Published online Sep 14, 2018. doi: 10.5306/wjco.v9.i5.74
Peer-review started: June 12, 2018
First decision: July 9, 2018
Revised: July 11, 2018
Accepted: August 6, 2018
Article in press: August 7, 2018
Published online: September 14, 2018
Processing time: 95 Days and 8.9 Hours
The neuropeptide hormone oxytocin, which is released from the posterior pituitary gland, is involved in a number of physiological processes. Understanding of its effects is gradually increasing due to new research in this area. While mostly recognized as a reproductive system hormone, oxytocin also regulates other organ systems such as the brain and cardiovascular system. Recently, research has focused on unraveling its involvement in cancer, and emerging evidence suggests a potential role for oxytocin as a cancer biomarker. This review summarizes observations linking oxytocin and cancer, with a special emphasis on prostate cancer, where it may promote cell proliferation. Research suggests that oxytocin effects may depend on cell type, concentration of the hormone, its interactions with other hormones in the microenvironment, and the precise localization of its receptor on the cell membrane. Future research is needed to further elucidate the involvement of oxytocin in cancer, and whether it could be a clinical cancer biomarker or therapeutic target.
Core tip: Oxytocin’s role outside of the reproductive system and social bonding has yet to be fully elucidated. Apparently, its role in cancer may vary depending on location and cell type. This review summarizes the current state of our understanding of the potential role of oxytocin in cancer.
- Citation: Lerman B, Harricharran T, Ogunwobi OO. Oxytocin and cancer: An emerging link. World J Clin Oncol 2018; 9(5): 74-82
- URL: https://www.wjgnet.com/2218-4333/full/v9/i5/74.htm
- DOI: https://dx.doi.org/10.5306/wjco.v9.i5.74
Oxytocin is a central nervous system (CNS) neuropeptide hormone, which is composed of nine amino acids. The synthesis of oxytocin begins in the hypothalamus, where the paraventricular nucleus and supra-optic neurons express high levels of oxytocin, which is released from the posterior pituitary gland[1]. Oxytocin is biologically similar to vasopressin (also known as antidiuretic hormone), and they are often studied in parallel, as both hormones also share some functions. Originally thought of as a hormone with a role limited to the uterus and milk ejection - oxytocin means “quick birth” in Greek[2] - further research has expanded understanding of its function across sexes and organ systems. Furthermore, it has become clear that in addition to physical function, oxytocin also wields important impact on social behaviors, which include stress and trust, anxiety, social interaction and bonding, and parental care[3], and thereby on neuropsychiatric disorders linked to these social behaviors. Interestingly, emerging evidence has linked oxytocin to somewhat conflicting roles in carcinogenesis, as oxytocin is implicated in either fostering development or, conversely, inhibition of cancer-related cellular functional phenomena.
Oxytocin exerts its effects primarily through a single receptor, which has been well characterized. The oxytocin receptor is a class-I G-protein-coupled receptor with seven transmembrane domains, and can be bound by several ligands, including oxytocin, oxytocin agonists and antagonists, as well as vasopressin[4]. These receptors are found in the endometrium, myometrium, trophoblast, osteoblasts, reproductive organs, and throughout the CNS (Table 1). Notably, oxytocin receptor has also been implicated in various cancers related to tissues in which it is expressed, including endometrial cancer, glioblastomas, neuroblastomas[5], and others. Several oxytocin receptor antagonists have been identified, with the most common being Atosiban[6,7]. While studies of oxytocin antagonist in cancer has been limited, atosiban has been implicated in inhibiting cell growth of DU145 prostate cancer[8] and various breast cancer cell lines[9]. However, little evidence indicates whether this strategy is efficacious in vivo.
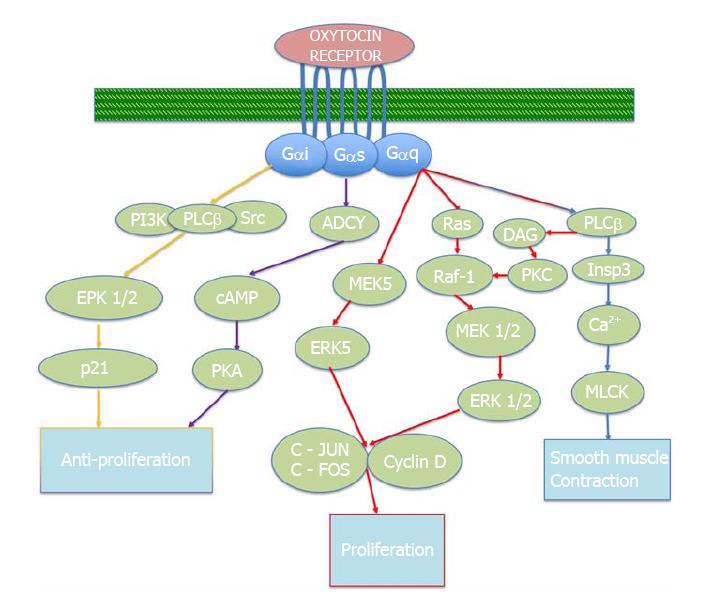
The effectors of the oxytocin receptor may vary. While the primary signaling mechanisms of oxytocin have not been fully elucidated, recent studies show that the main mitogenic signaling mechanism of the oxytocin receptor involves the Gq alpha subunit protein (Gαq)/phospholipase C (PLC)/inositol 1, 4, 5 triphosphate (InsP3) pathway. Through this activation, the G protein couples to PLC-B, resulting in the release of calcium from intracellular stores[10], triggering smooth muscle contractions[11], for example in the uterus or in the myoepithelial cells of the mammary gland. The main oxytocin pathways described in this paper are depicted in Figure 1.
Indeed, the first functional role attributed to oxytocin centered on the female reproductive system, specifically in uterine contraction and in lactation. Uterine sensitivity to oxytocin increases around the onset of labor, and upon labor oxytocin stimulation becomes more efficient. Therefore, exogenous administration of oxytocin is also clinically used to induce labor. Following parturition, the density of oxytocin receptors are declines. In lactation, the oxytocin pathway is activated when the infant begins sucking on the nipple[12]. A sensory impulse is sent from the nipple to the spinal cord, and from there transmitted to the oxytocinergic neurons in the hypothalamus. These neurons generate action potentials that lead to a substantial release of oxytocin into the blood stream, which subsequently elicits milk ejection via a contraction of myoepithelial cells[4]. Interestingly, this cascade can be triggered even before suckling occurs, by an event such as a baby crying[13], suggesting that it may involve reflex neural pathways. Increased plasma oxytocin has also been linked to an increased risk of pregnancy-induced hypertension[14], and exogenous oxytocin administration has been associated with angiotensin II-induced hypertension[15]. Interestingly, though, there is also a potential link between increased oxytocin and reduced risk of hypertension (which might be acquired in utero in association with intrauterine growth retardation)[16], so that oxytocin has been attributed with eliciting a reduction in blood pressure[17,18].
The reproductive function of oxytocin is not limited to females, as it stimulates contractility of the seminiferous tubules, epididymis, and the prostate gland[19]. Due to its production locale in the testes, oxytocin has been studied as a paracrine regulator of the prostate gland, specifically of growth and muscle contractility[20]. In males, oxytocin has been thought to induce erection and play a role in ejaculation[19,21]. Specifically, in the prostate, oxytocin has been suggested to induce prostatic smooth muscle cell contraction[22]. Its postulated involvement in ejaculation includes stimulation of the reproductive tract to promote sperm release[19]. Oxytocin’s role in aggravating and potentially facilitating the development of benign prostatic hyperplasia, and the oxytocin-induced proliferative effect, are likely mediated through the extracellular signal regulated kinase (ERK) pathway[23].
Given its ubiquitous distribution in the CNS, the role of oxytocin in cognition and social behavior has also been studied extensively, especially over the past decade. It has been shown that oxytocin can enhance positive social interactions, and importantly can enhance trust[24]. Oxytocin activity was found to be decreased in women who suffered abuse in their youth[25]. Conversely, and perhaps related to this, a study reported increased oxytocin levels in individuals enjoying heightened levels of partner support[26]. Oxytocin also has been reported to improve social cognition[27,28]. However, it must be noted that studies on the social effects of oxytocin are somewhat inconsistent[29], suggesting that at least socially, additional factors are likely to be at play.
One of the aims of oxytocin research stems from an attempt to establish it as a tool to predict, diagnose, and potentially treat neuropsychiatric disorders[30], and has mostly focused on anxiety and depression. Oxytocin intake has been shown to reduce anxiety symptoms[31,32]. In concordance with its pivotal role during birth, the majority of the research regarding depression in humans has revolved around pregnancies and the mother’s ability to recover from postnatal depression (PND). Studies in this field have discovered lowered oxytocin in mothers with PND, and that increased oxytocin levels bestow positive effects on mothers with PND and on their interactions with infants[33,34]. On the other hand, abnormal post-prandial oxytocin secretion has also been demonstrated in women with anorexia nervosa, possibly as an adaptive response to food-related symptoms of anxiety and depression[35]. One of the emerging “hot topics” in neuropsychiatric research links oxytocin with autism[36-38], with recent studies beginning to identify oxytocin as a potential medical therapy to alleviate social anxiety caused by autism[39].
The potential regulatory role of oxytocin in other organ systems has also raised considerable interest. Oxytocin contributes to several forms of cardiovascular regulation, as it has been shown that preconditioning rats with oxytocin reduces cardiac arrhythmias[40], and that oxytocin can lower blood pressure[41], increase anti-inflammatory and antioxidant activity, and exert beneficial metabolic effects. Therefore, its cardiovascular activity seems to aim largely at restoring homeostasis.
Notably, oxytocin seems to also exert cardiovascular regulation during elevated levels of physical activity. Oxytocin levels have been shown to rise in response to exercise[42], which activates oxytocinergic projections[43] and oxytocinergic modulatory loops that adjust cardiac output, assisting in keeping cardiovascular control over the blood supply[44]. The rise in oxytocin has been traced to the lumbar spinal cord[45]. Oxytocin also reduces the rise of exercise-induced adrenocorticotropic hormone (ACTH) and cortisol[46,47], furthering support on its effects on cortisol levels. Furthermore, exogenously administered oxytocin along with exercise have been shown to protect ovariectomized rats from myocardial infarction[48]. On the other hand, its contribution to cardiovascular regulation may depend on the type or intensity of exercise, because plasma oxytocin in cyclists remains unchanged during intense exercise[49].
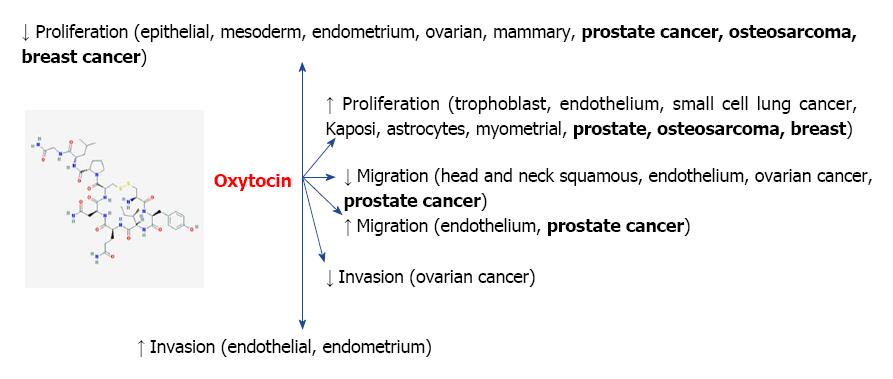
Less is understood about the connection between oxytocin and cancer, partly due to lack of adequate research in this area, and partly due to some inconsistency in the current data (Figure 2). The first link of oxytocin to cancer was reported in 1984, when oxytocin was described to be structurally and genomically related to vasopressin, an endogenous hormone that is also secreted by the pituitary, and that in addition to its physiological functions has been found to constitute a biomarker of small-cell lung cancer[50]. Furthermore, oxytocin and vasopressin are co-expressed in these cells, where they have been proposed to induce mitogenic effects[51]. Oxytocin’s link to vasopressin and its potential role as a biomarker was subsequently proposed in 1990[52]. Shortly thereafter, it was suggested that oxytocin may modulate growth in breast cancer[53], which was subsequently demonstrated[54]. These observations have instigated additional research into oxytocin’s potential involvement in various forms of cancer.
Interestingly, subsequent studies have shown that oxytocin in fact inhibits proliferation of breast cancer cell lines, such as MDA-MB231, MCF7, and T47D[55,56], as well as the canine mammary cell line CMT-U27[57], mouse mammary carcinoma cell line TS/A, and rat mammary carcinoma cell line D-R3230AC[9]. This effect was shown to be mediated via the cyclic adenosine monophosphate protein kinase A in human cell lines[58]. Importantly, anti-proliferative and tumor inhibitory properties were also observed in vivo in both rat and mouse experimental models, and attributed to both oxytocin and its analogue F314[9]. Recently, it was suggested that exercise training, by inducing oxytocin secretion, may reduce the expression of specific signaling proteins involved in breast cancer[59].
Lactation has long been linked to a reduced risk of cancer, with research dating back to as early as the 1950’s[60-63]. Worldwide, it has been shown that breastfeeding reduces the risk of both breast and uterine cancer, with prolonged durations of breastfeeding (usually involving multiple children breastfed) correlating with a progressive fall in the risks of both breast and uterine cancer[64-68]. The relationship with uterine cancer might be related to the action of oxytocin as a paracrine and endocrine hormone in lactation. Nevertheless, while the relationship between oxytocin, lactation, and breastfeeding with reduced risk of breast and uterine cancer are all well documented individually, more research needs to be conducted to determine if the relationship between oxytocin, lactation, and breastfeeding with reduced breast and uterine cancer is causal. Elucidating such a connection may establish new therapeutic targets in cancer.
In addition to breast and uterine cancer, the potential participation of oxytocin in the pathogenesis of other cancers in the reproductive system has been investigated. Oxytocin was found to inhibit the progression of ovarian carcinoma (Figure 2) both in vitro and in vivo. Using cell viability, invasion, and migration assays, it was demonstrated that oxytocin inhibited proliferation, migration and invasion of ovarian cancer cells in vitro, and its administration also attenuated the dissemination of ovarian cancer using mean tumor burden as a measure[69]. The same investigators had demonstrated in a previous study expression of the oxytocin receptor in various human ovarian carcinoma tissues and cell lines, and identified placental leucine aminopeptidase (P-LAP) as an oxytocin-degrading oxytocinase in certain adenocarcinoma tissues[70]. This team of investigators, therefore, proposed that a system involving P-LAP and oxytocin plays a role in the regulation of human endometrial adenocarcinoma, in which P-LAP exerts a functionally positive impact on carcinoma cell growth by degrading suppressive peptides such as oxytocin. More recently, these effects have also been linked with a cross-talk network between oxytocin and the stress hormone cortisol, whereby oxytocin reversed the carcinogenic effects of cortisol via autophagy (cellular self-degradation)[71]. Interestingly, pertinent to the postulated connection between oxytocin and symptoms of autism[39], oxytocin and cancer have also demonstrated an inverse relationship in autistic children[72].
Oxytocin receptors are expressed throughout the gastrointestinal (GI) tract[73], but little is known about their function in the GI tract, especially in relation to cancer. Some studies have suggested a link between oxytocin and its receptor in GI-related cancers, such as esophageal, gastric, and pancreatic cancers. For example, some studies showed an inverse relationship between the duration of breastfeeding and risk of esophageal cancer[74,75], gastric cancer[76], and pancreatic[77] cancer. In fact, Yu et al[78] showed a 54% decreased risk of developing esophageal cancer in women who breastfed for over 12 mo.
Unpublished data from our laboratory shows that the messenger ribonucleic acid (mRNA) expression of oxytocin is twofold higher in PANC-1 (a human pancreatic cancer cell line highly unresponsive to the chemotherapeutic agents, gemcitabine and 5-FU) compared to L3.6pl (a highly responsive human pancreatic cancer cell line). We also found that oxytocin receptor protein expression is also higher in PANC-1 than in L3.6pl. Further, inhibition of the oxytocin receptor decreased cell proliferation of PANC-1 and L3.6pl cells. Our analysis of data from the cBioPortal database revealed that up to 5% of pancreatic cancer patients included in The Cancer Genome Atlas showed genetic alterations (primarily upregulation of mRNA expression) in oxytocin and its receptor. Patients with these alterations had poorer survival outcomes as compared to those without these alterations. These interesting data warrant further investigation on the molecular mechanisms implicating oxytocin and its receptor in pancreatic cancer and other GI cancers.
As a role for oxytocin in the regulation of prostate function is established, its potential involvement in the development of prostate cancer has been proposed. Data from over two decades ago implicated oxytocin in the pathophysiology of benign prostatic hyperplasia, where the peptide might contribute to both the physical enlargement and dynamic tone of the gland[19]. More recently, immunohistochemical staining has detected oxytocin expression in stromal and epithelial cell lines and in tissue from patients with benign prostatic hyperplasia, which was significantly reduced in tissues of invasive prostate cancer in comparison to both benign prostatic hyperplasia tissues and normal human prostate epithelial cells[79]. This inverse relationship might implicate a fall in oxytocin levels in progression of prostate cancer. Within the prostate, oxytocin has been shown to affect gland growth both directly and via its interaction with androgen metabolism, and oxytocin concentrations are positively correlated with androgens[20,80]. Indeed, while in the absence of androgens oxytocin had no effect on prostate cancer cell lines (LNCaP and PC-3), in the presence of testosterone low oxytocin doses stimulated proliferation of PC-3 cells[81], supporting the notion that changes in levels of oxytocin in the prostate in aging and cancer may promote prostate epithelial cell proliferation. It is possible that increased levels of oxytocin might be involved in the mechanisms by which high ejaculation frequency is related to decreased risk of prostate cancer[82]. This hypothesis needs to be further investigated.
Conversely, a different study recently revealed that oxytocin increased the expression of APPL1, a protein with the ability to interact with tumor suppressor proteins. In vitro studies showed that oxytocin increased prostate cancer cell proliferation, and expression of APPL1. Analysis of serum and tissue samples identified increased oxytocin levels in the serum of prostate cancer patients, and high expression of oxytocin and its receptor in prostate tissues collected from prostate cancer patients in comparison to those collected from patients without prostate cancer. The oxytocin receptor has also been implicated in the migration of prostate cancer cells, and possibly modulation of prostate cancer metastasis[83]. Taken together, these observations of oxytocin in prostate cancer cells both in vivo and in vitro, suggest that oxytocin could serve as a prostate cancer biomarker[84].
Several explanations have been offered for the apparent differences in the data from different studies regarding the role of oxytocin in prostate cancer. One explanation is the notable difference in the numbers of participants involved in each study. Secondly, some of the studies included prostate cancer patients that had undergone neo-adjuvant therapy, which can affect oxytocin levels[85]. Thirdly, oxytocin is likely to activate a wide range of signaling mechanisms to elicit variable cellular responses, possibly depending on the density or precise localization of the oxytocin receptor on the plasma membrane[86]. This may also account for the dichotomy in the observations reported regarding the role of oxytocin in cancer. Clearly, additional studies are needed to elucidate the involvement of oxytocin and oxytocin receptor in progression or regression of human prostate cancer.
There is clearly some evidence implicating oxytocin in carcinogenesis, although its precise effect and underlying mechanisms are still unclear. It is possible that in some individuals, cell types, or types of cancers, oxytocin may not act as a sole regulator of carcinogenesis, but may mediate or modulate other coexisting factors in the microenvironment.
For example, research generally supports the hypothesis that exercise can inhibit the progression of cancer[87-89]. The positive impact of exercise in blunting development of cancer and facilitating recovery may potentially be partly mediated through oxytocin. For example, oxytocin has been proposed to mediate an exercise-induced reduction in the expression of specific signaling molecules involved in breast cancer[59]. It has also recently been shown in vivo that the combination of exogenous oxytocin with exercise improves cardiac function, which might be associated with improved cancer survival. Cardiac dysfunction and cancer have such a strong link that it has even spawned its own subspecialty in cardio-oncology[90-93], although much of the research has centered around long-term survival and cardiac complications in cancer patients. While this potential mediating effect of oxytocin has been hypothesized[94], it appears that little primary research has been conducted to address this postulation. The ability to knockout or silence oxytocin is available, and its social effects are already documented[95,96]. Thus, a thorough study is certainly possible. Similarly, oxytocin may mediate the inhibitory effects of lactation on development of breast cancer, and of ejaculation on development of prostate cancer, and additional studies could prove to be invaluable in revealing its involvement.
It must be noted that most systems and bodily processes in living organisms are tightly inter-connected. Untangling this complexity in the presence of confounding elements is often difficult, especially in relation to psychosocial factors. Therefore, interpretation of oxytocin’s expression and levels alone might be over-simplistic and under-informative. For example, instead of being increased as a direct response to exercise, oxytocin may be induced to assist its “companion” hormone, vasopressin, a well-known hormonal regulator of body fluid homeostasis[97]. Cortisol, the “stress hormone” and the rhythm surrounding its release has been linked to the progression and survival from various cancers[98-101], and has also been linked to oxytocin[71,72]. The ability to completely elucidate various pathways and mechanisms of actions would go a long way to showing if the established connection between the different hormones extends to cancer. Furthermore, while outside the scope of this review, there are several other diseases and pathologic states possibly linked to oxytocin. Pain, depression, and anxiety have all been linked to oxytocin[102,103]. Oxytocin’s role in depression management was mentioned previously in this article, but oxytocin also seems to be a promising target in pain management[104-107], and in immunotherapy, especially through its interactions in the gut[108].
Most knowledge of oxytocin centers on its role as a reproductive hormone. Since its discovery, its other roles have progressively become clearer, including involvement in social behavior, cardiovascular regulation, and carcinogenesis. While it is currently difficult to pinpoint and precisely define oxytocin’s oncogenic roles, it is hoped that this review will encourage greater intensity in researching the details of the role of oxytocin in cancer. Future research has a number of plausible and exciting directions to follow and will hopefully clarify some of the ambiguities concerning the role of oxytocin in cancer.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hosseini M, Vinh-Hung V, Wion D S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ
| 1. | Viero C, Shibuya I, Kitamura N, Verkhratsky A, Fujihara H, Katoh A, Ueta Y, Zingg HH, Chvatal A, Sykova E. REVIEW: Oxytocin: Crossing the bridge between basic science and pharmacotherapy. CNS Neurosci Ther. 2010;16:e138-e156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 2. | Grigor’eva ME, Golubeva MG. Oxytocin: Structure, synthesis, receptors, and basic effects. Neurochem J. 2010;4:75-83. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Lopatina O, Inzhutova A, Salmina AB, Higashida H. The roles of oxytocin and CD38 in social or parental behaviors. Front Neurosci. 2013;6:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2046] [Cited by in RCA: 2140] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 5. | Chatterjee O, Patil K, Sahu A, Gopalakrishnan L, Mol P, Advani J, Mukherjee S, Christopher R, Prasad TS. An overview of the oxytocin-oxytocin receptor signaling network. J Cell Commun Signal. 2016;10:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Kim SH, MacIntyre DA, Hanyaloglu AC, Blanks AM, Thornton S, Bennett PR, Terzidou V. The oxytocin receptor antagonist, Atosiban, activates pro-inflammatory pathways in human amnion via G(αi) signalling. Mol Cell Endocrinol. 2016;420:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Kim SH, Pohl O, Chollet A, Gotteland JP, Fairhurst AD, Bennett PR, Terzidou V. Differential Effects of Oxytocin Receptor Antagonists, Atosiban and Nolasiban, on Oxytocin Receptor-Mediated Signaling in Human Amnion and Myometrium. Mol Pharmacol. 2017;91:403-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Reversi A, Rimoldi V, Marrocco T, Cassoni P, Bussolati G, Parenti M, Chini B. The oxytocin receptor antagonist atosiban inhibits cell growth via a “biased agonist” mechanism. J Biol Chem. 2005;280:16311-16318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Cassoni P, Sapino A, Papotti M, Bussolati G. Oxytocin and oxytocin-analogue F314 inhibit cell proliferation and tumor growth of rat and mouse mammary carcinomas. Int J Cancer. 1996;66:817-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Vrachnis N, Malamas FM, Sifakis S, Deligeoroglou E, Iliodromiti Z. The oxytocin-oxytocin receptor system and its antagonists as tocolytic agents. Int J Endocrinol. 2011;2011:350546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Arrowsmith S, Wray S. Oxytocin: its mechanism of action and receptor signalling in the myometrium. J Neuroendocrinol. 2014;26:356-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 12. | Zingg HH, Lefebvre DL. Oxytocin and vasopressin gene expression during gestation and lactation. Brain Res. 1988;464:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 104] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | McNeilly AS, Robinson IC, Houston MJ, Howie PW. Release of oxytocin and prolactin in response to suckling. Br Med J (Clin Res Ed). 1983;286:257-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 151] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Gu H, Rong L, Sha JY. [Changes in blood oxytocin levels in cases of pregnancy induced hypertension]. Zhonghua Fuchanke Zazhi. 1994;29:268-270, 316. [PubMed] |
| 15. | Phie J, Haleagrahara N, Newton P, Constantinoiu C, Sarnyai Z, Chilton L, Kinobe R. Prolonged Subcutaneous Administration of Oxytocin Accelerates Angiotensin II-Induced Hypertension and Renal Damage in Male Rats. PLoS One. 2015;10:e0138048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Vargas-Martínez F, Schanler RJ, Abrams SA, Hawthorne KM, Landers S, Guzman-Bárcenas J, Muñoz O, Henriksen T, Petersson M, Uvnäs-Moberg K. Oxytocin, a main breastfeeding hormone, prevents hypertension acquired in utero: A therapeutics preview. Biochim Biophys Acta. 2017;1861:3071-3084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Light KC, Grewen KM, Amico JA. More frequent partner hugs and higher oxytocin levels are linked to lower blood pressure and heart rate in premenopausal women. Biol Psychol. 2005;69:5-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 229] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 18. | Petersson M, Alster P, Lundeberg T, Uvnäs-Moberg K. Oxytocin causes a long-term decrease of blood pressure in female and male rats. Physiol Behav. 1996;60:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 166] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Thackare H, Nicholson HD, Whittington K. Oxytocin--its role in male reproduction and new potential therapeutic uses. Hum Reprod Update. 2006;12:437-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Nicholson HD. Oxytocin: a paracrine regulator of prostatic function. Rev Reprod. 1996;1:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Andersson KE. Mechanisms of penile erection and basis for pharmacological treatment of erectile dysfunction. Pharmacol Rev. 2011;63:811-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 251] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 22. | Bodanszky M, Sharaf H, Roy JB, Said SI. Contractile activity of vasotocin, oxytocin, and vasopressin on mammalian prostate. Eur J Pharmacol. 1992;216:311-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Xu H, Fu S, Chen Y, Chen Q, Gu M, Liu C, Qiao Z, Zhou J, Wang Z. Oxytocin: its role in benign prostatic hyperplasia via the ERK pathway. Clin Sci (Lond). 2017;131:595-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Misrani A, Tabassum S, Long C. Oxytocin system in neuropsychiatric disorders: Old concept, new insights. Shengli Xuebao. 2017;69:196-206. [PubMed] |
