Copyright
©The Author(s) 2024.
World J Orthop. Dec 18, 2024; 15(12): 1191-1199
Published online Dec 18, 2024. doi: 10.5312/wjo.v15.i12.1191
Published online Dec 18, 2024. doi: 10.5312/wjo.v15.i12.1191
Figure 1 Clinical trial design.
The primary outcome measure for comparison between the two groups was the Oswestry disability index (ODI) score. Secondary outcome measures included the quality of life (QOL) score, the Barthel index, patient satisfaction with nursing services, incidence of postoperative complications, and adherence to rehabilitation. ERAS: Enhanced recovery after surgery; ICF: Informed consent form.
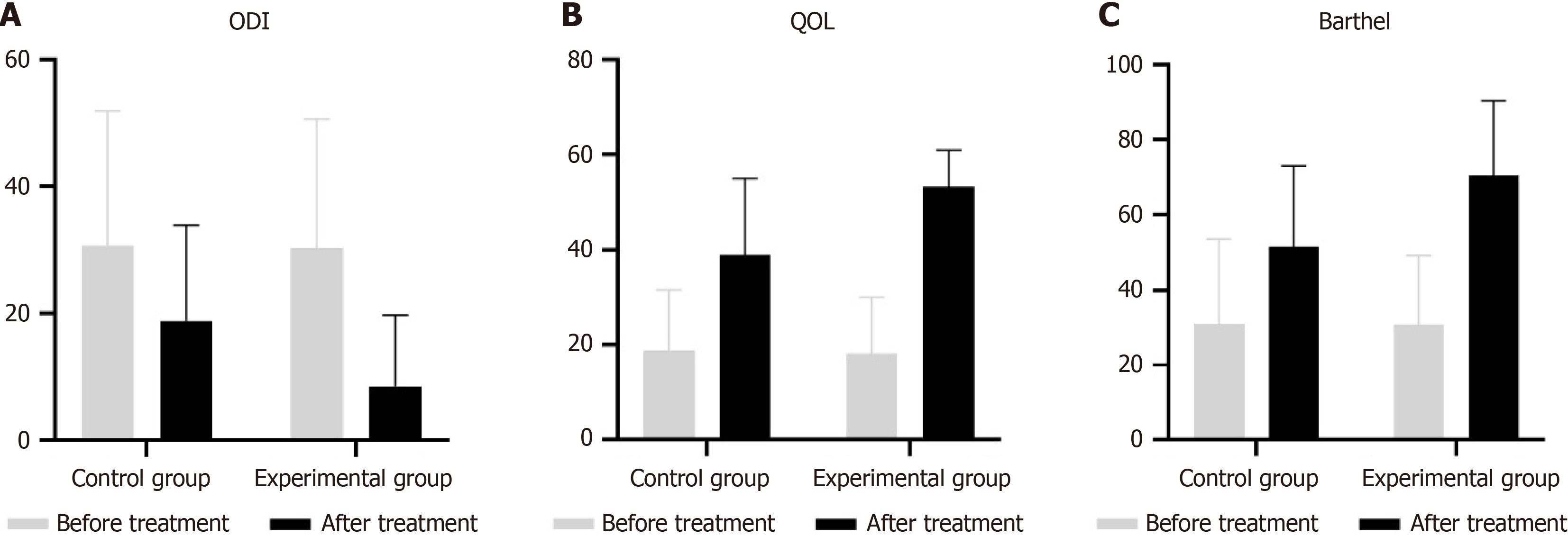
Figure 2 Outcomes before and after operation.
A: Oswestry disability index (ODI) score before and after operation for the control and experimental groups; B: Preoperative and postoperative quality of life (QOL) scores for the control and experimental groups; C: Barthel index scores for the control and experimental groups before and after surgery. ERAS: Enhanced recovery after surgery.
- Citation: Yan XJ, Zhang WH. Enhanced recovery after surgery protocols for minimally invasive treatment of Achilles tendon rupture: Prospective single-center randomized study. World J Orthop 2024; 15(12): 1191-1199
- URL: https://www.wjgnet.com/2218-5836/full/v15/i12/1191.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i12.1191