Published online Nov 10, 2018. doi: 10.5306/wjco.v9.i7.140
Peer-review started: April 30, 2018
First decision: June 6, 2018
Revised: August 6, 2018
Accepted: October 8, 2018
Article in press: October 8, 2018
Published online: November 10, 2018
Processing time: 194 Days and 16.4 Hours
To establish the Karnofsky performance status (KPS) categories which would facilitate the interconversion of the KPS scale to the Eastern Cooperative Oncology Group (ECOG) performance status (PS) scale.
This was a retrospective analysis of all patients attending the lung cancer clinic at a tertiary care center over a 5-year period (September 2009 to August 2014). All patients were assessed with both KPS and ECOG PS scales at each visit. Correlation between KPS and ECOG PS was assessed using Spearman’s correlation coefficient. KPS categories equivalent to ECOG PS scores were compared using hit rate and weighted kappa (κw).
A total of 1501 patients were assessed over the study period, providing 5844 paired KPS and ECOG PS assessments. The study cohort had a mean (standard deviation; SD) age of 58.4 (10.8) years, with the majority being current or ex-smokers (76.9%) and males (82.3%). Non-small cell lung cancer was the most common histological type (n = 1196, 79.7%) with the majority having advanced (stage IIIB/IV) disease (83.4%). Mean baseline KPS and ECOG PS scores were 77.6 (SD = 14.4) and 1.5 (SD = 1) respectively. The most frequent KPS score was 80 (29%), and the most frequent ECOG PS score was 1 (43%). The overall correlation between KPS and ECOG PS was good (Spearman r = -0.84, P < 0.0001) but ranged from -0.727 to -0.972 between visits. KPS categories derived from our cohort [10-40 (ECOG 4), 50-60 (ECOG 3), 70 (ECOG 2), 80-90 (ECOG 1), 100 (ECOG 0)] performed better [hit rate 78.1%, κw = 0.749 (0.736-0.762) P < 0.0001] than those suggested in the past literature.
The current study provides the largest set of paired KPS-ECOG assessments to date. We suggest that the KPS categories 10-40, 50-60, 70, 80-90, and 100 are equivalent to ECOG PS categories of 4, 3, 2, 1, and 0 respectively.
Core tip: Karnofsky performance status (KPS) scale and Eastern Cooperative Oncology Group (ECOG) scale are the most commonly used performance status (PS) tools worldwide for patients with cancer. Since the number of scoring points in each scale is different, these scales are not readily interconvertible. However, most clinical studies use only one of these two scales (either KPS or ECOG PS) to assess PS, rendering interpopulation comparisons difficult. In this study, we analyze the largest set of paired KPS-ECOG assessments to date in a cohort of lung cancer patients for a solution.
- Citation: Prasad KT, Kaur H, Muthu V, Aggarwal AN, Behera D, Singh N. Interconversion of two commonly used performance tools: An analysis of 5844 paired assessments in 1501 lung cancer patients. World J Clin Oncol 2018; 9(7): 140-147
- URL: https://www.wjgnet.com/2218-4333/full/v9/i7/140.htm
- DOI: https://dx.doi.org/10.5306/wjco.v9.i7.140
Performance status (PS) is a measure of a subject’s ability to perform the activities of daily living. PS has been shown to predict survival in patients with cancer[1-4]. Patients with poor PS often do not tolerate more aggressive treatment strategies like surgery or chemotherapy, and, hence, PS is often taken into account while deciding the therapeutic strategy for cancer. It is a well-known fact that patients with poor PS are excluded from most clinical trials in cancer[5,6].
Several tools are available for the assessment of PS, including the Karnofsky performance status (KPS) scale, the Eastern Cooperative Oncology Group (ECOG) PS scale, the palliative performance scale, and the Australia-modified KPS. Among these tools, the KPS scale and the ECOG PS scale are the most commonly used worldwide[7]. The KPS scale is an 11-point numerical scale, with scores ranging from 100 (normal functional status) to 0 (death), in decremental steps of 10[8]. The ECOG PS scale is a 6-point numerical scale, with scores ranging from 0 (normal functional status) to 5 (death), in incremental steps of 1[9]. No conclusive evidence exists in the literature to suggest that one scale is better than the other. However, the ECOG PS scale is often preferred, as it is simpler to apply with a smaller number of choices. Both the scores have been shown to be good predictors of mortality[1,4,10,11]. The ECOG PS may have a slightly better prognostic value compared to KPS[10]. Although both the scales have been used to predict treatment response in lung cancer, results have been variable[12].
Several studies have shown PS assessment made using the KPS and ECOG PS by different healthcare professionals (doctors, medical students, nurses) to have moderate to high interobserver correlation, albeit with considerable variation[13-16]. Assessments made by technical staff or patients have been shown to have a relatively larger variability compared to assessments by healthcare workers[11,13,16]. These differences could be attributed to variation in the level of overall training and exposure of the individual, which might affect the interpretation of the existing disability. Moreover, interobserver variability can get aggravated when assessments are made on patients with lower KPS scores[17]. Closer attention to certain behavioral issues might help to improve the PS assessments in such situations[17]. However, neither of these two scales have been shown to be consistently superior to the other with respect to interobserver variability[13-15].
Since PS is an important factor which can influence clinical outcomes, researchers making interpopulation comparisons across studies should ensure that the PSs of the populations analyzed are equivalent. However, most clinical trials usually utilize only one of the two scales in their study population (either KPS or ECOG PS)[7]. Hence, the comparison of PS between different patient populations is difficult as the two scales are not readily interconvertible, with different number of scoring points (11 vs 6). To overcome this hindrance, several attempts have been made to examine the possibility of interconversion between KPS and ECOG PS scales (Table 1)[7,10,18-20]. Despite these suggestions, confusion still exists regarding the optimal KPS categories for interconversion to ECOG. Herein, we attempt to determine the KPS categories equivalent to ECOG PS scores by analyzing data from a large cohort of lung cancer patients.
| Author, year | n and patients, assessments | Setting | KPS categories | ECOG |
| AJCC[34], 1977 | - | - | 90-100 | 0 |
| 70-80 | 1 | |||
| 50-60 | 2 | |||
| 30-40 | 3 | |||
| 10-20 | 4 | |||
| Minna et al[20], 1985 | - | - | 100 | 0 |
| 80-90 | 1 | |||
| 60-70 | 2 | |||
| 40-50 | 3 | |||
| 20-30 | 4 | |||
| Buccheri et al[10], 1996 | 536 (1656) | Subjects with lung cancer visiting a cancer clinic of a tertiary care centre | 80-100 | 0-1 |
| 60-70 | 2 | |||
| 10-50 | 3-4 | |||
| Ma et al[19], 2010 | 1385 (1385) | Subjects visiting an oncology palliative care clinic, or admitted to an acute cancer palliative care unit | 100 | 0 |
| 80-90 | 1 | |||
| 60-70 | 2 | |||
| 40-50 | 3 | |||
| 10-30 | 4 | |||
| de Kock et al[18], 2013 | 955 (674) | Subjects with advanced life-limiting illnesses (cancer and non-cancer) in acute care and community settings | 60-100 | 1 |
| 50 | 2 | |||
| 30-40 | 3 | |||
| 10-20 | 4 | |||
| Current study | 1501 (5844) | Subjects with lung cancer visiting a cancer clinic of a tertiary care center | 100 | 0 |
| 80-90 | 1 | |||
| 70 | 2 | |||
| 50-60 | 3 | |||
| 10-40 | 4 |
We performed a retrospective analysis of data collected in the lung cancer clinic of our center over a 5-year period (September 2009 to August 2014). Informed consent was obtained from all the subjects.
