Published online Jun 10, 2017. doi: 10.5306/wjco.v8.i3.300
Peer-review started: December 5, 2016
First decision: February 21, 2017
Revised: March 3, 2017
Accepted: April 18, 2017
Article in press: April 20, 2017
Published online: June 10, 2017
Processing time: 179 Days and 19.8 Hours
Stereotactic body radiotherapy (SBRT) is a widely accepted option for the treatment of medically inoperable early-stage non-small cell lung cancer (NSCLC). Herein, we highlight the importance of interfraction image guidance during SBRT. We describe a case of early-stage NSCLC associated with segmental atelectasis that translocated 15 mm anteroinferiorly due to re-expansion of the adjacent segmental atelectasis following the first fraction. The case exemplifies the importance of cross-sectional image-guided radiotherapy that shows the intended target, as opposed to aligning based on rigid anatomy alone, especially in cases associated with potentially “volatile” anatomic areas.
Core tip: This is a case of early-stage non-small cell lung cancer associated with segmental atelectasis that translocated owing to re-expansion of the adjacent segmental atelectasis following the first fraction. There are image-guidance systems that register solely based on rigid (bony) anatomy and others that also show soft tissue; if the former would have been used, the translocated target would have been missed. The case exemplifies the importance of cross-sectional image-guided radiotherapy that shows the intended target, as opposed to aligning based on rigid anatomy alone, in cases associated with potentially “volatile” anatomic areas.
- Citation: Mao B, Verma V, Zheng D, Zhu X, Bennion NR, Bhirud AR, Poole MA, Zhen W. Target migration from re-inflation of adjacent atelectasis during lung stereotactic body radiotherapy. World J Clin Oncol 2017; 8(3): 300-304
- URL: https://www.wjgnet.com/2218-4333/full/v8/i3/300.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i3.300
Among other indications, stereotactic body radiotherapy (SBRT) plays an important role in the treatment of early-stage non-small cell lung cancer (NSCLC), chiefly in medically inoperable candidates, or if patients refuse surgery[1-7]. It is well-known that target and respiratory motion management is critical, and that spatial uncertainty in SBRT can be caused by both internal motion with respiration and set-up errors. Therefore, image-guided radiotherapy (IGRT) before each treatment is strongly recommended for SBRT; IGRT can confirm that the gross tumor is consistently located within the pre-defined treatment volume.
SBRT may utilize one of two IGRT subtypes: Systems that rely on rigid bony anatomy, and those that provide soft tissue discrimination. For the first type, two in-room systems are specifically designed for stereotactic treatments and are widely used: CyberKnife (Accuray Inc., Sunnyvale, CA, United States) and ExacTrac (BrainLabAG, Feldkirchen, Germany). These systems use orthogonal kilovoltage (kV) electronic 2-D radiographs, generated by X-ray tubes combined with flat panel detectors, to align and verify the patient treatment position - usually according to bony anatomy. Though providing better soft-tissue contrast than its MV counterparts, 2D kV IGRT systems largely consider bony landmarks for registration instead of the internal target alignment, except in cases with bulky thoracic tumors or implanted fiducial markers. For the second type, good soft tissue cross-sectional visibility is provided by 3D images. Some of these systems use kV cone-beam computed tomography (CBCT) generated by linear-accelerator (LINAC)-integrated systems such as On-Board-Imager (Varian Medical Systems, Palo Alto, CA, United States) and X-ray-Volume-Imaging (Elekta Oncology Systems, Crawley, United Kingdom). Alternatively, kV computed tomography (CT) can also be implemented by CT-on-Rails (Siemens, Erlangen, Germany). These systems offer 3D images and soft-tissue-based target verification without fiducials for small lung lesions treated with SBRT[8]. The most common application of this IGRT scheme is a two-step verification process with an initial bony registration followed by a soft-tissue target alignment[9,10].
Though most studies report localization accuracy improvements of 3D-vs-2D on the order of a few millimeters for lung SBRT[11-13], we describe a patient with an early-stage NSCLC which translocated after the first fraction of SBRT owing to re-expansion of segmental atelectasis. We further discuss the role of IGRT systems that align to bone vs soft-tissue in detection and management of the resulting misalignment.
A 72-year-old man presented with a nonproductive cough; computed tomography (CT) scan showed a 2.5-cm right lower lobe nodule. He had a 50-year history of smoking and used 4 L of nighttime oxygen (ECOG performance status 3). On auscultation, he had diminished breath sounds; pulmonary function tests showed an FEV1 (forced expiratory volume, 1 s) 41% of the predicted value and a DLCO (diffusion capacity of carbon-monoxide) 29% of the predicted value. Subsequent positron emission tomography (PET) scan showed no other hypermetabolic foci. Needle biopsy revealed poorly-differentiated lung adenocarcinoma.
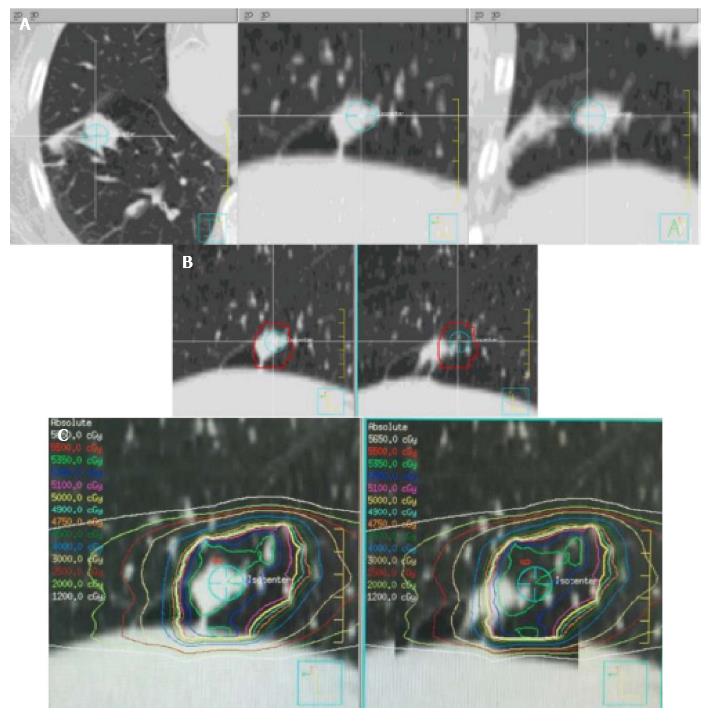
He was not a surgical candidate owing to poor pulmonary function, and was appropriate for SBRT. On CT simulation including a four-dimensional (4D) and free-breathing CT, new distal segmental atelectasis was noted near the nodule (Figure 1A). The target was delineated as an internal target volume (ITV) based on maximum intensity projection (MIP) generated by 4DCT simulation, with an additional 5 mm expansion to create the planning target volume (PTV). He then started Volumetric Modulated Arc Therapy to the right lower lobe PTV at a dose of 5000 cGy in 5 daily fractions. Daily kV CBCT was used for IGRT on a TrueBeamSTx LINAC. After the first dose, the tumor was found to have translocated 15mm anteroinferiorly due to re-expansion of segmental atelectasis as demonstrated by the kV CBCT prior to the second fraction. Of note, the movement was significant enough that it was only partially covered by the PTV. He underwent re-simulation, which confirmed the geometric target migration (Figure 1B and C). SBRT was re-adjusted for the new target location after migration. For subsequent fractions, daily kV CBCTs validated tumor position within the ITV; the remainder of therapy was completed uneventfully. Post-treatment CT showed resolution of disease at 4 mo post-SBRT.
Although surgery is currently the principal option for early-stage NSCLC patients that are medically operable, stereotactic body radiotherapy (SBRT) has emerged as the option of choice for patients who are medically inoperable. SBRT is a technique that administers high doses of radiation to the target while minimizing the dose to surrounding normal tissues. Reports show high local tumor control rates upwards of 90%, with severe toxicities well under 10%[14]. This has made it a favorable option for medically inoperable patients with stage I NSCLC, endorsed by both the National Comprehensive Cancer Network (NCCN) Guidelines and the European Society of Medical Oncology (ESMO) Clinical Practice Guidelines[15].
However, accounting for target and respiratory motion presents a challenge to proper delivery. Hence, in order to verify target location with high accuracy, high-fidelity IGRT is considered essential to SBRT. In our case report, the target translocated 15 mm anteriorly and inferiorly due to re-expansion of adjacent atelectasis after the first fraction. Without soft tissue discrimination in cross-sectional imaging, 2D IGRT based on bony anatomy only would have resulted in systematically missing the tumor in the remaining 4 fractions. These advantages of 3D IGRT are highlighted in anatomic areas liable to changes in morphology.
There have been several studies describing the utility of IGRT in SBRT. A study was carried out to evaluate the potential of image guidance, gating and real-time tumor tracking to improve accuracy in pulmonary SBRT. It illustrated that CBCT-based IGRT for pre-treatment verification of the target position and online correction of errors reduced safety margins most effectively in pulmonary SBRT[16]. Another recent study illustrated that application of continuous monitoring and intra-fraction target position correction during treatment improved the target coverage for patients in prostate SBRT. Without these IGRT techniques, intra-fractional motion would have significantly altered coverage in about 10% of patients[17]. These studies have demonstrated that inter-fractional, and possibly even intra-fractional IGRT, can improve SBRT delivery. We advocate for increased use of cross-sectional imaging IGRT with soft tissue definition, especially in cases of tumors near potentially “anatomically volatile” areas. While such large translocations such as reported here may be unlikely, intra-fractional real-time tumor tracking may provide additional benefit. An ionization radiation-free system using thoracic transducers and radiofrequency tracking using the Calypso system is under development (Varian Medical Systems, Palo Alto, CA, United States).
In summary, image guidance is a prerequisite for SBRT delivery, but 2D IGRT systems that solely align patients based on rigid bony anatomy may be notably inadequate in some cases. Instead, the use of imaging that provides cross-sectional soft tissue anatomical information to verify the target may prevent systematic misses from changes in target position.
A 72-year-old man of stage I non-small cell lung cancer associated with segmental atelectasis that translocated owing to re-expansion of the adjacent segmental atelectasis following the first dose of stereotactic body radiation therapy (SBRT).
Lung re-expansion of segmental atelectasis.
Diminished breath sounds, pulmonary function, positron emission tomography scan and needle biopsy.
The patient was not a surgical candidate owing to poor pulmonary function, and was appropriate for SBRT.
Re-simulation with high resolution computed tomography (CT) and image comparison using ridged image registration of primary CT simulation images confirmed geographic moves of the tumor due to re-expansion of an adjacent pulmonary atelectasis.
Needle biopsy showed poorly-differentiated lung adenocarcinoma.
Daily kV cone-beam computed tomography was used for IGRT during SBRT.
Related reports have demonstrated that inter-fractional, and possibly even intra-fractional IGRT, can improve SBRT delivery.
Non-small cell lung cancer is a deadly disease that may threaten people’s life.
Image guidance is extremely important for SBRT delivery.
This is an interesting case report worthy for publication. The authors reported on an early-stage lung tumor undergoing SBRT, translocating outside of the PTV after re-inflation of nearby atelectasis. The case herein presented highlight the risks of relying on IGRT system based on rigid anatomy alone. The manuscript is original, well-written and summarized in very explanatory figures.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Arcangeli S, Sugawara I S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Verma V. Stereotactic Radiotherapy Versus Surgery for Early-Stage Operable Lung Cancer: More Questions Than Answers. J Natl Compr Canc Netw. 2015;13:1293-1295. [PubMed] |
| 2. | Verma V, Zhen W. Treatment Costs of Early-Stage Lung Cancers Detected by Low-Dose Computed Tomography Screening. Int J Radiat Oncol Biol Phys. 2015;93:207-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Verma V, Beriwal S. Medicare Approves Coverage for Lung Cancer Screening: The Case for Symptomatic Screening. JAMA Oncol. 2015;1:1027-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Verma V. Lung cancer: Implementing lung-cancer screening--oncological ‘grey areas’. Nat Rev Clin Oncol. 2015;12:256-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Verma V, Shostrom VK, Kumar SS, Zhen W, Hallemeier CL, Braunstein SE, Holland J, Harkenrider MM, S Iskhanian A, Neboori HJ. Multi-institutional experience of stereotactic body radiotherapy for large (≥5 centimeters) non-small cell lung tumors. Cancer. 2017;123:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Verma V, McMillan MT, Grover S, Simone CB. Stereotactic Body Radiation Therapy and the Influence of Chemotherapy on Overall Survival for Large (≥5 Centimeter) Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2017;97:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Verma V, Shostrom VK, Zhen W, Zhang M, Braunstein SE, Holland J, Hallemeier CL, Harkenrider MM, Iskhanian A, Jabbour SK. Influence of Fractionation Scheme and Tumor Location on Toxicities Following Stereotactic Body Radiotherapy for Large (≥5 Centimeter) Non-Small Cell Lung Cancer: A Multi-Institutional Analysis. Int J Radiat Oncol Biol Phys. 2016;97:778-785. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Chang JY, Dong L, Liu H, Starkschall G, Balter P, Mohan R, Liao Z, Cox JD, Komaki R. Image-guided radiation therapy for non-small cell lung cancer. J Thorac Oncol. 2008;3:177-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Dahele M, Pearson S, Purdie T, Bissonnette JP, Franks K, Brade A, Cho J, Sun A, Hope A, Marshall A. Practical considerations arising from the implementation of lung stereotactic body radiation therapy (SBRT) at a comprehensive cancer center. J Thorac Oncol. 2008;3:1332-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Li Q, Mu J, Gu W, Chen Y, Ning Z, Jin J, Pei H. Frameless stereotactic body radiation therapy for multiple lung metastases. J Appl Clin Med Phys. 2014;15:4737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Yeung AR, Li JG, Shi W, Newlin HE, Chvetsov A, Liu C, Palta JR, Olivier K. Tumor localization using cone-beam CT reduces setup margins in conventionally fractionated radiotherapy for lung tumors. Int J Radiat Oncol Biol Phys. 2009;74:1100-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Grills IS, Hugo G, Kestin LL, Galerani AP, Chao KK, Wloch J, Yan D. Image-guided radiotherapy via daily online cone-beam CT substantially reduces margin requirements for stereotactic lung radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:1045-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 13. | Wang Z, Nelson JW, Yoo S, Wu QJ, Kirkpatrick JP, Marks LB, Yin FF. Refinement of treatment setup and target localization accuracy using three-dimensional cone-beam computed tomography for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2009;73:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone D. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2018] [Cited by in RCA: 1960] [Article Influence: 130.7] [Reference Citation Analysis (0)] |
| 15. | Senan S, Guckenberger M, Ricardi U. Stage I non-small cell lung cancer and oligometastatic disease. IASLC textbook multidisciplinary approach to thoracic oncology textbook. Aurora, CO: International Association for the study of Lung Cancer; 2014; . |
| 16. | Guckenberger M, Krieger T, Richter A, Baier K, Wilbert J, Sweeney RA, Flentje M. Potential of image-guidance, gating and real-time tracking to improve accuracy in pulmonary stereotactic body radiotherapy. Radiother Oncol. 2009;91:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Lovelock DM, Messineo AP, Cox BW, Kollmeier MA, Zelefsky MJ. Continuous monitoring and intrafraction target position correction during treatment improves target coverage for patients undergoing SBRT prostate therapy. Int J Radiat Oncol Biol Phys. 2015;91:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |