Copyright
©The Author(s) 2023.
World J Orthop. Jun 18, 2023; 14(6): 443-457
Published online Jun 18, 2023. doi: 10.5312/wjo.v14.i6.443
Published online Jun 18, 2023. doi: 10.5312/wjo.v14.i6.443
Figure 1 Study design profile and recruitment of patients.
Explanations for the participants excluded from the study were given in the Results section of this manuscript. GAH+CHS: 500 mg glucosamine hydrochloride and 400 mg chondroitin sulfate in one capsule; HOOS: Hip Disability and Osteoarthritis Outcome Score; KOOS: Knee Injury and Osteoarthritis Outcome Score; NSAID: Non-steroidal anti-inflammatory drugs.
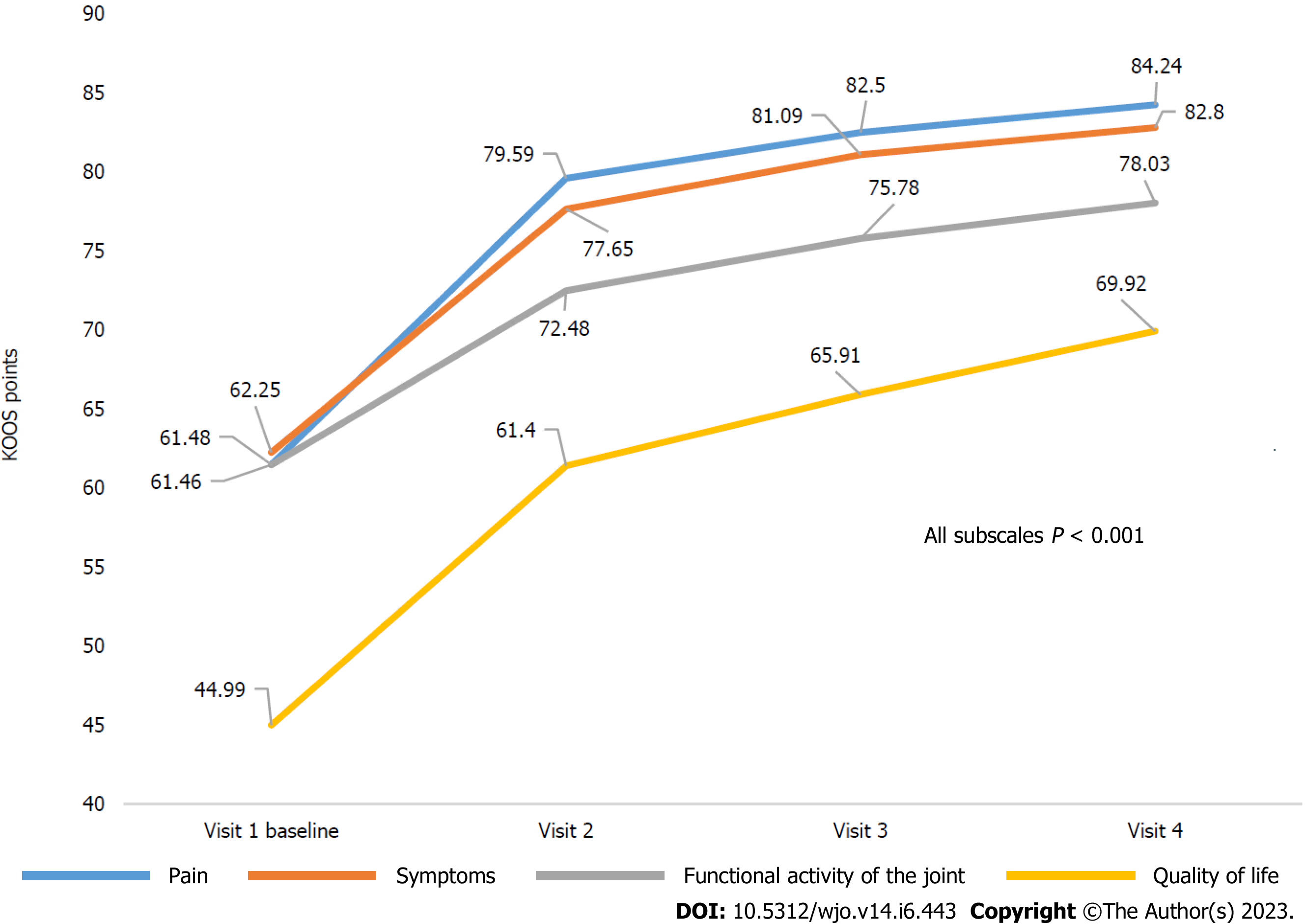
Figure 2 Dynamics of Knee Injury and Osteoarthritis Outcome Score subscale scores.
KOOS: Knee Injury and Osteoarthritis Outcome Score.
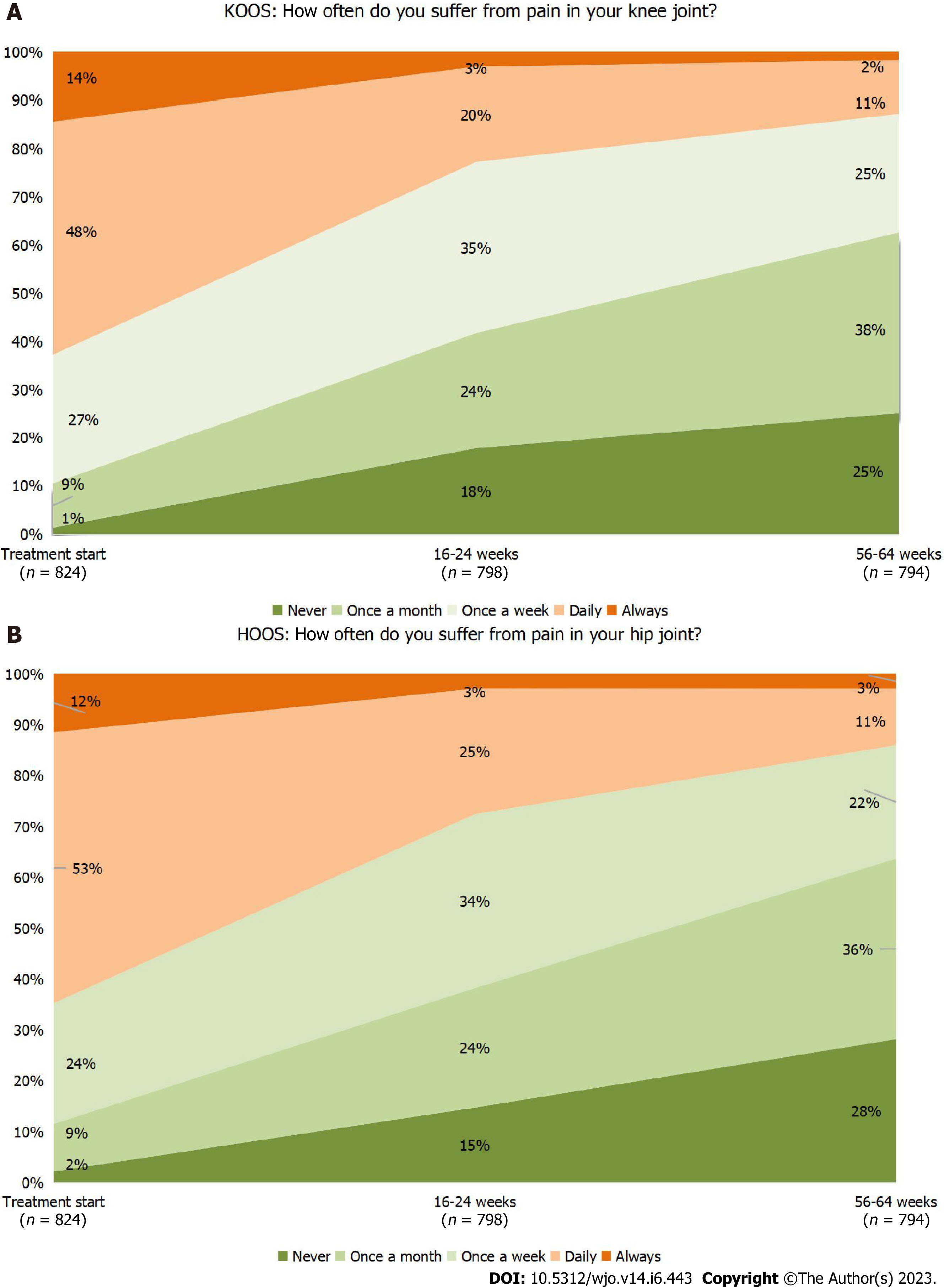
Figure 3 Dynamics of pain during the study (analysis of “Pain Frequency” questions in Knee Injury and Osteoarthritis Outcome Score and Hip Disability and Osteoarthritis Outcome Score).
A: Knee osteoarthritis; B: Hip osteoarthritis. HOOS: Hip Disability and Osteoarthritis Outcome Score; KOOS: Knee Injury and Osteoarthritis Outcome Score.
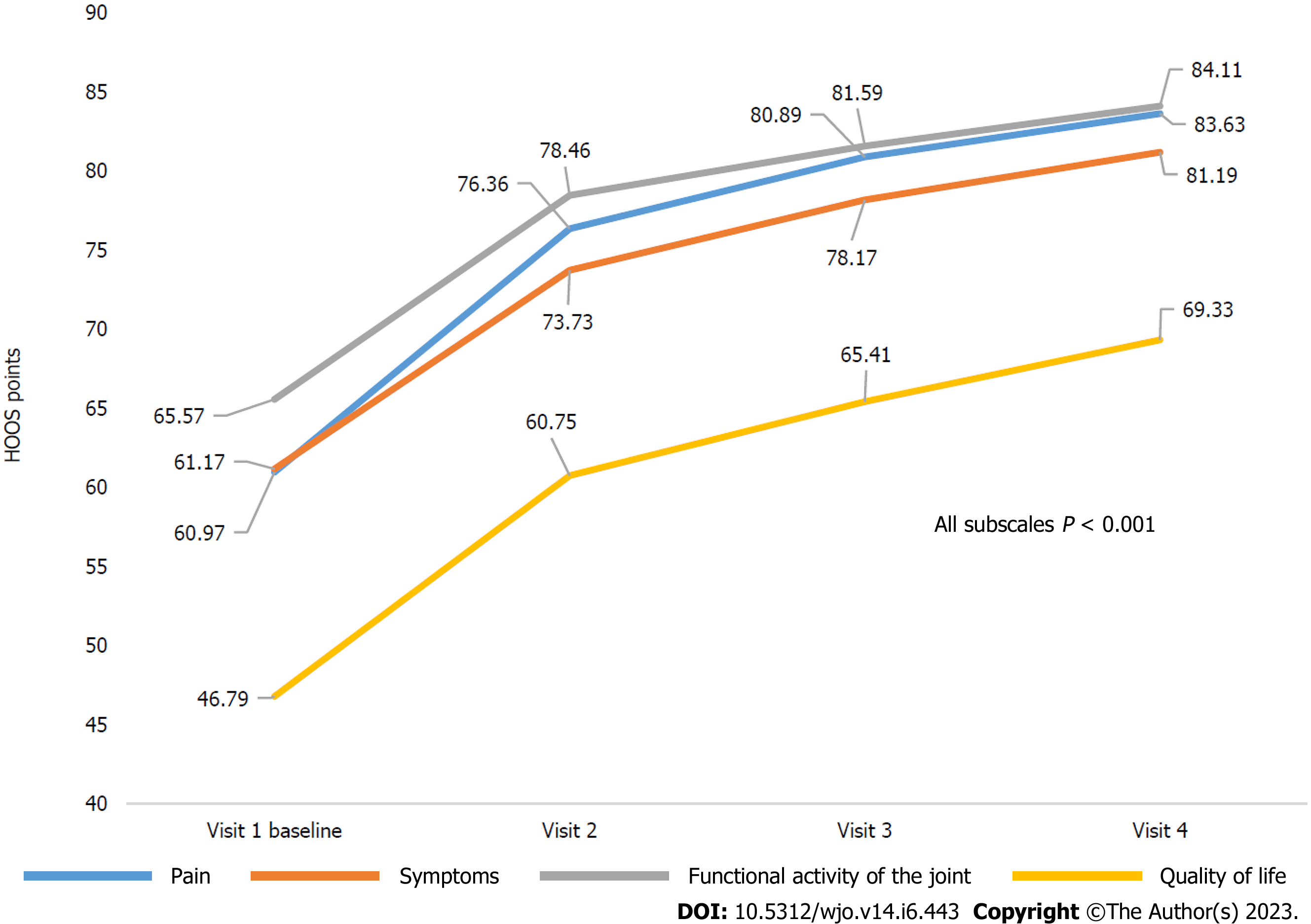
Figure 4 Dynamics of Hip Disability and Osteoarthritis Outcome Score subscale scores.
HOOS: Hip Disability and Osteoarthritis Outcome Score.
Figure 5 The need for concomitant symptomatic therapy with non-steroidal anti-inflammatory drugs for target joints at baseline and at the end of the observation period.
- Citation: Lila AM, Alekseeva LI, Baranov AA, Taskina EA, Kashevarova NG, Lapkina NA, Trofimov EA. Chondroitin sulfate and glucosamine combination in patients with knee and hip osteoarthritis: A long-term observational study in Russia. World J Orthop 2023; 14(6): 443-457
- URL: https://www.wjgnet.com/2218-5836/full/v14/i6/443.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i6.443