All subjects with lung cancer who visited the lung cancer clinic for chemotherapy and had at least one paired assessment of KPS and ECOG PS were included in the study. Subjects with a diagnosis of intrathoracic malignancy other than lung cancer were not included in this study. Subjects who did not receive chemotherapy and received only alternative forms of therapy, like surgery, radiotherapy, or targeted therapy, were also excluded from this study. The PS assessments made during the first 10 visits of each patient, starting from the date of the first cycle of chemotherapy, were included in this study.
The subjects were treated appropriately with chemotherapy, tyrosine kinase inhibitors, radiotherapy, or surgery as guided by the tumor histopathology, mutation status, and clinical status, as described previously[21-24]. Briefly, subjects with adenocarcinoma without any driver mutation were treated with pemetrexed-based platinum doublet followed by maintenance pemetrexed therapy until disease progression. Subjects with squamous histology were treated with docetaxel or gemcitabine-based platinum doublet. Subjects with small cell lung cancer received irinotecan-based platinum doublet. All patients receiving chemotherapy were administered at least four cycles of chemotherapy before response assessment. Subjects who showed a partial response received an additional two cycles, for a total of six cycles. Subjects with sensitizing EGFR gene mutation or ALK gene rearrangements were treated with appropriate EGFR tyrosine kinase or ALK inhibitors, respectively.
All subjects underwent a systematic assessment at baseline and follow-up as described previously[25-29]. At baseline, the following parameters were collected: age, gender, smoking status, body mass index, tumor histopathology, TNM stage (7th edition), KPS, and ECOG-PS[30]. The frequency of scheduled visits to the clinic by the patient varied depending upon the ongoing treatment modality. At every visit to the lung cancer clinic, the subjects were assessed with both the KPS and the ECOG PS scales by the treating physician.
Descriptive data are presented as numbers and percentages or mean ± SD unless otherwise stated. Correlation between KPS and ECOG PS was assessed using Spearman’s correlation coefficient (r), with values ranging from -1.00 to +1.00. An r-value of -1.00 was considered to represent a perfect negative correlation and a value of +1.00 was considered to represent a perfect positive correlation[31]. The original 11-point KPS scale was converted into a 5-point scale for comparison with the ECOG PS. The agreement between these KPS categories and the actual ECOG PS measurements was assessed using hit rate and the weighted kappa coefficient (κw). Hit rate was calculated as the proportion of assessments with a perfect agreement between the KPS categories and the measured ECOG PS scale. The κw was calculated to assess the level of agreement beyond chance. Agreement between the scales was classified based on the kappa values as poor (< 0.00), slight (0.00 to 0.20), fair (0.21 to 0.40), moderate (0.41 to 0.60), substantial (0.61 to 0.80), or almost perfect (0.81 to 1.00)[32]. Statistical analyses were performed with the help of Statistical Package for Social Sciences software (IBM SPSS Statistics, version 22; IBM Corporation, Armonk, NY, United States). All statistical tests were performed as two-sided. P < 0.05 was considered statistically significant.
During the study period, 1664 patients visited the lung cancer clinic. Among them, 150 patients did not receive any chemotherapy and were excluded. An additional 13 patients who had intrathoracic malignancies other than lung cancer were also excluded. A total of 1501 patients were assessed during the study period, providing 5844 paired PS assessments (KPS and ECOG PS). The mean (standard deviation; SD) age was 58.4 (10.8) years, with the majority being current or ex-smokers (76.9%) and males (82.3%) (Table 2). Non-small cell lung cancer was the most common histological type (n = 1196, 79.7%), with the majority having advanced (stage IIIB or IV) disease (83.4%).
| Characteristic | Total, n = 1501 |
| Age in yr | 58.4 (10.8) |
| Males | 1236 (82.3) |
| Smokers | 1155 (76.9) |
| Body mass index in kg/m2 | 20.2 (4.2) |
| Histopathology | |
| NSCLC: Squamous | 553 (36.8) |
| NSCLC: Adeno | 514 (34.2) |
| NSCLC: Undifferentiated | 87 (5.8) |
| NSCLC: Other | 42 (2.8) |
| SCLC | 305 (20.3) |
| NSCLC/SCLC stage1 | |
| I and II | 44 (2.9) |
| IIIA | 193 (12.9) |
| IIIB | 426 (28.4) |
| IV | 767 (51.1) |
| SCLC stage | |
| Limited disease | 145 (9.7) |
| Extensive disease | 160 (10.7) |
| Baseline performance status | |
| KPS | 77.6 (14.4) |
| ECOG PS | 1.5 (1) |
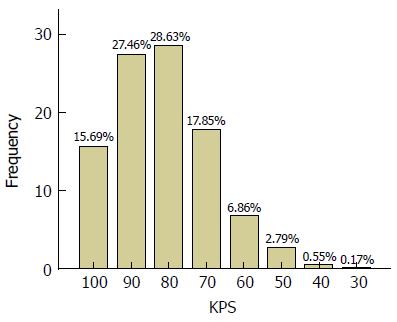
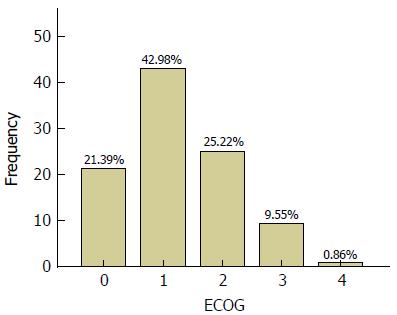
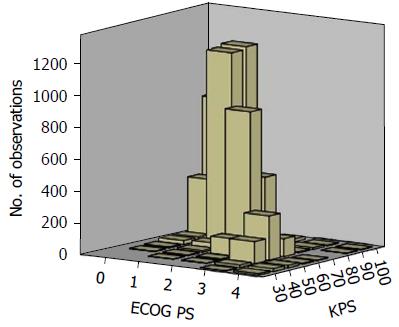
Mean baseline KPS and ECOG PS scores were 77.6 (SD = 14.4) and 1.5 (SD = 1) respectively. The most frequent KPS score was 80 (29%) (Figure 1) and the most frequent ECOG PS score was 1 (43%) (Figure 2). Among the total of 5844 paired PS assessments, a vast majority of the KPS scores were between 70 and 100 (89.6%) and similarly most ECOG PS scores were between 0 and 2 (89.6%). The overall correlation between KPS and ECOG PS was good [Spearman ρ = (-) 0.84, P < 0.0001] but ranged from -0.727 to -0.972 between visits (Table 3 and Figure 3). As expected, the number of assessments available at each progressive follow-up visit showed a reduction due to reduction in number of patients due to death or loss to follow-up.
| Visit number | Number of assessments | Spearman’s correlation coefficient1 |
| 1 | 1426 | -0.727 |
| 2 | 1099 | -0.890 |
| 3 | 957 | -0.876 |
| 4 | 823 | -0.863 |
| 5 | 602 | -0.858 |
| 6 | 464 | -0.867 |
| 7 | 270 | -0.901 |
| 8 | 129 | -0.854 |
| 9 | 54 | -0.773 |
| 10 | 20 | -0.972 |
| Overall | 5844 | -0.840 |
Visual inspection of cross-tabulated KPS and ECOG PS data was performed to arrive at the best possible KPS categories to allow conversion of KPS to ECOG PS scale, namely: 100 (ECOG 0), 80-90 (ECOG 1), 70 (ECOG 2), 50-60 (ECOG 3), and 10-40 (ECOG 4)[10]. We also analyzed the performance of KPS categories suggested in the past literature, on our cohort (Table 4). We found a substantial agreement with all the suggested conversion categories, except those suggested by de Kock et al[18] and the American Joint Committee on Cancer (AJCC), which had slight and fair agreements respectively. KPS categories derived from our cohort performed better (hit rate 78.4%, κw= 0.674) than those suggested in the past literature[19,33]. The highest hit rate (83.2%) was observed with the interconversion categories suggested by Buccheri et al[10]. The highest κw was observed with the categories derived from our cohort (κw= 0.749). The interconversion categories suggested by de Kock et al[18] had the worst hit rate (43.2%) and κw (0.079).
| Author, year | Hit rate1 | κw1 |
| AJCC et al[34], 1977 | 43.60% | 0.376 (0.363-0.389) P < 0.0001 |
| Minna et al[20], 1985 | 75.20% | 0.701 (0.687-0.714) P < 0.0001 |
| Buccheri et al[10], 1996 | 83.20% | 0.695 (0.679-0.711) P < 0.0001 |
| Ma et al[19], 2010 | 75.20% | 0.701 (0.687-0.714) P < 0.0001 |
| de Kock et al[18], 2013 | 43.20% | 0.079 (0.068-0.090) P < 0.0001 |
| Current study | 78.10% | 0.749 (0.736-0.762) P < 0.0001 |
In this study, we propose KPS categories which can aid interconversion of KPS scores to ECOG PS scores by retrospectively analyzing paired PS assessments (KPS and ECOG PS) made in a large cohort of lung cancer patients. We also compare the performance of KPS categories in previous literature for interconversion of KPS to ECOG PS on our cohort.
It is well-known that the PS is a predictor of mortality[1-4]. Hence, when comparing clinical characteristics or the outcomes of a therapeutic modality in different patient populations with lung cancer, it is essential that their PS is also matched. Failure to do so may result in erroneous conclusions. However, such comparisons are difficult when the tool used for assessment of PS in the study population is different (either KPS or ECOG PS). To overcome this difficulty, several investigators have suggested KPS categories for interconversion to ECOG PS scale (Table 1)[7,10,18-20].
The AJCC and Minna et al[20] were among the first to have suggested KPS categories for interconversion to ECOG PS[34]. However, these suggestions were not evidence-based. Verger et al[7] compared these two suggestions in a cohort of 150 patients with cancer attending a radiotherapy clinic and found that the categories suggested by Minna et al[20] performed better. They also stressed that at lower PS levels, interconversion between the two scores would be difficult as they observed a wide spread of values. Subsequently, Buccheri et al[10] studied 536 patients with lung cancer and found that a 3-point conversion scale for KPS resulted in the best agreement (hit rate 84%) between the two PS scales. Ma and colleagues had assessed KPS and ECOG PS in 1385 subjects with various cancers attending or admitted to an oncology palliative care clinic[19]. They analyzed several KPS categories and suggested a KPS category which closely resembled that suggested by Minna et al[20] and had the best combination of hit rate (75%) and κw (0.84, P < 0.0001). de Kock et al[18] studied 955 subjects with advanced life-limiting illnesses and suggested a 4-point scale, which had the best combination of hit rate (57%) and κ (0.7, 95%CI: 0.66-0.73).
Among all the interconversion categories for KPS suggested in the literature, the KPS categories which we suggest in this study had the best agreement with the actual PS measurements. Although, the categories suggested by Minna et al[20] and Ma et al[19] had the next best agreement, the categories suggested in our study appear more appropriate clinically. This can be illustrated by the following two examples. A patient who requires occasional assistance for daily activities (KPS 60), would be classified as ECOG 2 (capable of all self-care) using the categories suggested by Minna et al[20] or Ma et al[19], while they will be classified more appropriately as ECOG 3 (capable of limited self-care) using the categories suggested in the current study. Similarly, a patient who is disabled and requires special care and assistance (KPS 40) would be classified as ECOG 3 (capable of limited self-care) using the categories suggested by Minna et al[20] or Ma et al[19], while they will be classified more appropriately as ECOG 4 (cannot carry out any self-care) using the categories suggested in the current study. It should also be borne in mind that a change in the ECOG scale by a single score in the above situations entails a change in 1-year survival by at least 10%[4].
The highest hit rate (83.2%) was observed with the KPS categories suggested by Buccheri et al[10]. However, this could have been because of the consolidation of the KPS categories into a smaller number of groups (3 groups instead of 5): KPS 10-50 (ECOG PS 3-4), KPS 60-70 (ECOG PS 2), KPS 80-100 (ECOG PS 0-1). Despite this, the level of agreement was lower when compared to the current study (κw 0.645 vs 0.749).
We observed the lowest hit rate (43.2%) and the least agreement (κw= 0.079) with the KPS categories suggested by de Kock et al[18]. This observation could have been because a majority of the population in that study had a poor PS, which is known to affect the interobserver rating[17]. In fact, 42.7% of their subjects had an ECOG PS of 3, and 66.2% had a KPS score between 30 and 50. Additionally, the authors did not assign KPS categories for ECOG PS 0.
Our study is not without limitations. The inherent limitations of retrospective studies apply to our study as well. Since the PS assessments were made by various treating physicians, there could have been some variability in the assessments. However, it has been demonstrated that both the KPS and ECOG PS scored by physicians have substantial interobserver reliability[13-16]. Moreover, each paired assessment was made by a single observer. On the other hand, this presumed limitation could be considered as an advantage as our study is more likely to approximate a real-world scenario. A majority of our subjects had good PS (KPS score ≥ 70 and ECOG PS ≥ 2) as they visited the clinic for treatment purposes. Hence, the suggested KPS categories may not apply to patients with poorer PS (e.g., palliative care clinics). Finally, we did not have survival data for our cohort, as this was not the intended purpose of the study. However, despite these limitations, the current study provides the largest set of paired KPS-ECOG assessments to date in a real-world setting.
In conclusion, we suggest that the KPS categories 10-40, 50-60, 70, 80-90, and 100 are equivalent to ECOG PS categories of 4, 3, 2, 1, and 0 respectively. These categories may be useful for interconversion of KPS to ECOG PS scale when attempting to compare patient populations across different studies in whom investigators have used one of the two different scales (KPS or ECOG PS) for assessment of PS.
Performance status (PS) is an estimate of a subject’s ability to perform activities of daily living. Several tools are available to estimate the PS. Among them, the Karnofsky performance status (KPS) scale and the Eastern Cooperative Oncology Group (ECOG) scale are the most commonly used PS scales worldwide for patients with cancer. The KPS scale is an 11-point numerical scale, with scores ranging from 100 (normal functional status) to 0 (death), in decremental steps of 10. The ECOG PS scale is a 6-point numerical scale, with scores ranging from 0 (normal functional status) to 5 (death), in incremental steps of 1. Since the number of scoring points in each scale is different, these scales are not readily interconvertible.
PS is an important clinical factor which affects prognosis and influences treatment decisions in subjects with lung cancer. Hence, researchers who attempt to compare clinical characteristics or outcomes across different patient populations should ensure that their PS levels are matched. Failure to do so may result in erroneous conclusions. Most clinical studies employ only one of these two scales (either KPS or ECOG PS) in their study population for assessment of PS. When the PS scale used in the studies are different (either KPS or ECOG PS) this may lead to difficulty. Several investigators have tried to overcome this hindrance by suggesting KPS categories for interconversion to the ECOG PS scale. However, the performance of these suggested KPS categories has been variable.
We attempted to establish the KPS categories which would facilitate the interconversion of the KPS scale to the ECOG PS scale.
We retrospectively analyzed the data of 1501 patients from a lung cancer clinic. In these patients, at every visit, paired assessments of PS had been made using both the KPS and ECOG PS scales by physicians. We also studied the performance of other KPS categories suggested in the literature, on our patient cohort. We used statistical methods called hit rate and weighted kappa to test the agreement between the KPS categories and the actual observations.
We found that the KPS categories 10-40, 50-60, 70, 80-90, and 100 were equivalent to ECOG PS categories of 4, 3, 2, 1, and 0 respectively. We also found that the agreement between the KPS categories suggested in the past literature (for interconversion to ECOG PS) and the paired KPS-ECOG PS assessments made in our cohort was variable.
The current study is the largest set of paired KPS-ECOG assessments published in the literature in patients with lung cancer to date. The suggested KPS categories will facilitate interconversion of the KPS to the ECOG PS scale and will enhance communication between researchers utilizing either of the two scales.
The KPS categories suggested in our study may be prospectively evaluated to test their validity. The applicability of the suggested categories may be evaluated in other populations to study the effect of cultural and regional variations.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Couñago F, Guan YS, Wang K S- Editor: Cui LJ L- Editor: Filipodia E- Editor: Bian YN
| 1. | Stanley KE. Prognostic factors for survival in patients with inoperable lung cancer. J Natl Cancer Inst. 1980;65:25-32. [PubMed] |
| 2. | Downing M, Lau F, Lesperance M, Karlson N, Shaw J, Kuziemsky C, Bernard S, Hanson L, Olajide L, Head B. Meta-analysis of survival prediction with Palliative Performance Scale. J Palliat Care. 2007;23:245-252; discussion 252-254. [PubMed] |
| 3. | Maltoni M, Caraceni A, Brunelli C, Broeckaert B, Christakis N, Eychmueller S, Glare P, Nabal M, Viganò A, Larkin P. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations--a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23:6240-6248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 488] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 4. | Kawaguchi T, Takada M, Kubo A, Matsumura A, Fukai S, Tamura A, Saito R, Maruyama Y, Kawahara M, Ignatius Ou SH. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol. 2010;5:620-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 247] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 5. | Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:2895-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 466] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 6. | Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, von Pawel J, Gottfried M, Bondarenko I, Liao M. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15:143-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 722] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 7. | Verger E, Salamero M, Conill C. Can Karnofsky performance status be transformed to the Eastern Cooperative Oncology Group scoring scale and vice versa? Eur J Cancer. 1992;28A:1328-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 969] [Cited by in RCA: 1073] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 9. | Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7038] [Cited by in RCA: 7988] [Article Influence: 190.2] [Reference Citation Analysis (0)] |
| 10. | Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32A:1135-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 451] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 11. | Blagden SP, Charman SC, Sharples LD, Magee LR, Gilligan D. Performance status score: do patients and their oncologists agree? Br J Cancer. 2003;89:1022-1027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 162] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Yogananda MN, Muthu V, Prasad KT, Kohli A, Behera D, Singh N. Utility of the revised Edmonton Symptom Assessment System (ESAS-r) and the Patient-Reported Functional Status (PRFS) in lung cancer patients. Support Care Cancer. 2018;26:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | de Borja MT, Chow E, Bovett G, Davis L, Gillies C. The correlation among patients and health care professionals in assessing functional status using the karnofsky and eastern cooperative oncology group performance status scales. Support Cancer Ther. 2004;2:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Taylor AE, Olver IN, Sivanthan T, Chi M, Purnell C. Observer error in grading performance status in cancer patients. Support Care Cancer. 1999;7:332-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Roila F, Lupattelli M, Sassi M, Basurto C, Bracarda S, Picciafuoco M, Boschetti E, Milella G, Ballatori E, Tonato M. Intra and interobserver variability in cancer patients’ performance status assessed according to Karnofsky and ECOG scales. Ann Oncol. 1991;2:437-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Conill C, Verger E, Salamero M. Performance status assessment in cancer patients. Cancer. 1990;65:1864-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Péus D, Newcomb N, Hofer S. Appraisal of the Karnofsky Performance Status and proposal of a simple algorithmic system for its evaluation. BMC Med Inform Decis Mak. 2013;13:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 258] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 18. | de Kock I, Mirhosseini M, Lau F, Thai V, Downing M, Quan H, Lesperance M, Yang J. Conversion of Karnofsky Performance Status (KPS) and Eastern Cooperative Oncology Group Performance Status (ECOG) to Palliative Performance Scale (PPS), and the interchangeability of PPS and KPS in prognostic tools. J Palliat Care. 2013;29:163-169. [PubMed] |
| 19. | Ma C, Bandukwala S, Burman D, Bryson J, Seccareccia D, Banerjee S, Myers J, Rodin G, Dudgeon D, Zimmermann C. Interconversion of three measures of performance status: an empirical analysis. Eur J Cancer. 2010;46:3175-3183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Minna JD, Higgins GA, Glatstein EJ. Cancer of the lung. VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Philadelphia: J. B. Lippincott Co 1985; . |
| 21. | Singh N, Aggarwal AN, Behera D. Management of advanced lung cancer in resource-constrained settings: a perspective from India. Expert Rev Anticancer Ther. 2012;12:1479-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Singh N, Aggarwal AN, Kaur J, Behera D. Association of Graded Folic Acid Supplementation and Total Plasma Homocysteine Levels With Hematological Toxicity During First-line Treatment of Nonsquamous NSCLC Patients With Pemetrexed-based Chemotherapy. Am J Clin Oncol. 2017;40:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Singh N, Aggarwal AN, Behera D, Jindal SK. Intercycle delays during chemotherapy of non-small cell lung cancer in a health care resource-constrained setting and their effect on overall survival. J Thorac Oncol. 2010;5:236-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Baldi M, Behera D, Kaur J, Kapoor R, Singh N. Rationale and Design of PEMVITASTART-An Open-label Randomized Trial Comparing Simultaneous Versus Standard Initiation of Vitamin B12 and Folate Supplementation in Nonsquamous, Non-Small-cell Lung Cancer Patients Undergoing First-line Pemetrexed-based Chemotherapy. Clin Lung Cancer. 2017;18:432-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Singh N, Aggarwal AN, Gupta D, Behera D, Jindal SK. Unchanging clinico-epidemiological profile of lung cancer in north India over three decades. Cancer Epidemiol. 2010;34:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Singh N, Aggarwal AN, Gupta D, Behera D, Jindal SK. Quantified smoking status and non-small cell lung cancer stage at presentation: analysis of a North Indian cohort and a systematic review of literature. J Thorac Dis. 2012;4:474-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 27. | Singh N, Aggarwal AN, Gupta D, Behera D. Prevalence of low body mass index among newly diagnosed lung cancer patients in North India and its association with smoking status. Thorac Cancer. 2011;2:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Kaur H, Sehgal IS, Bal A, Gupta N, Behera D, Das A, Singh N. Evolving epidemiology of lung cancer in India: Reducing non-small cell lung cancer-not otherwise specified and quantifying tobacco smoke exposure are the key. Indian J Cancer. 2017;54:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Singh N, Singh PS, Aggarwal AN, Behera D. Comorbidity Assessment Using Charlson Comorbidity Index and Simplified Comorbidity Score and Its Association With Clinical Outcomes During First-Line Chemotherapy for Lung Cancer. Clin Lung Cancer. 2016;17:205-213.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L; International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2389] [Cited by in RCA: 2581] [Article Influence: 143.4] [Reference Citation Analysis (0)] |
| 31. | Sedgwick P. Correlation. BMJ-BRIT MED J. 2012;345. |
| 32. | Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43944] [Cited by in RCA: 41871] [Article Influence: 872.3] [Reference Citation Analysis (0)] |
| 33. | American Joint Committee on Cancer. AJCC Cancer Staging Manual. 8th ed. New York: Springer International Publishing, 2017. . |
| 34. | American Joint Committee for Cancer Staging and End-Results Reporting. Manual for staging of cancer. 1st ed. Chicago, 1977. . |