Published online Oct 10, 2017. doi: 10.5306/wjco.v8.i5.405
Peer-review started: October 23, 2016
First decision: December 20, 2016
Revised: July 6, 2017
Accepted: September 1, 2017
Article in press: September 1, 2017
Published online: October 10, 2017
Processing time: 339 Days and 7.3 Hours
To determine factors independently influencing response to ingenol mebutate therapy and assess efficacy on clinical setting of non-hypertrophic non-hyperkeratotic actinic keratosis (AK).
Consecutive patients affected by non-hypertrophic non-hyperkeratotic AKs of the face or scalp were enrolled to receive ingenol mebutate 0.015% gel on a selected skin area of 25 cm2 for 3 consecutive days. Local skin reactions were calculated at each follow up visit using a validated composite score. Efficacy was evaluated by the comparison of clinical and dermoscopic pictures before the treatment and at day 57, and classified as complete, partial and poor response.
A number of 130 patients were enrolled, of which 101 (77.7%) were treated on the face, while 29 (22.3%) on the scalp. The great majority of our study population (n = 119, 91.5%) reached at least a 75% clearance of AKs and, in particular, 58 patients (44.6%) achieved a complete response while 61 (46.9%) a partial one. Logistic backward multivariate analysis showed that facial localization, level of local skin reaction (LSR) at day 2, the highest LSR values and level of crusts at day 8 were factors independently associated with the achievement of a complete response.
Ingenol mebutate 0.015% gel, when properly applied, is more effective on the face than on the scalp and efficacy is directly associated to LSR score.
Core tip: Ingenol mebutate 0.015% gel is an effective treatment for non-hypertrophic non-hyperkeratotic actinic keratosis of face and scalp. Facial lesions are more prone to achieve a complete response to this therapy than those located on the scalp. Facial localization and the highest levels of local skin reaction, in particular the amount of crusting, are predictive for complete response to ingenol mebutate 0.015% gel therapy in a real clinical setting.
- Citation: Skroza N, Proietti I, Bernardini N, Balduzzi V, Mambrin A, Marchesiello A, Tolino E, Zuber S, La Torre G, Potenza C. Factors influencing response to ingenol mebutate therapy for actinic keratosis of face and scalp. World J Clin Oncol 2017; 8(5): 405-411
- URL: https://www.wjgnet.com/2218-4333/full/v8/i5/405.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i5.405
For a long time dermatologists have questioned if actinic keratosis (AK) should be considered as a precancerous lesion or an early squamous cell carcinoma (SCC). Apart from academic debate, it is actually clear that AKs have a low but definite potential to become invasive and even metastatic and that this risk increases over time[1].
Since it is impossible to predict which AK will progress to SCC and given the high prevalence of AKs in people with fair photo-types, chronically exposed to ultraviolet (UV) rays, treatment is recommended[2].
Conventional treatments for AK include cryotherapy, laser-therapy, surgical excision, photodynamic therapy, diclofenac 3% gel, imiquimod 5% and 5-fluorouracil creams[3,4].
Ingenol mebutate 0.015% gel, obtained by the sap of the plant Euphorbia peplus, has been recently approved in Europe for the treatment of non-hypertrophic non-hyperkeratotic AKs of face and scalp, which mainly correspond to I and II histopathologic categories[5,6].
The mechanism of action of ingenol mebutate has been partially explained with a rapid cytotoxic activity at higher concentration and with the activation of immune system at lower concentration[7]. The long-lasting immune surveillance and the clearance of single tumour cell clones within cancerization field, could justify the low recurrence rates of AKs observed after treatment[8].
To the best of our knowledge, no studies have assessed factors independently influencing the response to ingenol mebutate therapy. Efficacy data of phase III trials have not been widely confirmed on a large real clinical setting to date[9-11].
These studies reported a higher efficacy of ingenol mebutate 0.015% gel in patients experiencing more severe local skin reactions (LSRs); however they didn’t investigate how the single components of the composite LSR score could influence the response to treatment.
