Published online Feb 18, 2019. doi: 10.5312/wjo.v10.i2.63
Peer-review started: October 7, 2018
First decision: October 19, 2018
Revised: December 26, 2018
Accepted: January 5, 2019
Article in press: January 6, 2019
Published online: February 18, 2019
Processing time: 135 Days and 17.8 Hours
Total knee arthroplasty is a common procedure, with extremely good clinical results. Despite this success, it produces 20% unsatisfactory results. Among the causes of these failures is metal hypersensitivity. Metal sensitization is higher in patients with a knee arthroplasty than in the general population and is even higher in patients undergoing revision surgery. However, a clear correlation between metal sensitization and symptomatic knee after surgery has not been ascertained. Surely, patients with a clear history of metal allergy must be carefully examined through dermatological and laboratory testing before surgery. There is no globally accepted diagnostic algorithm or laboratory test to diagnose metal hypersensitivity or metal reactions. The patch test is the most common test to determine metal hypersensitivity, though presenting some limitations. Several laboratory assays have been developed, with a higher sensitivity compared to patch testing, yet their clinical availability is not widespread, due to high costs and technical complexity. Symptoms of a reaction to metal implants present across a wide spectrum, ranging from pain and cutaneous dermatitis to aseptic loosening of the arthroplasty. However, although cutaneous and systemic hypersensitivity reactions to metals have arisen, thereby increasing concern after joint arthroplasties, allergies against implant materials remain quite rare and not a well-known problem. The aim of the following paper is to provide an overview on diagnosis and management of metal hypersensitivity in patients who undergo a total knee arthroplasty in order clarify its real importance.
Core tip: Metal hypersensitivity may be a cause of failure for total knee arthroplasty, although a clear correlation between metal sensitization and symptomatic knee after surgery has not been ascertained. Patients with a clear history of metal allergy, must be carefully examined through dermatological and laboratory testing before surgery. However, despite the increase in cutaneous and systemic hypersensitivity reactions to metals, which raise concern about joint arthroplasties, allergies against implant materials remain quite rare and an unexplored issue.
- Citation: Saccomanno MF, Sircana G, Masci G, Cazzato G, Florio M, Capasso L, Passiatore M, Autore G, Maccauro G, Pola E. Allergy in total knee replacement surgery: Is it a real problem? World J Orthop 2019; 10(2): 63-70
- URL: https://www.wjgnet.com/2218-5836/full/v10/i2/63.htm
- DOI: https://dx.doi.org/10.5312/wjo.v10.i2.63
Osteoarthritis is a degenerative disease affecting an important part of the population aged 65 or older, with a reported prevalence higher than 30% in the United States[1]. Its incidence increases with age and overweight status[2]. Its aetiology has not been completely clarified, but likely entails a multifactorial interplay of mechanical and biological causes. Various molecular mediators involved in the development of osteoarthritis have been identified[3-4], although their clinical relevance has yet to be demonstrated. Surgical therapy is required in cases of advanced osteoarthritis.
Total knee arthroplasty (TKA) is a common procedure performed in increasing numbers worldwide[5]. Despite its great success, up to 20% of patients still complain of unsatisfactory results[6]. Although several causes, such as infection or malalignment, have been shown to be most commonly responsible for dissatisfaction and poor outcomes, in the last decades another possible explanation has gained popularity: implant-related metal (or less frequently cement) hypersensitivity. In other words, an immunological reaction to the metallic portion of TKA components or to the bone cement[7,8]. The hypothesis is supported by studies showing a higher prevalence of positive patch tests after TKA implantation[9-11]. However, even if local and systemic exposure to metals deriving from the implanted device can cause sensitization, the positivity of a metal test cannot be held as a proof of symptom causality[12]. As a matter of fact, it has also been shown that many patients who underwent TKA did not develop any complication after surgery, despite being sensitive to metal[13].
Looking at the epidemiologic data, 10% to 15% of the general population present dermatologic symptoms caused by metal hypersensitivity. Nickel is responsible in the majority of cases, while cobalt, chromium, beryllium and less frequently tantalum, titanium and vanadium are responsible for dermal symptomatology in a smaller number of cases[14]. However, taking into consideration patients undergoing TKA revision, it has been reported that less than 2% showed “metal related pathologies”[15]. Bone cement hypersensitivity is even more rare[16]. Therefore, it appears quite clear that the role of metal or cement allergies in TKA failures remains a controversial issue. The aim of the following paper is to provide an overview on diagnosis and management of metal hypersensitivity in patients who undergo a TKA in order clarify its real importance.
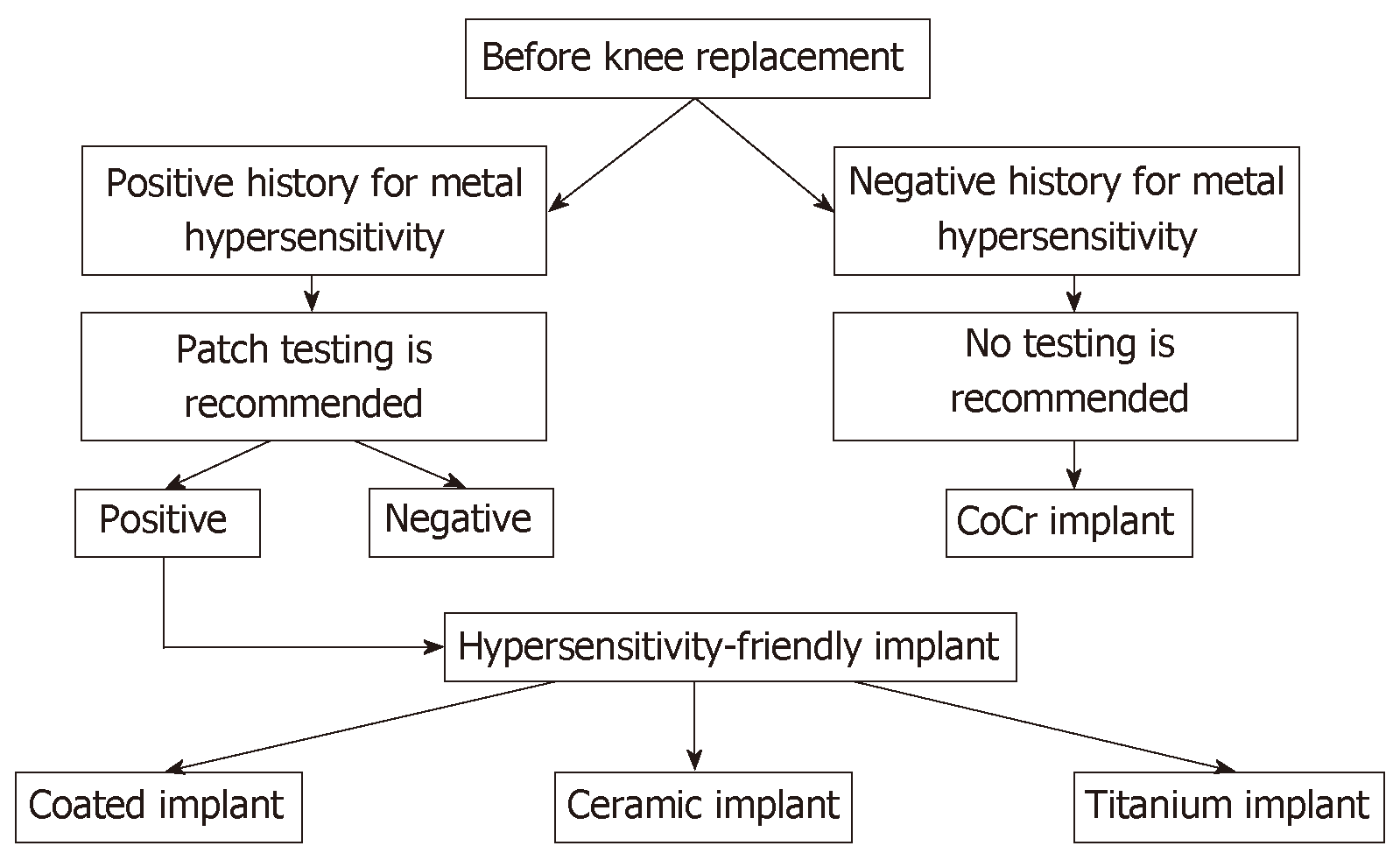
Patient-reported allergies and history (family history, exposure, occupation, and self-reported allergies) are of utmost importance as a first diagnostic step. Although there are no guidelines, routine preoperative evaluation in patients reporting no history of adverse cutaneous reactions to metals or history of adverse events related to previous implant of metallic devices is not necessary and therefore not recommended. Moreover, use of conventional cobalt-chromium implants is also allowed without additional preoperative investigation, even in patients reporting only mild cutaneous reactions. An opposite consideration is that it is mandatory for patients reporting substantial cutaneous or systemic reactions to undergo patch testing before TKA[9,17] (Figure 1).
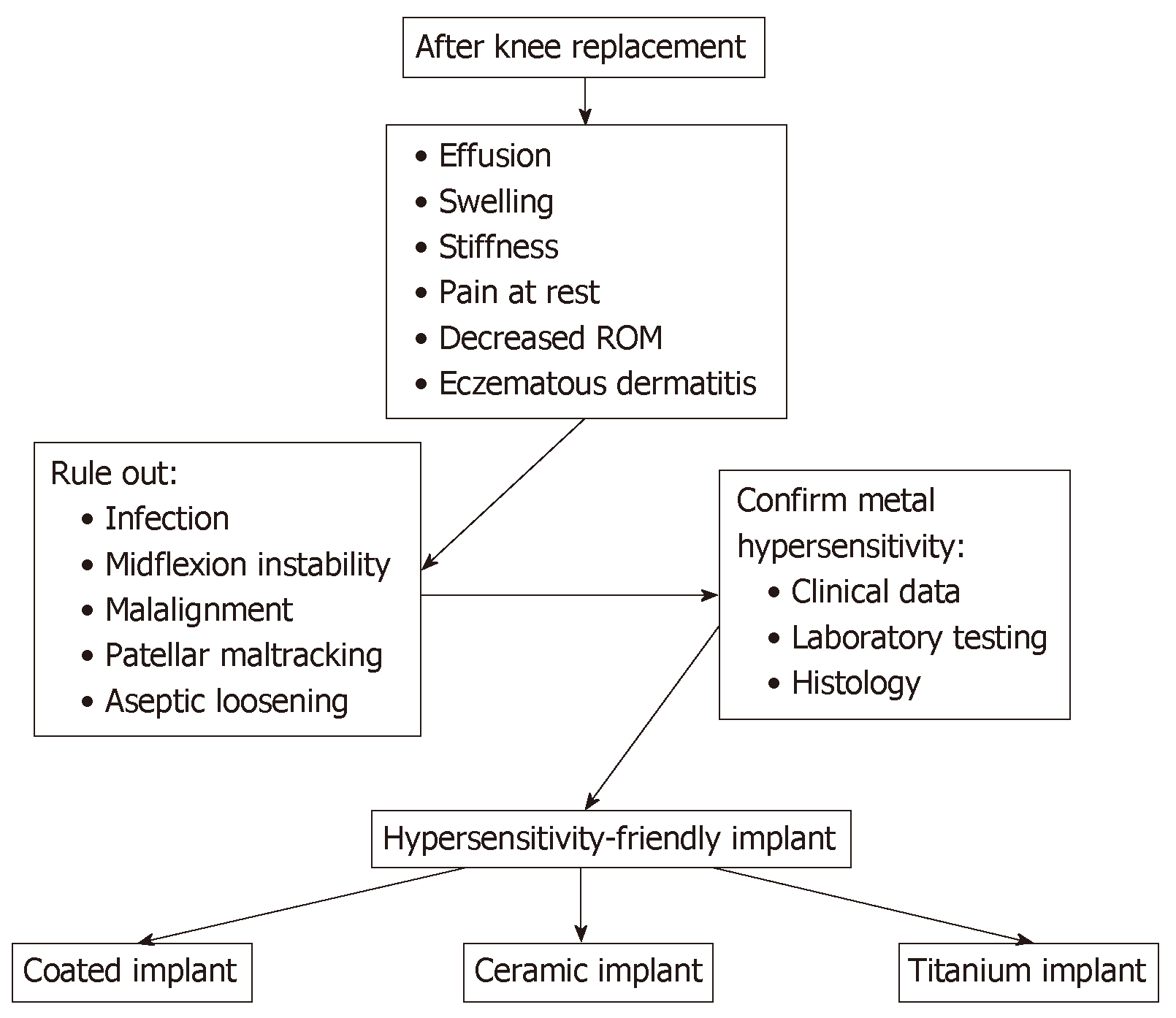
After TKA implantation, clinical presentation of metal hypersensitivity is unspecific and symptoms are common to other complications. Metal hypersensitivity is a very rare condition and is usually a diagnosis of exclusion. The most common symptoms are joint effusion, swelling, stiffness, persistent pain at rest and decreased range of motion; less frequently, it is characterized by eczematous dermatitis, which can be local or generalized, extended to the neck, buttock and extremities[18]. Rarely, a general complication may occur, such as rhinitis, itching or asthma, hair loss and alopecia. The time range of first symptoms is variable, from 4 wk to 2 yr[19].
As a matter of fact, residual pain after TKA has many causes that need to be ruled out before a metal hypersensitivity is taken into consideration (Figure 2). An infection must be first ruled out by blood tests (erythrocyte sedimentation rate and C-reactive protein) and joint synovial fluid aspirations. Moreover, other common causes of pain, such as midflexion instability, component malalignment with patellar maltracking, crepitation and patellar clunk, can be ruled out by physical examination. Less common causes are early aseptic loosening and avascular necrosis of the patella[20]. Surely, a metal hypersensitivity should be suspected in patients with a clear self-reported history of metal reactions.
Radiographic images can show osteolytic lesions in the proximity of the femoral and tibial components, which formed as a result of the inflammatory response and can lead to aseptic loosening of the implant, loss of tibial posterior slope and setting of the tibial base plate into varus, as compared to the previous images taken after surgery[21]. The diagnostic algorithm is based on metal hypersensitivity aetiology. The immunological reactive mechanism to metallic components is still an unclear and debated issue. Metal hypersensitivity is generally a type IV allergic reaction, meaning that immune response acts through a delayed cell-mediated response, with activation of specific CD4+ T lymphocytes, macrophages, dendritic cells and other immune cells found within the synovial tissue. This response produces tissue inflammation and periprosthetic tissue damage, powered by the incretion of cytokines involved in the pro-inflammatory pathway, including interleukin (IL)-1, IL-12, IL-6, tumour necrosis factor and interferon-gamma. In particular, it must be highlighted that this mechanism of metal hypersensitivity is different from those that characterize lymphocyte-dominated non-septic vasculitis-associated lesions and pseudotumours that are reported as adverse local tissue reactions after metal-on-metal total hip arthtroplasty[18].
No generally accepted and reliable tests are available for the clinical diagnosis of metal hypersensitivity after TKA[22]. Patch test is the most frequent method used to diagnose contact allergy to metals and is, up to now, considered the gold standard. However, its validity faces a lot of controversy, and its sensitivity and specificity are 77% and 71%, respectively[18]. It is an in vivo test, widely available and easy to apply, so it can provide results within a few days. The hypothesis that cutaneous and systemic hypersensitivities are strictly related to the presence of metal after TKA is supported by a series of studies that found an important prevalence of positive patch tests after implantation of metallic TKA components. As already highlighted, the most common sensitizations to metal are with nickel, chromium and cobalt[19]. Patch test can be performed not only for metals but also for cement components, by using adhesive patches loaded with a known concentration of specific allergens compared with vaseline. However, patch tests also present some important limitations, namely skin reactions that are different compared with deep tissue layers and the joint environment, with the potential of an antigen-presenting mechanism being altered thereby. Patch tests also have different preparation and plots which can differ from subject to subject and tester to tester[23]. According to the published guidelines of the German Contact Allergy Group, patch testing should be exclusively regarded as a mean to “verify or exclude metal allergy in patients with a corresponding history”[24]. The American Contact Dermatitis Society has defined criteria for the diagnosis of post-implantation metal hypersensitivity contact dermatitis[25]. In particular, they proposed four major and six minor criteria, reported here in Table 1.
| Major criteria |
| Eruption overlying the metal implant |
| Positive patch test reaction to a metal used in the implant |
| Complete recovery after removal of the offending implant |
| Chronic dermatitis beginning weeks to months after metallic implantation |
| Minor criteria |
| Unexplained pain and/or failure of the offending implant |
| Dermatitis reaction is resistant to therapy |
| Morphology consistent with dermatitis (erythema, induration, papules, vesicles) |
| Systemic allergic dermatitis reaction |
| Histology consistent with allergic contact dermatitis |
| Positive in vitro test to metals (e.g., lymphocytes transformation test) |
Several laboratory assays have been proposed over the years. Lymphocyte transformation test is an in vitro test which analyses the proliferation of lymphocytes obtained by peripheral blood draw after contact with a metallic implant. It compares peripheral blood lymphocyte proliferation upon a 7 d incubation period, with and without the addition of metal antigen. Lymphocyte transformation test sensitivity is higher than patch testing, and provides quantifiable data and is very reproducible. Despite these positive aspects, this test is poorly available and there are a limited number of allergens that can be tested[19].
Even if many cytokines may be overexpressed in other conditions and diseases, enzyme-linked immunosorbent assay (commonly known as ELISA) testing can be used for measurement of cytokines released by stimulated cells.
Other in vitro tests are the Modified Lymphocyte Stimulation Test, the Lymphocyte Activation Test and the Leukocyte Migration Inhibition Test. The Modified Lymphocyte Stimulation Test has been described as a reliable test to diagnose systemic metal hypersensitivity, but it is currently impossible to use in large-scale settings because of its costs and limited availability[26]. The Lymphocyte Activation Test quantifies the expression of specific receptors (CD69) on circulating mononuclear cells after stimulation with metals. The Leukocyte Migration Inhibition Test measures the speed of migration of leukocytes after contact with sensitizing allergens. Another technique, even if not yet disseminated, is confocal microscopy, which can demonstrate intracellular abnormalities of the stimulated cells after contact with metals by 3D images obtained by computer tomography[23].
If the implant has to be removed, intra-operative biopsies and a consequential histopathological work-up can be used to confirm implant-related hypersensitivity. In those cases, periprosthetic membranes are characterized by a pronounced lymphocytes’ infiltration[19]. At the histologic analysis of the synovial membrane, the characteristic pattern is granulation tissue and fibrosis, along with numerous giant cells and calcification. In support of the hypothesis that a chronic inflammatory response is the cause of synovitis, lymphocytic and plasma cell infiltrates in the surrounding synovial tissue have also been reported[18].
Management of patients with a suspicion of metal hypersensitivity is still not well defined. Once again, several authors have reported that patients with an ascertained diagnosis of metal allergy who underwent knee replacement with a metal-containing prosthetic device present no clinical evidence of metal hypersensitivity[27], and that no correlation between metal hypersensitivity and complications connected with the prosthesis could be found[28]. Therefore, although implant removal is surely a definitive solution, it must be considered very carefully, as a last option.
Good results have been reported after short-term therapy including topical steroids in the treatment of cutaneous dermatitis and non-steroidal anti-inflammatory drugs or physical therapy to manage pain caused by synovitis[29,30]. By the end, if the symptoms do not resolve, one-stage revision surgery with a hypoallergenic implant should be considered. Resolution of symptoms after revision surgery is expected in 2-3 mo after surgery.
When a metal hypersensitivity has been diagnosed, surgeons need safe implants; many “hypersensitivity-friendly” knee arthroplasty components are currently available from various manufacturers. They can be divided in two categories: Coated implants and non-allergenic implants[23,31].
Cobalt chrome (CoCr) implants coated with a hypersensitivity-friendly thin layer provide the advantage of retaining part of the superior tribological properties of CoCr; yet, the hypersensitivity-friendly layer, after scratching or wear can become damaged, exposing the underlying allergenic alloy[31]. Non-allergenic implants are made of non-CoCr alloys; while reducing the risk of exposure to allergenic metals, they usually show inferior physical properties compared to CoCr alloys[31]. They can be made of oxidized zirconium, pure titanium or ceramic femoral components associated with a tibial tray made of titanium or polyethylene[23].
The aim of the development of the Titanium Nitride (TiN) coating of knee implants was to improve their biocompatibility and mechanical properties[32]. TiN is applied as a 3–4 μm layer on CoCr implants[32]. In biomechanical studies, it has been shown to provide a 98% reduction of polyethylene wear[33]. Specifically, TiN showed a high resistance to adhesive wear and less adhesion to polyethylene; in addition, while CoCr catalyses polyethylene degradation, TiN is inert[34]. Sealing the CoCr surface, TiN reduces the cobalt and chromium ions release[32,35], avoiding hypersensitivity reactions. Despite this theoretical advantage, in a clinical trial, no difference in metal sensitization and blood ion concentration has been found between coated and uncoated arthroplasties[26]. TiN-coated implants have shown good clinical and radiological results[36]. They were shown to have a 95.1% survival rate for any reason at 10 yr. When compared to a standard CoCr implant, they showed no difference in functional outcome, range of motion, revision rate and postoperative pain[37]. The titanium niobium nitride-coated implants showed similar results; comparison of the titanium niobium nitride implants with standard CrCo implants resulted in no statistical difference in clinical outcome scores, range of motion or radiographic evaluation at 1-yr follow-up[38].
Zirconium is a metal with physical properties resembling those of titanium. Its oxide, named zirconia, is a ceramic material. The coupled zirconium-oxidized zirconium has been used as a hybrid material to produce knee arthroplasty implants. It is composed of a core of solid metal, surrounded by a ceramic zirconium oxide (ZrOx) layer which cannot be considered as a coating but instead as the surface of the metal alloy[39]. This material couples the superficial wear characteristics of the ceramic zirconia and the strength of the internal metal. ZrOx causes less wear of the polyethylene than CoCr components and shows a better resistance to abrasion[40]. In an in vitro study, a reduction of 42% of polyethylene wear was demonstrated[41]. Containing no nickel, it is absolutely safe in metal-sensitive patients[37]. The ZrOx femoral component is usually coupled with a pure titanium tibial baseplate. A survival rate of 95%-98.7% has been reported at a 5-10 yr follow-up[42,43]. No radiographic failures have been found in short-term[44] or long-term[43] follow up. In a clinical study comparing the results of ZrOx and CoCr arthroplasties implanted in patients undergoing simultaneous bilateral knee replacement, 44% of the patients perceived their knees as equivalent at a 5-yr follow-up[45].
Ceramic implants, being bioinert, represent a further choice for patients with metal hypersensitivity. These materials have a load to scratch that is 5 times greater than that of ZrOx and 10 times greater than CoCr, resulting in less wear of the surface and, consequently, less third-body wear of the polyethylene[46]. Among ceramics, zirconia is especially suitable for development of implants because of its tensile stress resistance and the possibility to shape it with a thickness similar to that of CoCr components[47]. Good clinical and radiographic results have been reported with these implants[47-49]; at 2-yr and 5-yr follow-up, neither clinical or radiological outcome nor revision rate difference between the CoCr and ceramic implants could be found[48,49], and a survival rate of 97.4% at 10 yr and 94% at 15 yr has been reported[50,51].
All-polyethylene tibial implants should provide the advantages of a thinner bone resection, thicker polyethylene implant and absence of locking mechanisms[52]. In a meta-analysis, no increased revision rates were found at 5 yr and 10 yr, when compared to metal-backed tibial components. No difference could be found for clinical and functional outcomes as well[53]. Should the patient be allergic to a substance contained in the cement, cementless implants are available. Advantages of cementless fixation are preservation of bone stock, provision of a biological fixation of the implant to the bone and avoidance of cement and its wear particles[54]. At 10 yr, a survival rate of 98.9% has been found[55]. In a recent meta-analysis, no difference could be found in terms of implant survivorship, clinical outcomes, radiological outcomes and complications between cemented and cementless implants[54].
Metal hypersensitivity is a rare condition. Routine allergy testing or patch testing prior to TKA is not recommended, unless a clear history of local or systemic reactions has been reported. In cases of positive history and positive tests, a hypersensitivity-friendly implant should be considered. However, there is still a lack of evidence regarding correlation between metal hypersensitivity and implant-related complications. As a matter of fact, after TKA, one-stage revision surgery for metal or bone cement hypersensitivity should be considered only after ruling out most common causes of TKA failure and even after a short-term therapy aiming to solve cutaneous dermatitis and pain. In this paper, we report the up-to-date knowledge on metal hypersensitivity, suggesting how to make diagnosis of metal hypersensitivity, to treat the symptoms, and to avoid its presentation. Further studies are needed in order to reach a definitive conclusion on the role of metal ions in sensitization and development of implant-related metal hypersensitivity.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Anand A, Musumeci G, Papachristou G, Yu B S- Editor: Yan JP L- Editor: A E- Editor: Wu YXJ
| 1. | Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. 2006;20:3-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 765] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 2. | Jüni P, Hari R, Rutjes AW, Fischer R, Silletta MG, Reichenbach S, da Costa BR. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;CD005328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 189] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 3. | Giunta S, Castorina A, Marzagalli R, Szychlinska MA, Pichler K, Mobasheri A, Musumeci G. Ameliorative effects of PACAP against cartilage degeneration. Morphological, immunohistochemical and biochemical evidence from in vivo and in vitro models of rat osteoarthritis. Int J Mol Sci. 2015;16:5922-5944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Musumeci G, Castrogiovanni P, Trovato FM, Imbesi R, Giunta S, Szychlinska MA, Loreto C, Castorina S, Mobasheri A. Physical activity ameliorates cartilage degeneration in a rat model of aging: a study on lubricin expression. Scand J Med Sci Sports. 2015;25:e222-e230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Castorina S, Guglielmino C, Castrogiovanni P, Szychlinska MA, Ioppolo F, Massimino P, Leonardi P, Maci C, Iannuzzi M, Di Giunta A, Musumeci G. Clinical evidence of traditional vs fast track recovery methodologies after total arthroplasty for osteoarthritic knee treatment. A retrospective observational study. Muscles Ligaments Tendons J. 2018;7:504-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Pacheco KA. Allergy to Surgical Implants. J Allergy Clin Immunol Pract. 2015;3:683-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Bloemke AD, Clarke HD. Prevalence of self-reported metal allergy in patients undergoing primary total knee arthroplasty. J Knee Surg. 2015;28:243-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Razak A, Ebinesan AD, Charalambous CP. Metal Hypersensitivity in Patients with Conventional Orthopaedic Implants. JBJS Rev. 2014;2:pii: 01874474-201402000-00004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Atanaskova Mesinkovska N, Tellez A, Molina L, Honari G, Sood A, Barsoum W, Taylor JS. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch Dermatol. 2012;148:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Basko-Plluska JL, Thyssen JP, Schalock PC. Cutaneous and systemic hypersensitivity reactions to metallic implants. Dermatitis. 2011;22:65-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Schalock PC, Menné T, Johansen JD, Taylor JS, Maibach HI, Lidén C, Bruze M, Thyssen JP. Hypersensitivity reactions to metallic implants - diagnostic algorithm and suggested patch test series for clinical use. Contact Dermatitis. 2012;66:4-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Thomsen M, Rozak M, Thomas P. Pain in a chromium-allergic patient with total knee arthroplasty: disappearance of symptoms after revision with a special surface-coated TKA--a case report. Acta Orthop. 2011;82:386-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Middleton S, Toms A. Allergy in total knee arthroplasty: a review of the facts. Bone Joint J. 2016;98-B:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Frigerio E, Pigatto PD, Guzzi G, Altomare G. Metal sensitivity in patients with orthopaedic implants: a prospective study. Contact Dermatitis. 2011;64:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Australian Orthopaedic Association National Joint Replacement Registry. Annual Report. Adelaide: AOA 2014; . |
| 16. | Stathopoulos IP, Andrianopoulos N, Paschaloglou D, Tsarouchas I. Revision total knee arthroplasty due to bone cement and metal hypersensitivity. Arch Orthop Trauma Surg. 2017;137:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Granchi D, Cenni E, Giunti A, Baldini N. Metal hypersensitivity testing in patients undergoing joint replacement: a systematic review. J Bone Joint Surg Br. 2012;94:1126-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Akil S, Newman JM, Shah NV, Ahmed N, Deshmukh AJ, Maheshwari AV. Metal hypersensitivity in total hip and knee arthroplasty: Current concepts. J Clin Orthop Trauma. 2018;9:3-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 19. | Faschingbauer M, Renner L, Boettner F. Allergy in Total Knee Replacement. Does It Exist?: Review Article. HSS J. 2017;13:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Park CN, White PB, Meftah M, Ranawat AS, Ranawat CS. Diagnostic Algorithm for Residual Pain After Total Knee Arthroplasty. Orthopedics. 2016;39:e246-e252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Gupta R, Phan D, Schwarzkopf R. Total Knee Arthroplasty Failure Induced by Metal Hypersensitivity. Am J Case Rep. 2015;16:542-547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Lachiewicz PF, Watters TS, Jacobs JJ. Metal Hypersensitivity and Total Knee Arthroplasty. J Am Acad Orthop Surg. 2016;24:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Innocenti M, Vieri B, Melani T, Paoli T, Carulli C. Metal hypersensitivity after knee arthroplasty: fact or fiction? Acta Biomed. 2017;88:78-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Wawrzynski J, Gil JA, Goodman AD, Waryasz GR. Hypersensitivity to Orthopedic Implants: A Review of the Literature. Rheumatol Ther. 2017;4:45-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Schalock PC, Crawford G, Nedorost S, Scheinman PL, Atwater AR, Mowad C, Brod B, Ehrlich A, Watsky KL, Sasseville D, Silvestri D, Worobec SM, Elliott JF, Honari G, Powell DL, Taylor J, DeKoven J. Patch Testing for Evaluation of Hypersensitivity to Implanted Metal Devices: A Perspective From the American Contact Dermatitis Society. Dermatitis. 2016;27:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Lützner J, Hartmann A, Dinnebier G, Spornraft-Ragaller P, Hamann C, Kirschner S. Metal hypersensitivity and metal ion levels in patients with coated or uncoated total knee arthroplasty: a randomised controlled study. Int Orthop. 2013;37:1925-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Carlsson A, Möller H. Implantation of orthopaedic devices in patients with metal allergy. Acta Derm Venereol. 1989;69:62-66. [PubMed] |
| 28. | Webley M, Kates A, Snaith ML. Metal sensitivity in patients with a hinge arthroplasty of the knee. Ann Rheum Dis. 1978;37:373-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Pinson ML, Coop CA, Webb CN. Metal hypersensitivity in total joint arthroplasty. Ann Allergy Asthma Immunol. 2014;113:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Verma SB, Mody B, Gawkrodger DJ. Dermatitis on the knee following knee replacement: a minority of cases show contact allergy to chromate, cobalt or nickel but a causal association is unproven. Contact Dermatitis. 2006;54:228-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Ajwani SH, Charalambous CP. Availability of Total Knee Arthroplasty Implants for Metal Hypersensitivity Patients. Knee Surg Relat Res. 2016;28:312-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Wisbey A, Gregson PJ, Tuke M. Application of PVD TiN coating to Co-Cr-Mo based surgical implants. Biomaterials. 1987;8:477-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 45] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Pappas MJ, Makris G, Buechel FF. Titanium nitride ceramic film against polyethylene. A 48 million cycle wear test. Clin Orthop Relat Res. 1995;64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 34. | Galetz MC, Seiferth SH, Theile B, Glatzel U. Potential for adhesive wear in friction couples of UHMWPE running against oxidized zirconium, titanium nitride coatings, and cobalt-chromium alloys. J Biomed Mater Res B Appl Biomater. 2010;93:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Goldberg JR, Gilbert JL. The electrochemical and mechanical behavior of passivated and TiN/AlN-coated CoCrMo and Ti6Al4V alloys. Biomaterials. 2004;25:851-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Choi NY, In Y, Bae JH, Do JH, Chung SJ, Koh IJ. Are Midterm Patient-Reported Outcome Measures Between Rotating-Platform Mobile-Bearing Prosthesis and Medial-Pivot Prosthesis Different? A Minimum of 5-Year Follow-Up Study. J Arthroplasty. 2017;32:824-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | van Hove RP, Brohet RM, van Royen BJ, Nolte PA. No clinical benefit of titanium nitride coating in cementless mobile-bearing total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23:1833-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Thienpont E. Titanium niobium nitride knee implants are not inferior to chrome cobalt components for primary total knee arthroplasty. Arch Orthop Trauma Surg. 2015;135:1749-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Ries MD, Salehi A, Widding K, Hunter G. Polyethylene wear performance of oxidized zirconium and cobalt-chromium knee components under abrasive conditions. J Bone Joint Surg Am. 2002;84-A Suppl 2:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 40. | Patel AM, Spector M. Tribological evaluation of oxidized zirconium using an articular cartilage counterface: a novel material for potential use in hemiarthroplasty. Biomaterials. 1997;18:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Ezzet KA, Hermida JC, Colwell CW, D'Lima DD. Oxidized zirconium femoral components reduce polyethylene wear in a knee wear simulator. Clin Orthop Relat Res. 2004;120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Innocenti M, Civinini R, Carulli C, Matassi F, Villano M. The 5-year results of an oxidized zirconium femoral component for TKA. Clin Orthop Relat Res. 2010;468:1258-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Hofer JK, Ezzet KA. A minimum 5-year follow-up of an oxidized zirconium femoral prosthesis used for total knee arthroplasty. Knee. 2014;21:168-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Laskin RS. An oxidized Zr ceramic surfaced femoral component for total knee arthroplasty. Clin Orthop Relat Res. 2003;191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Hui C, Salmon L, Maeno S, Roe J, Walsh W, Pinczewski L. Five-year comparison of oxidized zirconium and cobalt-chromium femoral components in total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Solarino G, Piconi C, De Santis V, Piazzolla A, Moretti B. Ceramic Total Knee Arthroplasty: Ready to Go? Joints. 2017;5:224-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Bergschmidt P, Bader R, Ganzer D, Hauzeur C, Lohmann C, Rüther W, Tigani D, Rani N, Prats FL, Zorzi C, Madonna V, Rigotti S, Benazzo F, Rossi SM, Kundt G, Bloch HR, Mittelmeier W. Ceramic femoral components in total knee arthroplasty - two year follow-up results of an international prospective multi-centre study. Open Orthop J. 2012;6:172-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Bergschmidt P, Ellenrieder M, Bader R, Kluess D, Finze S, Schwemmer B, Mittelmeier W. Prospective comparative clinical study of ceramic and metallic femoral components for total knee arthroplasty over a five-year follow-up period. Knee. 2016;23:871-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Bergschmidt P, Bader R, Kluess D, Zietz C, Schwemmer B, Kundt G, Mittelmeier W. Total knee replacement system with a ceramic femoral component versus two traditional metallic designs: a prospective short-term study. J Orthop Surg (Hong Kong). 2013;21:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Nakamura S, Kobayashi M, Ito H, Nakamura K, Ueo T, Nakamura T. The Bi-Surface total knee arthroplasty: minimum 10-year follow-up study. Knee. 2010;17:274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Nakamura S, Ito H, Nakamura K, Kuriyama S, Furu M, Matsuda S. Long-Term Durability of Ceramic Tri-Condylar Knee Implants: A Minimum 15-Year Follow-Up. J Arthroplasty. 2017;32:1874-1879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Gioe TJ, Maheshwari AV. The all-polyethylene tibial component in primary total knee arthroplasty. J Bone Joint Surg Am. 2010;92:478-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Nouta KA, Verra WC, Pijls BG, Schoones JW, Nelissen RG. All-polyethylene tibial components are equal to metal-backed components: systematic review and meta-regression. Clin Orthop Relat Res. 2012;470:3549-3559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Zhou K, Yu H, Li J, Wang H, Zhou Z, Pei F. No difference in implant survivorship and clinical outcomes between full-cementless and full-cemented fixation in primary total knee arthroplasty: A systematic review and meta-analysis. Int J Surg. 2018;53:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 55. | Napier RJ, O'Neill C, O'Brien S, Doran E, Mockford B, Boldt J, Beverland DE. A prospective evaluation of a largely cementless total knee arthroplasty cohort without patellar resurfacing: 10-year outcomes and survivorship. BMC Musculoskelet Disord. 2018;19:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |