Copyright
©2014 Baishideng Publishing Group Inc.
World J Orthop. Jul 18, 2014; 5(3): 304-311
Published online Jul 18, 2014. doi: 10.5312/wjo.v5.i3.304
Published online Jul 18, 2014. doi: 10.5312/wjo.v5.i3.304
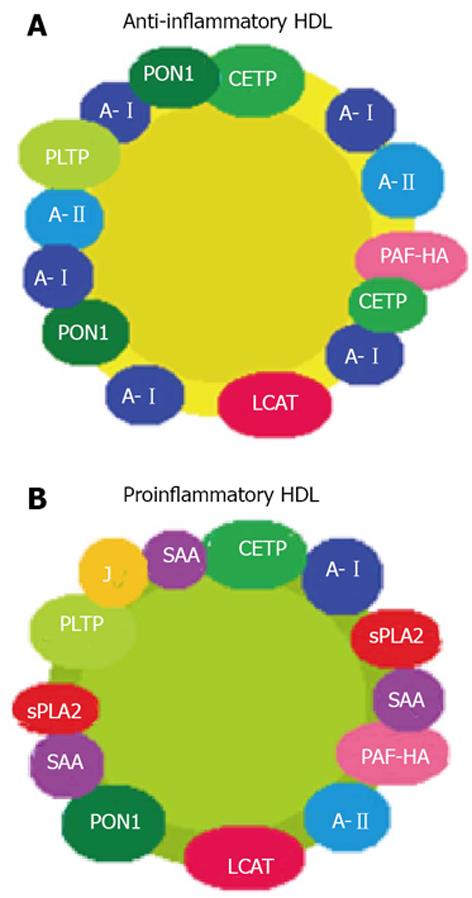
Figure 1 Structural change from normal protective anti-inflammatory high-density lipoproteins (A) to proinflammatory high-density lipoproteins (B) in the context of inflammation.
A-I: Apolipoprotein AI; A-II: Apolipoprotein AII; J: Apolipoprotein J; PON1: Paraoxonase 1; PLTP: Phospholipid transfer protein; CETP: Cholesteryl ester transfer protein; PAF-HA: Hydrolyzes platelet-activating factor; LCAT: Lecitin cholesterol acil transferasa; sPLA2: Pancreatic phospholipase A2; SAA: Serum amyloid protein A.
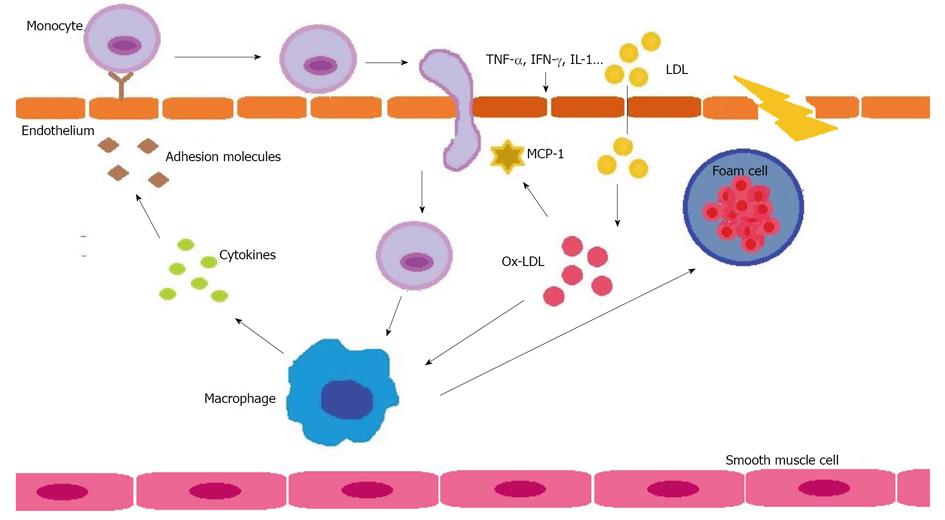
Figure 2 Oxidation of low-density lipoprotein in the context of inflammation to produce atherosclerosis (see text).
Ox-LDL: Oxidation of low-density lipoprotein; MCP-1: Monocyte chemotactic protein-1; TNF-α: Tumor necrosis factor α; IFN-γ: Interferon γ; IL-1: Interleukin-1.
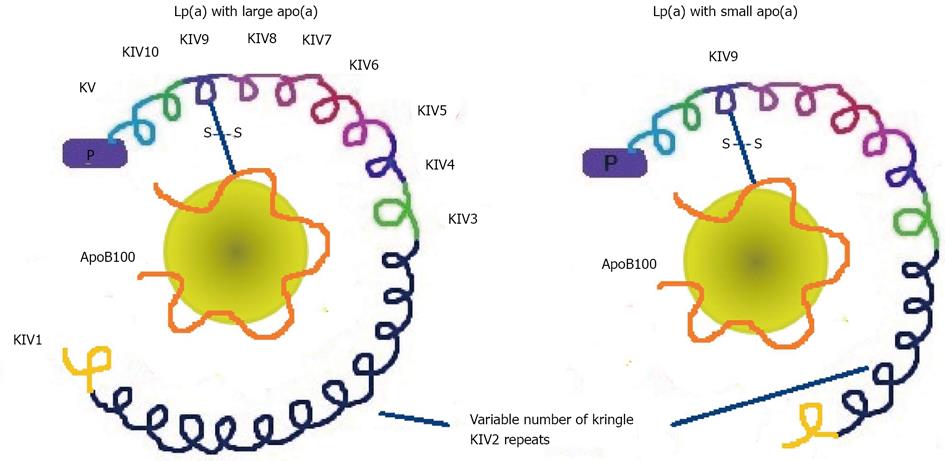
Figure 3 Outline of the different lipoprotein (a) sizes depending on apolipoprotein (a) size, which in turn depends on the number of copies of one of the domains of the protein, the Kringle IV type 2.
Apo(a) is formed by 10 different types of plasminogen Kringle IV-like repeats and also contains other regions that are homologous to plaminogen, the Kringle V and protease (P) regions. Fynally, apo(a) is linked in its kringle IV type 9 domain to the apolipoprotein B100 (apoB100) by a single disulfide bond (S-S). Lp(a): lipoprotein (a); Apo(a): Apolipoprotein (a); KV2: Kringle IV type 2.
- Citation: García-Gómez C, Bianchi M, de la Fuente D, Badimon L, Padró T, Corbella E, Pintó X. Inflammation, lipid metabolism and cardiovascular risk in rheumatoid arthritis: A qualitative relationship? World J Orthop 2014; 5(3): 304-311
- URL: https://www.wjgnet.com/2218-5836/full/v5/i3/304.htm
- DOI: https://dx.doi.org/10.5312/wjo.v5.i3.304