We conducted a prospective study to determine which factors, among age, gender, head site and LSR score, could independently predict the response to 0.015% ingenol mebutate treatment and to assess the efficacy of this therapy in a real clinical setting.
We (GLV and RP) enrolled consecutive patients, aged ≥ 18 years, affected by non-hypertrophic non-hyperkeratotic AKs of face and scalp, who were attending our outpatient clinic from April 2014 to March 2015.
The diagnosis of AK was performed both clinically and dermoscopically, respectively based on the presence of erythematous macular lesions with or without a slightly scaly surface, and on the identification of the typical red pseudonetwork, corresponding to grade I AK, or strawberry pattern, corresponding to grade II AK[12].
The presence of a skin cancer other than AK in the selected skin area was considered as an exclusion criteria. Furthermore, if at least one AK of the selected area had been treated by non-ablative methods within the previous year, patient was excluded from the study.
Ingenol mebutate 0.015% gel was applied by the same physician (GLV) for 3 consecutive days on a selected skin area of 25 cm2, which included 4 to 8 AKs.
Each enrolled patient gave written informed consent for clinical and dermoscopic digital documentation and the ethical committee approval was waived.
Clinical and dermoscopic pictures were collected at baseline and at each control visit (day 2, 3, 8, 15, 29 and 57).
Local skin reactions (LSR) score was calculated at each control visit, using a validated composite score (ranging from 0 to 24) given by the sum of 6 single scores for erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation and erosion/ulceration; with grade 0 representing no reaction while grade 4 indicating a skin reaction extending beyond the treated are[13].
Efficacy was evaluated comparing clinical and dermoscopic pictures at baseline and at day 57 and response was classified as complete, partial (≥ 75% clearance) or poor (< 75% clearance).
Statistical analyses were performed using the IBM SPSS 21.0 package (Statistical Package for Social Sciences, SPSS Inc., Chicago, Ill.).
Data is expressed as mean standard deviation. To analyse factors influencing efficacy of 0.015% ingenol mebutate therapy, we used Spearman’s rho coefficient to assess significant correlations, which were subsequently quantified via univariate logistic regression. Furthermore, a logistic multivariate regression backward model was constructed to identify major independent factors that showed a significant difference (P < 0.10) on univariate analysis, that have an influence on complete response. The statistical significance was set at P < 0.05.
Demographic and efficacy data are listed in Table 1. A number of 130 patients were enrolled, 91 (70.0%) were males and 39 (30.0%) were females, with a mean age (standard deviation) of 72.2 (10.3) years. All the patients completed the 3 applications of ingenol mebutate 0.015% gel, as scheduled; the majority, 101 (77.7%) were treated on the face, while 29 (22.3%) on the scalp.
| Factors | Value | |
| Age (yr), mean ± SD | 72.2 ± 10.3 | |
| Gender | M | 91 (70) |
| F | 39 (30) | |
| Total | 130 | |
| Head site | Face | 101 (77.7) |
| Scalp | 29 (22.3) | |
| Total | 130 | |
| Response | Poor | 11 (8.5) |
| Partial | 61 (46.9) | |
| Complete | 58 (44.6) | |
| Total | 130 |
Regarding efficacy, the great majority of our study population (119, 91.5%) reached at least a 75% clearance of AKs, in particular 58 patients (44.6%) achieved a complete response and 61 (46.9%) a partial one; while poor responders were only 11 (8.5%).
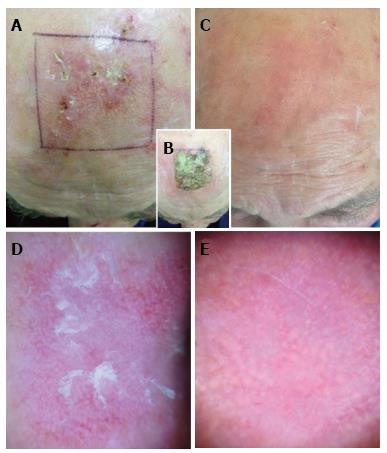
Figure 1 shows the clinical and dermoscopic pictures of a patient treated on the scalp, before and after the therapy.