| 25. | Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol Psychiatry. 2009;14:954-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 335] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 26. | Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom Med. 2005;67:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 250] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 27. | Guastella AJ, Mitchell PB, Mathews F. Oxytocin enhances the encoding of positive social memories in humans. Biol Psychiatry. 2008;64:256-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 261] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 28. | Bartz JA, Zaki J, Bolger N, Hollander E, Ludwig NN, Kolevzon A, Ochsner KN. Oxytocin selectively improves empathic accuracy. Psychol Sci. 2010;21:1426-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 290] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 29. | Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 556] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 30. | Marazziti D, Catena Dell’osso M. The role of oxytocin in neuropsychiatric disorders. Curr Med Chem. 2008;15:698-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | MacDonald K, Feifel D. Oxytocin’s role in anxiety: a critical appraisal. Brain Res. 2014;1580:22-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 32. | Alvares GA, Chen NT, Balleine BW, Hickie IB, Guastella AJ. Oxytocin selectively moderates negative cognitive appraisals in high trait anxious males. Psychoneuroendocrinology. 2012;37:2022-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Moura D, Canavarro MC, Figueiredo-Braga M. Oxytocin and depression in the perinatal period-a systematic review. Arch Womens Ment Health. 2016;19:561-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Mah BL. Oxytocin, Postnatal Depression, and Parenting: A Systematic Review. Harv Rev Psychiatry. 2016;24:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Lawson EA, Holsen LM, Santin M, DeSanti R, Meenaghan E, Eddy KT, Herzog DB, Goldstein JM, Klibanski A. Postprandial oxytocin secretion is associated with severity of anxiety and depressive symptoms in anorexia nervosa. J Clin Psychiatry. 2013;74:e451-e457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Vanya M, Szucs S, Vetro A, Bartfai G. The potential role of oxytocin and perinatal factors in the pathogenesis of autism spectrum disorders - review of the literature. Psychiatry Res. 2017;247:288-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Zhang R, Zhang HF, Han JS, Han SP. Genes Related to Oxytocin and Arginine-Vasopressin Pathways: Associations with Autism Spectrum Disorders. Neurosci Bull. 2017;33:238-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 38. | Ooi YP, Weng SJ, Kossowsky J, Gerger H, Sung M. Oxytocin and Autism Spectrum Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Pharmacopsychiatry. 2017;50:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Kanat M, Spenthof I, Riedel A, van Elst LT, Heinrichs M, Domes G. Restoring effects of oxytocin on the attentional preference for faces in autism. Transl Psychiatry. 2017;7:e1097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 40. | Faghihi M, Alizadeh AM, Khori V, Khodayari V, Moradi S. Preconditioning effects of oxytocin in reducing cardiac arrhythmias in a rat heart regional ischemia-reperfusion model. Physiol Pharmacol. 2012;16:393-403. |
| 41. | Gutkowska J, Jankowski M, Antunes-Rodrigues J. The role of oxytocin in cardiovascular regulation. Braz J Med Biol Res. 2014;47:206-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 42. | Hew-Butler T, Noakes TD, Soldin SJ, Verbalis JG. Acute changes in endocrine and fluid balance markers during high-intensity, steady-state, and prolonged endurance running: unexpected increases in oxytocin and brain natriuretic peptide during exercise. Eur J Endocrinol. 2008;159:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Martins AS, Crescenzi A, Stern JE, Bordin S, Michelini LC. Hypertension and exercise training differentially affect oxytocin and oxytocin receptor expression in the brain. Hypertension. 2005;46:1004-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Michelini LC. Differential effects of vasopressinergic and oxytocinergic pre-autonomic neurons on circulatory control: reflex mechanisms and changes during exercise. Clin Exp Pharmacol Physiol. 2007;34:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Stebbins CL, Ortiz-Acevedo A. The exercise pressor reflex is attenuated by intrathecal oxytocin. Am J Physiol. 1994;267:R909-R915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 46. | Coiro V, Passeri M, Davoli C, Bacchi-Modena A, Bianconi L, Volpi R, Chiodera P. Oxytocin reduces exercise-induced ACTH and cortisol rise in man. Acta Endocrinol (Copenh). 1988;119:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Cardoso C, Ellenbogen MA, Orlando MA, Bacon SL, Joober R. Intranasal oxytocin attenuates the cortisol response to physical stress: a dose-response study. Psychoneuroendocrinology. 2013;38:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 48. | Bulut EC, Abueid L, Ercan F, Süleymanoğlu S, Ağırbaşlı M, Yeğen BÇ. Treatment with oestrogen-receptor agonists or oxytocin in conjunction with exercise protects against myocardial infarction in ovariectomized rats. Exp Physiol. 2016;101:612-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 49. | Chicharro JL, Hoyos J, Bandrés F, Gómez Gallego F, Pérez M, Lucía A. Plasma oxytocin during intense exercise in professional cyclists. Horm Res. 2001;55:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Sausville E, Carney D, Battey J. The human vasopressin gene is linked to the oxytocin gene and is selectively expressed in a cultured lung cancer cell line. J Biol Chem. 1985;260:10236-10241. [PubMed] |
| 51. | Péqueux C, Keegan BP, Hagelstein MT, Geenen V, Legros JJ, North WG. Oxytocin- and vasopressin-induced growth of human small-cell lung cancer is mediated by the mitogen-activated protein kinase pathway. Endocr Relat Cancer. 2004;11:871-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Legros JJ, Geenen V, Carvelli T, Martens H, Andre M, Corhay JL, Radermecker M, Zangerle PF, Sassolas G, Gharib C. Neurophysins as markers of vasopressin and oxytocin release. A study in carcinoma of the lung. Horm Res. 1990;34:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 53. | Taylor AH, Ang VT, Jenkins JS, Silverlight JJ, Coombes RC, Luqmani YA. Interaction of vasopressin and oxytocin with human breast carcinoma cells. Cancer Res. 1990;50:7882-7886. [PubMed] |
| 54. | Cassoni P, Sapino A, Negro F, Bussolati G. Oxytocin inhibits proliferation of human breast cancer cell lines. Virchows Arch. 1994;425:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Cassoni P, Sapino A, Marrocco T, Chini B, Bussolati G. Oxytocin and oxytocin receptors in cancer cells and proliferation. J Neuroendocrinol. 2004;16:362-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Cassoni P, Catalano MG, Sapino A, Marrocco T, Fazzari A, Bussolati G, Fortunati N. Oxytocin modulates estrogen receptor alpha expression and function in MCF7 human breast cancer cells. Int J Oncol. 2002;21:375-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 57. | Benavente MA, Bianchi CP, Imperiale F, Aba MA. Antiproliferative Effects of Oxytocin and Desmopressin on Canine Mammary Cancer Cells. Front Vet Sci. 2016;3:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Cassoni P, Sapino A, Fortunati N, Munaron L, Chini B, Bussolati G. Oxytocin inhibits the proliferation of MDA-MB231 human breast-cancer cells via cyclic adenosine monophosphate and protein kinase A. Int J Cancer. 1997;72:340-344. [PubMed] |
| 59. | Alizadeh AM, Heydari Z, Rahimi M, Bazgir B, Shirvani H, Alipour S, Heidarian Y, Khalighfard S, Isanejad A. Oxytocin mediates the beneficial effects of the exercise training on breast cancer. Exp Physiol. 2018;103:222-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 60. | King AG. Suppression of lactation and its relation to cancer of the breast. J Med Assoc Ga. 1958;47:342-344. [PubMed] |
| 61. | Lewison EF. Breast cancer and pregnancy or lactation. Int Abstr Surg. 1954;99:417-424. [PubMed] |
| 62. | Huggins C, Dao TL. Lactation induced by luteotrophin in women with mammary cancer; growth of the breast of the human male following estrogenic treatment. Cancer Res. 1954;14:303-306. [PubMed] |
| 63. | Abrao A, Da Silva Neto JB, Mirra AP. Cancer of the breast in pregnancy and lactation; study of 10 cases. Rev Paul Med. 1954;45:563-570. [PubMed] |
| 64. | Sugawara Y, Kakizaki M, Nagai M, Tomata Y, Hoshi R, Watanabe I, Nishino Y, Kuriyama S, Tsuji I. Lactation pattern and the risk for hormone-related female cancer in Japan: the Ohsaki Cohort Study. Eur J Cancer Prev. 2013;22:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 65. | Su D, Pasalich M, Lee AH, Binns CW. Ovarian cancer risk is reduced by prolonged lactation: a case-control study in southern China. Am J Clin Nutr. 2013;97:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Jordan I, Hebestreit A, Swai B, Krawinkel MB. Breast cancer risk among women with long-standing lactation and reproductive parameters at low risk level: a case-control study in Northern Tanzania. Breast Cancer Res Treat. 2013;142:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 67. | Zheng T, Holford TR, Mayne ST, Owens PH, Zhang Y, Zhang B, Boyle P, Zahm SH. Lactation and breast cancer risk: a case-control study in Connecticut. Br J Cancer. 2001;84:1472-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Purwanto H, Sadjimin T, Dwiprahasto I. Lactation and the risk of breast cancer. Gan To Kagaku Ryoho. 2000;27 Suppl 2:474-481. [PubMed] |
| 69. | Morita T, Shibata K, Kikkawa F, Kajiyama H, Ino K, Mizutani S. Oxytocin inhibits the progression of human ovarian carcinoma cells in vitro and in vivo. Int J Cancer. 2004;109:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Suzuki Y, Shibata K, Kikkawa F, Kajiyama H, Ino K, Nomura S, Tsujimoto M, Mizutani S. Possible role of placental leucine aminopeptidase in the antiproliferative effect of oxytocin in human endometrial adenocarcinoma. Clin Cancer Res. 2003;9:1528-1534. [PubMed] |
| 71. | Mankarious A, Dave F, Pados G, Tsolakidis D, Gidron Y, Pang Y, Thomas P, Hall M, Karteris E. The pro-social neurohormone oxytocin reverses the actions of the stress hormone cortisol in human ovarian carcinoma cells in vitro. Int J Oncol. 2016;48:1805-1814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 72. | Corbett BA, Bales KL, Swain D, Sanders K, Weinstein TA, Muglia LJ. Comparing oxytocin and cortisol regulation in a double-blind, placebo-controlled, hydrocortisone challenge pilot study in children with autism and typical development. J Neurodev Disord. 2016;8:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Monstein HJ, Grahn N, Truedsson M, Ohlsson B. Oxytocin and oxytocin-receptor mRNA expression in the human gastrointestinal tract: a polymerase chain reaction study. Regul Pept. 2004;119:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 74. | Lindblad M, García Rodríguez LA, Chandanos E, Lagergren J. Hormone replacement therapy and risks of oesophageal and gastric adenocarcinomas. Br J Cancer. 2006;94:136-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 75. | Cronin-Fenton DP, Murray LJ, Whiteman DC, Cardwell C, Webb PM, Jordan SJ, Corley DA, Sharp L, Lagergren J; Barrett’s Esophagus, Adenocarcinoma Consortium (BEACON) Investigators. Reproductive and sex hormonal factors and oesophageal and gastric junction adenocarcinoma: a pooled analysis. Eur J Cancer. 2010;46:2067-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 76. | Inoue H, Matsuyama A, Mimori K, Ueo H, Mori M. Prognostic score of gastric cancer determined by cDNA microarray. Clin Cancer Res. 2002;8:3475-3479. [PubMed] |
| 77. | Skinner HG, Michaud DS, Colditz GA, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Parity, reproductive factors, and the risk of pancreatic cancer in women. Cancer Epidemiol Biomarkers Prev. 2003;12:433-438. [PubMed] |
| 78. | Yu H, Liu G, Zhao P, Zhu L. Hormonal and reproductive factors and risk of esophageal cancer in Chinese postmenopausal women: a case-control study. Asian Pac J Cancer Prev. 2011;12:1953-1956. [PubMed] |
| 79. | Whittington K, Assinder S, Gould M, Nicholson H. Oxytocin, oxytocin-associated neurophysin and the oxytocin receptor in the human prostate. Cell Tissue Res. 2004;318:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 80. | Biswas M, Thackare H, Jones MK, Bowen-Simpkins P. Lymphocytic hypophysitis and headache in pregnancy. BJOG. 2002;109:1184-1186. [PubMed] |
| 81. | Whittington K, Connors B, King K, Assinder S, Hogarth K, Nicholson H. The effect of oxytocin on cell proliferation in the human prostate is modulated by gonadal steroids: implications for benign prostatic hyperplasia and carcinoma of the prostate. Prostate. 2007;67:1132-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 82. | Leitzmann MF, Platz EA, Stampfer MJ, Willett WC, Giovannucci E. Ejaculation frequency and subsequent risk of prostate cancer. JAMA. 2004;291:1578-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 83. | Zhong M, Boseman ML, Millena AC, Khan SA. Oxytocin induces the migration of prostate cancer cells: involvement of the Gi-coupled signaling pathway. Mol Cancer Res. 2010;8:1164-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 84. | Xu H, Fu S, Chen Q, Gu M, Zhou J, Liu C, Chen Y, Wang Z. The function of oxytocin: a potential biomarker for prostate cancer diagnosis and promoter of prostate cancer. Oncotarget. 2017;8:31215-31226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 85. | Nicholson HD, Jenkin L. Oxytocin and prostatic function. Adv Exp Med Biol. 1995;395:529-538. [PubMed] |
| 86. | Rimoldi V, Reversi A, Taverna E, Rosa P, Francolini M, Cassoni P, Parenti M, Chini B. Oxytocin receptor elicits different EGFR/MAPK activation patterns depending on its localization in caveolin-1 enriched domains. Oncogene. 2003;22:6054-6060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 87. | Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. J Clin Oncol. 2005;23:3830-3842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 393] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 88. | Giovannucci EL, Liu Y, Leitzmann MF, Stampfer MJ, Willett WC. A prospective study of physical activity and incident and fatal prostate cancer. Arch Intern Med. 2005;165:1005-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 89. | Liu Y, Hu F, Li D, Wang F, Zhu L, Chen W, Ge J, An R, Zhao Y. Does physical activity reduce the risk of prostate cancer? A systematic review and meta-analysis. Eur Urol. 2011;60:1029-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 90. | Tajiri K, Aonuma K, Sekine I. Cardio-oncology: a multidisciplinary approach for detection, prevention and management of cardiac dysfunction in cancer patients. Jpn J Clin Oncol. 2017;47:678-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 91. | Ryzhov S, Francis S, Sawyer DB. Cardiac Dysfunction Due to Cancer Therapy: Finding New Directions. Circ Res. 2016;119:1055-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 92. | Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, Gravdehaug B, von Knobelsdorff-Brenkenhoff F, Bratland Å, Storås TH, Hagve TA, Røsjø H, Steine K, Geisler J, Omland T. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671-1680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 491] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 93. | Ewer MS, Swain SM, Cardinale D, Fadol A, Suter TM. Cardiac dysfunction after cancer treatment. Tex Heart Inst J. 2011;38:248-252. [PubMed] |
| 94. | Imanieh MH, Bagheri F, Alizadeh AM, Ashkani-Esfahani S. Oxytocin has therapeutic effects on cancer, a hypothesis. Eur J Pharmacol. 2014;741:112-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 95. | Winslow JT, Insel TR. The social deficits of the oxytocin knockout mouse. Neuropeptides. 2002;36:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 200] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 96. | Nakajima M, Görlich A, Heintz N. Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons. Cell. 2014;159:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 97. | el-Sayed MS, Davies B, Morgan DB. Vasopressin and plasma volume response to submaximal and maximal exercise in man. J Sports Med Phys Fitness. 1990;30:420-425. [PubMed] |
| 98. | Fabre B, Grosman H, Gonzalez D, Machulsky NF, Repetto EM, Mesch V, Lopez MA, Mazza O, Berg G. Prostate Cancer, High Cortisol Levels and Complex Hormonal Interaction. Asian Pac J Cancer Prev. 2016;17:3167-3171. [PubMed] |
| 99. | Schrepf A, Thaker PH, Goodheart MJ, Bender D, Slavich GM, Dahmoush L, Penedo F, DeGeest K, Mendez L, Lubaroff DM. Diurnal cortisol and survival in epithelial ovarian cancer. Psychoneuroendocrinology. 2015;53:256-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 100. | Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, Spiegel D, Salmon P. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun. 2013;30 Suppl:S163-S170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 193] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 101. | Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. 2010;6:1863-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 300] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 102. | Smith HR. Depression in cancer patients: Pathogenesis, implications and treatment (Review). Oncol Lett. 2015;9:1509-1514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 280] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 103. | Li XM, Xiao WH, Yang P, Zhao HX. Psychological distress and cancer pain: Results from a controlled cross-sectional survey in China. Sci Rep. 2017;7:39397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 104. | Rash JA, Aguirre-Camacho A, Campbell TS. Oxytocin and pain: a systematic review and synthesis of findings. Clin J Pain. 2014;30:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 105. | Bos PA, Montoya ER, Hermans EJ, Keysers C, van Honk J. Oxytocin reduces neural activity in the pain circuitry when seeing pain in others. Neuroimage. 2015;113:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 106. | Rash JA, Campbell TS. Future directions for the investigation of intranasal oxytocin and pain: Comment on: Oxytocin nasal spray in fibromyalgic patients (Rheumatol Int. E-pub ahead of print. doi: 10.1007/s00296-014-2953-y). Rheumatol Int. 2014;34:1177-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 107. | Poisbeau P, Grinevich V, Charlet A. Oxytocin Signaling in Pain: Cellular, Circuit, System, and Behavioral Levels. Curr Top Behav Neurosci. 2018;35:193-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 108. | Poutahidis T, Kearney SM, Levkovich T, Qi P, Varian BJ, Lakritz JR, Ibrahim YM, Chatzigiagkos A, Alm EJ, Erdman SE. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS One. 2013;8:e78898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 214] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 109. | Péqueux C, Breton C, Hagelstein MT, Geenen V, Legros JJ. Oxytocin receptor pattern of expression in primary lung cancer and in normal human lung. Lung Cancer. 2005;50:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 110. | Thibonnier M, Conarty DM, Preston JA, Plesnicher CL, Dweik RA, Erzurum SC. Human vascular endothelial cells express oxytocin receptors. Endocrinology. 1999;140:1301-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 152] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 111. | Amrani Y, Syed F, Huang C, Li K, Liu V, Jain D, Keslacy S, Sims MW, Baidouri H, Cooper PR. Expression and activation of the oxytocin receptor in airway smooth muscle cells: Regulation by TNFalpha and IL-13. Respir Res. 2010;11:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 112. | Amico JA, Finn FM, Haldar J. Oxytocin and vasopressin are present in human and rat pancreas. Am J Med Sci. 1988;296:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 113. | Szeto A, Sun-Suslow N, Mendez AJ, Hernandez RI, Wagner KV, McCabe PM. Regulation of the macrophage oxytocin receptor in response to inflammation. Am J Physiol Endocrinol Metab. 2017;312:E183-E189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 114. | Di Benedetto A, Sun L, Zambonin CG, Tamma R, Nico B, Calvano CD, Colaianni G, Ji Y, Mori G, Grano M. Osteoblast regulation via ligand-activated nuclear trafficking of the oxytocin receptor. Proc Natl Acad Sci USA. 2014;111:16502-16507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |