;87:1531-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Kealey WD, Moore AJ, Cook S, Cosgrove AP. Deprivation, urbanisation and Perthes' disease in Northern Ireland. J Bone Joint Surg Br. 2000;82:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 21. | Perry DC, Bruce CE, Pope D, Dangerfield P, Platt MJ, Hall AJ. Legg-Calvé-Perthes disease in the UK: geographic and temporal trends in incidence reflecting differences in degree of deprivation in childhood. Arthritis Rheum. 2012;64:1673-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Perry DC, Bruce CE, Pope D, Dangerfield P, Platt MJ, Hall AJ. Perthes' disease of the hip: socioeconomic inequalities and the urban environment. Arch Dis Child. 2012;97:1053-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Perry DC, Bruce CE, Pope D, Dangerfield P, Platt MJ, Hall AJ. Perthes' disease: deprivation and decline. Arch Dis Child. 2011;96:1124-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Hall AJ, Barker DJ. Perthes' disease in yorkshire. J Bone Joint Surg Br. 1989;71:229-233. [PubMed] |
| 25. | Hall AJ, Barker DJ, Dangerfield PH, Taylor JF. Perthes' disease of the hip in Liverpool. Br Med J (Clin Res Ed). 1983;287:1757-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Margetts BM, Perry CA, Taylor JF, Dangerfield PH. The incidence and distribution of Legg-Calvé-Perthes' disease in Liverpool, 1982-95. Arch Dis Child. 2001;84:351-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Metcalfe D, Van Dijck S, Parsons N, Christensen K, Perry DC. A Twin Study of Perthes Disease. Pediatrics. 2016;137:e20153542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Lappin K, Kealey D, Cosgrove A, Graham K. Does low birthweight predispose to Perthes' disease? Perthes' disease in twins. J Pediatr Orthop B. 2003;12:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 29. | Wiig O, Terjesen T, Svenningsen S, Lie SA. The epidemiology and aetiology of Perthes' disease in Norway. A nationwide study of 425 patients. J Bone Joint Surg Br. 2006;88:1217-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Loder RT, Schwartz EM, Hensinger RN. Behavioral characteristics of children with Legg-Calvé-Perthes disease. J Pediatr Orthop. 1993;13:598-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Perry DC, Bruce CE, Pope D, Dangerfield P, Platt MJ, Hall AJ. Comorbidities in Perthes' disease: a case control study using the General Practice Research database. J Bone Joint Surg Br. 2012;94:1684-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Perry DC, Pope D, Bruce CE, Dangerfield P, Hall AJ, Platt MJ. Hyperactivity and the psychological burden of Perthes disease: a case-control study. J Pediatr Orthop. 2013;33:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Podeszwa DA, Richard HM, Nguyen DC, De La Rocha A, Shapiro EL. Preoperative psychological findings in adolescents undergoing hip preservation surgery. J Pediatr Orthop. 2015;35:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Berman J, Aran A, Berenstein-Weyel T, Lebel E. Exploring the Association between Legg-Calvé-Perthes Disease and Attention Deficit Hyperactivity Disorder in Children. Isr Med Assoc J. 2016;18:652-654. [PubMed] |
| 35. | Hailer YD, Montgomery S, Ekbom A, Nilsson O, Bahmanyar S. Legg-Calvé-Perthes disease and the risk of injuries requiring hospitalization: a register study involving 2579 patients. Acta Orthop. 2012;83:572-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Hailer YD, Nilsson O. Legg-Calvé-Perthes disease and the risk of ADHD, depression, and mortality. Acta Orthop. 2014;85:501-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Türkmen I, Poyanlı O, Unay K, Kemah B, Akan K, Ulusan S. Association between attention deficit and hyperactivity disorder and Perthes disease. Acta Orthop Traumatol Turc. 2014;48:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Neal DC, Alford TH, Moualeu A, Jo CH, Herring JA, Kim HK. Prevalence of Obesity in Patients With Legg-Calvé-Perthes Disease. J Am Acad Orthop Surg. 2016;24:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Lee JH, Zhou L, Kwon KS, Lee D, Park BH, Kim JR. Role of leptin in Legg-Calvé-Perthes disease. J Orthop Res. 2013;31:1605-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | O'Sullivan M, O'Rourke SK, MacAuley P. Legg-Calvé-Perthes disease in a family: genetic or environmental. Clin Orthop Relat Res. 1985;179-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Livesey JP, Hay SM, Bell MJ. Perthes disease affecting three female first-degree relatives. J Pediatr Orthop B. 1998;7:230-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Miyamoto Y, Matsuda T, Kitoh H, Haga N, Ohashi H, Nishimura G, Ikegawa S. A recurrent mutation in type II collagen gene causes Legg-Calvé-Perthes disease in a Japanese family. Hum Genet. 2007;121:625-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Al-Omran AK, Sadat-Ali M. Legg-Calve-Perthes disease in two generations of male family members: a case report. J Orthop Surg (Hong Kong). 2013;21:258-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Kannu P, Irving M, Aftimos S, Savarirayan R. Two novel COL2A1 mutations associated with a Legg-Calvé-Perthes disease-like presentation. Clin Orthop Relat Res. 2011;469:1785-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Su P, Li R, Liu S, Zhou Y, Wang X, Patil N, Mow CS, Mason JC, Huang D, Wang Y. Age at onset-dependent presentations of premature hip osteoarthritis, avascular necrosis of the femoral head, or Legg-Calvé-Perthes disease in a single family, consequent upon a p.Gly1170Ser mutation of COL2A1. Arthritis Rheum. 2008;58:1701-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Li N, Yu J, Cao X, Wu QY, Li WW, Li TF, Zhang C, Cui YX, Li XJ, Yin ZM, Xia XY. A novel p. Gly630Ser mutation of COL2A1 in a Chinese family with presentations of Legg-Calvé-Perthes disease or avascular necrosis of the femoral head. PLoS One. 2014;9:e100505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Woratanarat P, Thaveeratitharm C, Woratanarat T, Angsanuntsukh C, Attia J, Thakkinstian A. Meta-analysis of hypercoagulability genetic polymorphisms in Perthes disease. J Orthop Res. 2014;32:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Srzentić S, Nikčević G, Spasovski D, Baščarević Z, Živković Z, Terzic-Šupić Z, Matanović D, Djordjević V, Pavlović S, Spasovski V. Predictive genetic markers of coagulation, inflammation and apoptosis in Perthes disease—Serbian experience. Eur J Pediatr. 2015;174:1085-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Suehiro M, Hirano T, Shindo H. Osteonecrosis induced by standing in growing Wistar Kyoto rats. J Orthop Sci. 2005;10:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Liu R, Fan L, Yin L, Wang K, Miao W, Song Q, Dang X, Gao H, Bai C. Comparative study of serum proteomes in Legg-Calve-Perthes disease. BMC Musculoskelet Disord. 2015;16:281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Srzentić S, Spasovski V, Spasovski D, Zivković Z, Matanović D, Bascarević Z, Supić ZT, Stojiljković M, Karan-Djurasević T, Stanković B, Pavlović S, Nikcević G, Vukasinović Z. Association of gene variants in TLR4 and IL-6 genes with Perthes disease. Srp Arh Celok Lek. 2014;142:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Kamiya N, Yamaguchi R, Adapala NS, Chen E, Neal D, Jack O, Thoveson A, Gudmundsson P, Brabham C, Aruwajoye O, Drissi H, Kim HK. Legg-Calvé-Perthes disease produces chronic hip synovitis and elevation of interleukin-6 in the synovial fluid. J Bone Miner Res. 2015;30:1009-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 53. | Catterall A, Pringle J, Byers PD, Fulford GE, Kemp HB, Dolman CL, Bell HM, McKibbin B, Rális Z, Jensen OM, Lauritzen J, Ponseti IV, Ogden J. A review of the morphology of Perthes' disease. J Bone Joint Surg Br. 1982;64:269-275. [PubMed] |
| 54. | Jonsater S. Coxa plana; a histo-pathologic and arthrografic study. Acta Orthop Scand Suppl. 1953;12:5-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Su P, Zhang L, Peng Y, Liang A, Du K, Huang D. A histological and ultrastructural study of femoral head cartilage in a new type II collagenopathy. Int Orthop. 2010;34:1333-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Calver R, Venugopal V, Dorgan J, Bentley G, Gimlette T. Radionuclide scanning in the early diagnosis of Perthes' disease. J Bone Joint Surg Br. 1981;63-B:379-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Catterall A, Pringle J, Byers PD, Fulford GE, Kemp HB. Perthes' disease: is the epiphysial infarction complete? J Bone Joint Surg Br. 1982;64:276-281. [PubMed] |
| 58. | Ponseti IV, Maynard JA, Weinstein SL, Ippolito EG, Pous JG. Legg-Calvé-Perthes disease. Histochemical and ultrastructural observations of the epiphyseal cartilage and physis. J Bone Joint Surg Am. 1983;65:797-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 41] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Gershuni DH, Hargens AR, Lee YF, Greenberg EN, Zapf R, Akeson WH. The questionable significance of hip joint tamponade in producing osteonecrosis in Legg-Calvé-Perthes syndrome. J Pediatr Orthop. 1983;3:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 60. | Royle SG, Galasko CS. The irritable hip. Scintigraphy in 192 children. Acta Orthop Scand. 1992;63:25-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Lamer S, Dorgeret S, Khairouni A, Mazda K, Brillet PY, Bacheville E, Bloch J, Penneçot GF, Hassan M, Sebag GH. Femoral head vascularisation in Legg-Calvé-Perthes disease: comparison of dynamic gadolinium-enhanced subtraction MRI with bone scintigraphy. Pediatr Radiol. 2002;32:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Atsumi T, Yamano K, Muraki M, Yoshihara S, Kajihara T. The blood supply of the lateral epiphyseal arteries in Perthes' disease. J Bone Joint Surg Br. 2000;82:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | de Camargo FP, de Godoy RM, Tovo R. Angiography in Perthes' disease. Clin Orthop Relat Res. 1984;216-220. [PubMed] |
| 64. | Théron J. Angiography in Legg-Calvé-Perthes disease. Radiology. 1980;135:81-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 65. | Pinheiro M, Dobson CA, Perry D, Fagan MJ. New insights into the biomechanics of Legg-Calvé-Perthes' disease: The Role of Epiphyseal Skeletal Immaturity in Vascular Obstruction. Bone Joint Res. 2018;7:148-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 66. | Cheon JE, Yoo WJ, Kim IO, Kim WS, Choi YH. Effect of arterial deprivation on growing femoral epiphysis: quantitative magnetic resonance imaging using a piglet model. Korean J Radiol. 2015;16:617-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 67. | Li X, Hu J, Zhen H, Tang L, Xu A. Early reversible ischemia of femoral head epiphysis in piglets on gadolinium-enhanced MRI: an experimental study. J Huazhong Univ Sci Technolog Med Sci. 2006;26:482-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 68. | Babyn PS, Kim HK, Gahunia HK, Lemaire C, Salter RB, Fornasier V, Pritzker KP. MRI of the cartilaginous epiphysis of the femoral head in the piglet hip after ischemic damage. J Magn Reson Imaging. 1998;8:717-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 69. | Li X, Qi J, Xia L, Li H, Hu J, Yu C, Pen W, Guan J, Hu D. Diffusion MRI in ischemic epiphysis of the femoral head: an experimental study. J Magn Reson Imaging. 2008;28:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Levin D, Norman D, Zinman C, Misselevich I, Reis DN, Boss JH. Osteoarthritis-like disorder in rats with vascular deprivation-induced necrosis of the femoral head. Pathol Res Pract. 1999;195:637-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 71. | Kandzierski G. Remarks on the etiology and pathogenesis of Perthes' disease: an experiment-based hypothesis. Ortop Traumatol Rehabil. 2004;6:553-560. [PubMed] |
| 72. | Suehiro M, Hirano T, Mihara K, Shindo H. Etiologic factors in femoral head osteonecrosis in growing rats. J Orthop Sci. 2000;5:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 73. | Vaverka M, Návrat TS, Vrbka M, Florian Z, Fuis V. Stress and strain analysis of the hip joint using FEM. Technol Health Care. 2006;14:271-279. [PubMed] |
| 74. | Naito M, Schoenecker PL, Owen JH, Sugioka Y. Acute effect of traction, compression, and hip joint tamponade on blood flow of the femoral head: an experimental model. J Orthop Res. 1992;10:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 75. | Trueta J. The normal vascular anatomy of the human femoral head during growth. J Bone Joint Surg Br. 1957;39-B:358-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 76. | Perry DC, Green DJ, Bruce CE, Pope D, Dangerfield P, Platt MJ, Hall AJ, Jones H. Abnormalities of vascular structure and function in children with Perthes disease. Pediatrics. 2012;130:e126-e131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Kim HK, Kaste S, Dempsey M, Wilkes D. A comparison of non-contrast and contrast-enhanced MRI in the initial stage of Legg-Calvé-Perthes disease. Pediatr Radiol. 2013;43:1166-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 78. | Kitoh H, Kitakoji T, Katoh M, Takamine Y. Delayed ossification of the proximal capital femoral epiphysis in Legg-Calvé-Perthes' disease. J Bone Joint Surg Br. 2003;85:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 79. | Kocjančič B, Moličnik A, Antolič V, Mavčič B, Kralj-Iglič V, Vengust R. Unfavorable hip stress distribution after Legg-Calvé-Perthes syndrome: a 25-year follow-up of 135 hips. J Orthop Res. 2014;32:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 80. | Pinheiro MDS, Dobson C, Clarke NM, Fagan M. The potential role of variations in juvenile hip geometry on the development of Legg-Calvé-Perthes disease: a biomechanical investigation. Comput Methods Biomech Biomed Engin. 2018;21:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 81. | Zhang W, Yuan Z, Pei X, Ma R. In vivo and in vitro characteristic of HIF-1α and relative genes in ischemic femoral head necrosis. Int J Clin Exp Pathol. 2015;8:7210-7216. [PubMed] |
| 82. | Kim HK, Bian H, Aya-ay J, Garces A, Morgan EF, Gilbert SR. Hypoxia and HIF-1alpha expression in the epiphyseal cartilage following ischemic injury to the immature femoral head. Bone. 2009;45:280-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 83. | Neidel J, Zander D, Hackenbroch MH. Low plasma levels of insulin-like growth factor I in Perthes' disease. A controlled study of 59 consecutive children. Acta Orthop Scand. 1992;63:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 84. | Matsumoto T, Enomoto H, Takahashi K, Motokawa S. Decreased levels of IGF binding protein-3 in serum from children with Perthes' disease. Acta Orthop Scand. 1998;69:125-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 85. | Grasemann H, Nicolai RD, Hauffa BP, Reinhardt W, Nicolai H, Hövel M. Skeletal immaturity, IGF-I and IGFBP-3 serum concentrations in Legg-Calvé-Perthes disease (skeletal immaturity, IGF-I and IGFBP-3 in LCPD). Klin Padiatr. 1996;208:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 86. | Neidel J, Schönau E, Zander D, Rütt J, Hackenbroch MH. Normal plasma levels of IGF binding protein in Perthes' disease. Follow-up of previous report. Acta Orthop Scand. 1993;64:540-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 87. | Mullan CJ, Thompson LJ, Cosgrove AP. The Declining Incidence of Legg-Calve-Perthes' Disease in Northern Ireland: An Epidemiological Study. J Pediatr Orthop. 2017;37:e178-e182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 88. | Vielwerth SE, Jensen RB, Larsen T, Greisen G. The impact of maternal smoking on fetal and infant growth. Early Hum Dev. 2007;83:491-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 89. | Jaddoe VW, Verburg BO, de Ridder MA, Hofman A, Mackenbach JP, Moll HA, Steegers EA, Witteman JC. Maternal smoking and fetal growth characteristics in different periods of pregnancy: the generation R study. Am J Epidemiol. 2007;165:1207-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 90. | Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3710] [Cited by in RCA: 3658] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 91. | Chen XX, Yang T. Roles of leptin in bone metabolism and bone diseases. J Bone Miner Metab. 2015;33:474-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 92. | Garonna E, Botham KM, Birdsey GM, Randi AM, Gonzalez-Perez RR, Wheeler-Jones CP. Vascular endothelial growth factor receptor-2 couples cyclo-oxygenase-2 with pro-angiogenic actions of leptin on human endothelial cells. PLoS One. 2011;6:e18823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 93. | Gimble JM. Leptin's balancing act between bone and fat. J Bone Miner Res. 2011;26:1694-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 94. | Zhou L, Jang KY, Moon YJ, Wagle S, Kim KM, Lee KB, Park BH, Kim JR. Leptin ameliorates ischemic necrosis of the femoral head in rats with obesity induced by a high-fat diet. Sci Rep. 2015;5:9397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 95. | Ma HZ, Zeng BF, Li XL. Upregulation of VEGF in subchondral bone of necrotic femoral heads in rabbits with use of extracorporeal shock waves. Calcif Tissue Int. 2007;81:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 96. | Ruiz M, Pettaway C, Song R, Stoeltzing O, Ellis L, Bar-Eli M. Activator protein 2alpha inhibits tumorigenicity and represses vascular endothelial growth factor transcription in prostate cancer cells. Cancer Res. 2004;64:631-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 97. | Catterall A. The natural history of Perthes' disease. J Bone Joint Surg Br. 1971;53:37-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 284] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 98. | Guille JT, Lipton GE, Szöke G, Bowen JR, Harcke HT, Glutting JJ. Legg-Calvé-Perthes disease in girls. A comparison of the results with those seen in boys. J Bone Joint Surg Am. 1998;80:1256-1263. [PubMed] |
| 99. | Georgiadis AG, Seeley MA, Yellin JL, Sankar WN. The presentation of Legg-Calvé-Perthes disease in females. J Child Orthop. 2015;9:243-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 100. | Zheng P, Yang T, Ju L, Jiang B, Lou Y. Epigenetics in Legg-Calvé-Perthes disease: A study of global DNA methylation. J Int Med Res. 2015;43:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 101. | Kenet G, Ezra E, Wientroub S, Steinberg DM, Rosenberg N, Waldman D, Hayek S. Perthes' disease and the search for genetic associations: collagen mutations, Gaucher's disease and thrombophilia. J Bone Joint Surg Br. 2008;90:1507-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 102. | Pouya F, Kerachian MA. Avascular Necrosis of the Femoral Head: Are Any Genes Involved? Arch Bone Jt Surg. 2015;3:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 103. | Baltzer HL, Riester S, Moran SL. Bilateral Legg-Calve-Perthes Disease and Kienbock's Disease in a Child With Factor V Leiden Thrombophilia: A Case Report. Hand (N Y). 2016;11:NP16-NP19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1881] [Cited by in RCA: 3214] [Article Influence: 292.2] [Reference Citation Analysis (0)] |
| 105. | Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369-2380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1942] [Cited by in RCA: 2189] [Article Influence: 104.2] [Reference Citation Analysis (0)] |