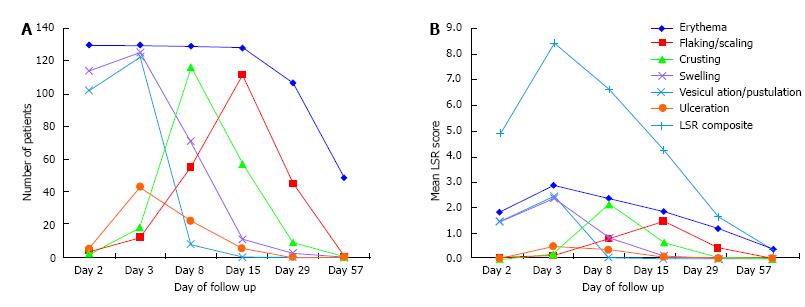
Figure 2 and Table 2 report data about the "number of patients with positive scores" and "mean values" of LSR composite and single scores at each follow up visit.
| Day 2 | Day 3 | Day 8 | Day 15 | Day 29 | Day 57 | ||
| Erythema | n (%) | 130 (100) | 130 (100) | 129 (99.2) | 128 (98.5) | 107 (82.3) | 49 (37.7) |
| mean ± SD | 1.82 ± 0.68 | 2.87 ± 0.58 | 2.38 ± 0.78 | 1.86 ± 0.81 | 1.19 ± 0.77 | 0.38 ± 0.50 | |
| Flaking/scaling | n (%) | 4 (3.1) | 12 (9.2) | 55 (42.3) | 112 (86.2) | 45 (34.6) | 0 |
| mean ± SD | 0.05 ± 0.28 | 0.11 ± 0.39 | 0.78 ± 1.00 | 1.49 ± 0.87 | 0.43 ± 0.65 | 0 | |
| Crusting | n (%) | 2 (1.5) | 18 (13.8) | 116 (89.2) | 57 (43.8) | 9 (6.9) | 0 |
| mean ± SD | 0.02 ± 0.12 | 0.17 ± 0.45 | 2.16 ± 0.98 | 0.65 ± 0.89 | 0.10 ± 0.39 | 0 | |
| Swelling | n (%) | 114 (87.7) | 125 (96.2) | 71 (54.6) | 11 (8.5) | 2 (1.5) | 0 |
| mean ± SD | 1.48 ± 0.82 | 2.38 ± 1.08 | 0.85 ± 0.98 | 0.13 ± 0.55 | 0.02 ± 0.12 | 0 | |
| Vesiculation/pustulation | n (%) | 102 (78.5) | 123 (94.6) | 8 (6.2) | 0 | 0 | 0 |
| mean ± SD | 1.48 ± 0.93 | 2.45 ± 0.86 | 0.06 ± 0.24 | 0 | 0 | 0 | |
| Ulceration | n (%) | 5 (3.8) | 43 (33.1) | 22 (16.9) | 5 (3.8) | 0 | 0 |
| mean ± SD | 0.04 ± 0.19 | 0.48 ± 0.74 | 0.36 ± 0.90 | 0.08 ± 0.45 | 0 | 0 | |
| LSR composite | mean ± SD | 4.89 ± 2.14 | 8.43 ± 2.38 | 6.62 ± 2.44 | 4.25 ± 1.72 | 1.66 ± 1.28 | 0.35 ± 0.49 |
Each patient enrolled experienced at least one LSR, but no one reported systemic symptoms.
The highest number of patients involved and the highest mean scores were reached at day 3 for both composite and all single scores, with the exception of crusting and flaking/scaling, reaching the highest level at day 8 and 15, respectively.
These 2 components were the less represented at day 2 [4 patients (3.1%) had flaking/scaling and only 2 (1.5%) had crusts, with mean values of 0.05 ± 0.28 and 0.02 ± 0.12, respectively] and totally disappeared in the whole population since day 57.
Erythema was the only LSR component involving the entire study population (at day 2 and 3) and the only, still present at day 57 in 49 patients (37.7%), with a mean score of 0.38 ± 0.50.
Swelling reached the highest levels at day 2 and 3 [114 (87.7%) and 125 (96.2%) patients, with 1.48 ± 0.82 and 2.38 ± 1.08 mean scores, respectively], but quickly reduced afterward, becoming totally absent since day 57.
Grade 4 swelling was observed in 23 (17.7%) patients and presented as periorbital edema following the application of ingenol mebutate gel on forehead and temporal areas; it resolved within day 15 in all cases.
Vesiculation/pustulation were the first signs to disappear, being widely present at day 2 and 3 [102 (78.5%) and 123 (94.6%) patients, with 1.48 ± 0.93 and 2.45 ± 0.86 mean scores, respectively], but only observable in 8 patients (6.2%) at day 8 and completely absent since day 15.
Ulceration was the least observed LSR component, being present in a maximum of 43 patients (33.1%) at day 3 and early disappearing in the entire population since day 29.
Spearman rho analysis highlighted significant correlations among response and gender, head site and the maximum level of the LSR composite score (ρ = 0.189, P = 0.031; ρ = -0.258, P = 0.003; ρ = 0.449, P < 0.001, respectively).
Furthermore, all the maximum levels of single scores, but flaking/scaling, resulted to be correlated to response (erythema: ρ = 0.351, P < 0.001; vesiculation: ρ = 0.329, P < 0.001; crusting: ρ = 0.255, P = 0.003; swelling: ρ = 0.365, P < 0.001; ulceration: ρ = 0.194, P = 0.027).
Regarding the single follow up visits, a significant correlation with response was reported for LSR composite score at day 2, 3, 8 and 15 (ρ = 0.455, P < 0.001; ρ = 0.484, P < 0.001; ρ = 0.325, P < 0.001; ρ = 0.234, P = 0.007, respectively), for erythema at every follow up visit (day 2: ρ = 0.400, P < 0.001; day 3: ρ = 0.351, P < 0.001; day 8: ρ = 0.314, P < 0.001; day 15: ρ = 0.270, P = 0.002; day 29: ρ = 0.282, P = 0.001; day 57: ρ = 0.189, P = 0.032), for crusting, swelling, vesiculation/pustulation and ulceration at days 3 (ρ = 0.180, P = 0.041; ρ = 0.372, P < 0.001; ρ = 0.329, P < 0.001; ρ = 0.215, P = 0.014, respectively) for swelling and vesiculation at day 2 (ρ = 0.357, P < 0.001; ρ = 0.418, P < 0.001, respectively) and for crusting and swelling at day 8 (ρ = 0.288, P = 0.001; ρ = 0.237, P = 0.007, respectively).
The univariate logistic regression analysis confirmed that the factors highlighted by Spearman’s correlation were all good predictors of complete response to ingenol mebutate 0.015% therapy, with the exclusion of crusting at day 3 and the highest values of ulceration (OR = 2.32, 95%CI: 0.99-5.46, P = 0.053 and OR = 1.35, 95%CI: 0.93-1.96, P = 0.113, respectively) (Table 3).
| OR | 95%CI for OR | P value | ||||
| Lower | Upper | |||||
| Gender | 2.30 | 1.07 | 4.94 | 0.033a | ||
| Head site | 4.07 | 1.53 | 10.83 | 0.005a | ||
| Max values | LSR composite | 1.55 | 1.27 | 1.89 | < 0.001a | |
| Erythema | 3.90 | 1.86 | 8.19 | < 0.001a | ||
| Crusting | 1.85 | 1.18 | 2.92 | 0.008a | ||
| Swelling | 2.24 | 1.50 | 3.35 | < 0.001a | ||
| Vesiculation/pustulation | 2.76 | 1.55 | 4.94 | 0.001a | ||
| Day 2 | LSR composite | 1.70 | 1.36 | 2.12 | < 0.001a | |
| Erythema | 3.83 | 2.05 | 7.17 | < 0.001a | ||
| Vesiculation/pustulation | 2.82 | 1.74 | 4.56 | < 0.001a | ||
| Swelling | 2.73 | 1.64 | 4.55 | < 0.001a | ||
| Day 3 | LSR composite | 1.65 | 1.34 | 2.05 | < 0.001a | |
| Erythema | 3.90 | 1.86 | 8.19 | < 0.001a | ||
| Vesiculation/pustulation | 2.76 | 1.55 | 4.94 | 0.001a | ||
| Swelling | 2.22 | 1.51 | 3.28 | < 0.001a | ||
| Ulceration | 1.72 | 1.06 | 2.79 | 0.028a | ||
| Day 8 | LSR composite | 1.25 | 1.07 | 1.47 | 0.006a | |
| Erythema | 2.44 | 1.45 | 4.13 | 0.001a | ||
| Swelling | 1.55 | 1.06 | 2.25 | 0.022a | ||
| Crusting | 1.76 | 1.17 | 2.64 | 0.006a | ||
| Day 15 | LSR composite | 1.27 | 1.02 | 1.59 | 0.030a | |
| Erythema | 1.93 | 1.22 | 3.05 | 0.005a | ||
| Day 29 | Erythema | 2.06 | 1.26 | 3.37 | 0.004a | |
| Day 57 | Erythema | 2.03 | 1.01 | 4.09 | 0.047a | |
More specifically, females were 2 times more likely to risk facial lesions than males, and were almost 4 times more likely to achieve a complete response than scalp ones.
Concerning local skin reactions, both the maximum levels and the values at day 2, 3, 8 and 15 of the composite score were associated with increased odds to achieve a complete response, ranging from 1.27 to 1.70.
Similarly, for erythema, both the maximum values and the levels at each follow up visit were associated with a complete response.
The maximum levels of crusting, swelling and vesiculation/pustulation gave also an increased odd to achieve a complete response, as well as the scores of swelling and vesiculation/pustulation at day 2 and 3 and of swelling and crusting at day 8.
Finally, ulceration at day 3 was also predictive of complete response to therapy.
Multivariate backward logistic regression analysis showed that patients with facial lesions were almost 5 times more likely to achieve a complete response than those treated on the scalp (OR = 5.19, 95%CI: 1.51-17.86, P = 0.009); LSR composite score at day 2 resulted as a predictive factor of complete response, with 14.6% higher odds for each point of score added (OR = 1.46, 95%CI: 1.08-1.97, P = 0.014). Furthermore, also the maximum level of LSR composite score was associated with complete response to ingenol mebutate therapy, but with a lower statistical significance (OR = 1.50, 95%CI: 1.02-2.21, P = 0.038). Finally, regarding single scores, we found that patients with higher crusting reactions at day 8 were more likely to achieve a complete response, with 19.4% higher odds for each point of score added (OR = 1.94, 95%CI: 1.18-3.20, P = 0.009) (Table 4).
Ingenol mebutate gel was recently introduced as a safe and effective therapeutic option for non-hypertrophic non-hyperkeratotic AK at the dosage of 0.015% for face and scalp[14,15].
Phase III trials reported complete clearance rates of 42.2% and partial response rates of 63.9%, for the treatment of facial and scalp AKs with ingenol mebutate, 5 however less is known about the factors influencing the response to treatment[16].
In the present study, we achieved complete and partial responses in 44.6% and 46.9% of cases, respectively; furthermore, ingenol mebutate 0.015% gel therapy resulted to be independently related to both the head site and the level of LSR, with a higher efficacy on facial lesions, compared to scalp ones and in case of more severe LSRs. Level of crusting at day 8 was independently associated with the achievement of a complete response.
Previous studies showed a greater efficacy of ingenol mebutate on AKs located on the face compared to scalp lesions, but the reason has not been clarified so far. In our opinion a possible explanation could be related to the lower rate of self-application errors on face than on scalp; however, in the present study, we obtained the same results even performing a physician-assisted application[17]. Therefore, other factors should be investigated to explain these findings, such as local differences in skin architecture, microbiota and ph.
Regarding the LSR composite score, we observed that both the highest levels and the values at day 2 were independently associated to complete response. The vast majority of our study population reached the highest values of LSR composite score at day 2.
The weight of each component of the composite score at each follow up visit was further evaluated and related to drug efficacy.
Erythema was the only component present at each evaluation and it was closely associated with response in univariate logistic regression analysis. Intriguingly, in multivariate analysis, when the weight of each variable was mutually adjusted for all variables in the model, erythema no longer could be associated with the response to therapy.
The highest levels of swelling and vesiculation/pustulation and the levels of these components reported in the first week after treatment were significantly associated to response in univariate analysis, but not in the multivariate model.
Conversely, the level of crusting at day 8 was the only single component of LSR composite score independently associated with the achievement of a complete response to ingenol mebutate therapy. A possible explanation of this finding could be related to the fact that the other parameters, in particular swelling and vesiculation/pustulation, probably reached their peak between day 3 and 8 follow up visits, so we couldn’t register the highest levels of these reactions. This is also supported by the fact that crusts are strictly related to the occurrence of vesicles and pustules, resulting from the drying of their fluid content.
Differently from phase III trials in which the first follow up was set at day 4, we evaluated LSRs at day 2 and 3, during physician-assisted application of ingenol.
Physician assisted application seems to be very effective in limiting withdrawal due to LSRs therefore improving adherence, in particular in elderly patients; however, a direct comparison with self-application was not performed.
Other limitations of the present study were the absence of long term efficacy, safety and cosmetic data, the absence of a quantitative evaluation of symptoms, such as pruritus, burn and pain and the low number of patients treated on the scalp, compared to the face group. However, facial localization demonstrated to be independently associated to complete response in multivariate analysis; whereas, this was not the case for patients treated on the scalp, due to the low number of patients that were treated. To obtain a more reliable result a test should be made on a higher number of patients.
On the basis of our findings we suggest that physician-assisted application of ingenol mebutate, at least for the first 2 d, could be very effective in order to improve adherence and patient satisfaction, maximize the results and minimize the risk of application errors. The severity of LSRs at day 2 and the level of crusting at day 8 should be considered as the best predictors of response to treatment.
In conclusion, our experience demonstrates that ingenol mebutate 0.015% gel is safe and effective when applied correctly. This treatment seems to be more effective on the face than on the scalp and the efficacy seems to be directly related to the level of LSR.
Actinic keratosis (AK) is considered an in situ squamous cell carcinoma, therefore treatment is mandatory.
Ingenol mebutate 0.015% gel was recently approved for the treatment of non-hypertrophic non-hyperkeratotic AK of face and scalp.
This study considers severe local skin reaction (LSR) the most important factor influencing the response to ingenol mebutate therapy for actinic keratosis.
This study demonstrates that ingenol mebutate 0.015% gel is safe and effective when applied correctly. This treatment seems to be more effective on the face than on the scalp and the efficacy seems to be directly related to the level of LSR.
This is an interesting study regarding the use of ingenol mebutate therapy for actinic keratosis of face and scalp, and the factors which may affect the treatment response. The study was well-performed, the results are novel and interesting, and the findings should be clinically relevant and useful.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aksoy B, Hu SCS S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 2. | Kirby JS, Scharnitz T, Seiverling EV, Ahrns H, Ferguson S. Actinic Keratosis Clinical Practice Guidelines: An Appraisal of Quality. Dermatol Res Pract. 2015;2015:456071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Bonerandi JJ, Beauvillain C, Caquant L, Chassagne JF, Chaussade V, Clavère P, Desouches C, Garnier F, Grolleau JL, Grossin M, Jourdain A, Lemonnier JY, Maillard H, Ortonne N, Rio E, Simon E, Sei JF, Grob JJ, Martin L; French Dermatology Recommendations Association (aRED). Guidelines for the diagnosis and treatment of cutaneous squamous cell carcinoma and precursor lesions. J Eur Acad Dermatol Venereol. 2011;25 Suppl 5:1-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Haque T, Rahman KM, Thurston DE, Hadgraft J, Lane ME. Topical therapies for skin cancer and actinic keratosis. Eur J Pharm Sci. 2015;77:279-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Lebwohl M, Swanson N, Anderson LL, Melgaard A, Xu Z, Berman B. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 319] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 6. | Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One. 2014;9:e96829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Rosen RH, Gupta AK, Tyring SK. Dual mechanism of action of ingenol mebutate gel for topical treatment of actinic keratoses: rapid lesion necrosis followed by lesion-specific immune response. J Am Acad Dermatol. 2012;66:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Micali G, Lacarrubba F, Nasca MR, Schwartz RA. Topical pharmacotherapy for skin cancer: part I. Pharmacology. J Am Acad Dermatol. 2014;70:965.e1-12; quiz 977-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Lebwohl M, Shumack S, Stein Gold L, Melgaard A, Larsson T, Tyring SK. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratoses. JAMA Dermatol. 2013;149:666-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Augustin M, Tu JH, Knudsen KM, Erntoft S, Larsson T, Hanke CW. Ingenol mebutate gel for actinic keratosis: the link between quality of life, treatment satisfaction, and clinical outcomes. J Am Acad Dermatol. 2015;72:816-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Garbe C, Basset-Seguin N, Poulin Y, Larsson T, Østerdal ML, Venkata R, Lear JT. Efficacy and safety of follow-up field treatment of actinic keratosis with ingenol mebutate 0·015% gel: a randomized, controlled 12-month study. Br J Dermatol. 2016;174:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Zalaudek I, Piana S, Moscarella E, Longo C, Zendri E, Castagnetti F, Pellacani G, Lallas A, Argenziano G. Morphologic grading and treatment of facial actinic keratosis. Clin Dermatol. 2014;32:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Rosen R, Marmur E, Anderson L, Welburn P, Katsamas J. A new, objective, quantitative scale for measuring local skin responses following topical actinic keratosis therapy with ingenol mebutate. Dermatol Ther (Heidelb). 2014;4:207-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Werner RN, Jacobs A, Rosumeck S, Erdmann R, Sporbeck B, Nast A. Methods and Results Report - Evidence and consensus-based (S3) Guidelines for the Treatment of Actinic Keratosis -International League of Dermatological Societies in cooperation with the European Dermatology Forum. J Eur Acad Dermatol Venereol. 2015;29:e1-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Martin G, Swanson N. Clinical findings using ingenol mebutate gel to treat actinic keratoses. J Am Acad Dermatol. 2013;68:S39-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Micali G, Lacarrubba F, Nasca MR, Ferraro S, Schwartz RA. Topical pharmacotherapy for skin cancer: part II. Clinical applications. J Am Acad Dermatol. 2014;70:979.e1-12; quiz 9912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Stockfleth E, Peris K, Guillen C, Cerio R, Basset-Seguin N, Foley P, Sanches J, Culshaw A, Erntoft S, Lebwohl M. A consensus approach to improving patient adherence and persistence with topical treatment for actinic keratosis. Int J Dermatol. 2015;54:509-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |