Published online Jun 10, 2017. doi: 10.5306/wjco.v8.i3.289
Peer-review started: February 12, 2017
First decision: March 8, 2017
Revised: March 29, 2017
Accepted: April 23, 2017
Article in press: April 24, 2017
Published online: June 10, 2017
Processing time: 124 Days and 4 Hours
The oncocytic variant of prostatic adenocarcinoma is exceptionally rare with only 4 cases reported in the English literature. Little is known about the clinical behavior of this variant of prostatic adenocarcinoma, because of the exceptionally low number of reported cases. The 2016 World Health Organization Classification of Tumors of Prostate does not recognize the oncocytic variant, again likely related to the exceptional paucity of reported cases. Here, we report the fifth case of the oncocytic variant of acinar type prostatic adenocarcinoma in an asymptomatic 64-year-old Caucasian American male with elevated serum prostate specific antigen (7.33 ng/mL; normal range 0-4.00 ng/mL) during routine blood screening for diabetes mellitus. At subsequent transrectal prostate biopsy, the right side of prostate was infiltrated by adenocarcinoma with tumor cells forming variably differentiated glands, including some poorly differentiated. Tumor cell nuclear:cytoplasmic ratio was low, with small to intermediate sized vesicular nuclei and only rare discernable small nucleoli. Cellular cytoplasm was characteristically granular pink with sharply defined cell membranes. Positive AMACR (P504S) epithelial immunohistochemical staining and absence of staining for prostatic basal cells confirmed the tumor to be primary prostatic adenocarcinoma. AMACR immunohistochemical staining was also helpful with accurate grading of the tumor due to the difficulty of differentiating tumor cells from residual prostate myocytes at routine hematoxylin and eosin (HE) staining. This new case adds to the exceptionally small number of previously reported cases of the oncocytic variant of primary prostatic adenocarcinoma. It also highlights the difficulty associated with Gleason scoring of the oncocytic variant by routine HE evaluation and the usefulness of AMACR (P504S) immunostaining for accurate grading of prostatic adenocarcinoma in the oncocytic variant.
Core tip: The oncocytic variant of prostatic adenocarcinoma is exceptionally rare with only 4 cases reported so far. Through reporting this new case, the oncocytic variant is being highlighted and challenges associated with its accurate diagnosis and staging discussed. The use of immunohistochemistry to confirm prostatic origin of this tumor for accurate grading of this lesion is also highlighted. It is also postulated that the tumor cells may be difficult to locate for their presence and organization at hematoxylin and eosin evaluation, potentially resulting in inaccurate grading of the tumor, the tumor likely behaves no different from the usual/typical variant of acinar-type adenocarcinoma if appropriately graded.
- Citation: Klairmont MM, Zafar N. Prostatic adenocarcinoma oncocytic variant: Case report and literature review. World J Clin Oncol 2017; 8(3): 289-292
- URL: https://www.wjgnet.com/2218-4333/full/v8/i3/289.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i3.289
Prostatic adenocarcinoma is a common malignancy, however, the 2016 World Health Organization (WHO) Classification of Prostatic Tumors[1] does not mention the oncocytic variant of acinar-type adenocarcinoma, likely due to the very small number of reported cases in the literature. There is a paucity of data concerning the clinical behavior of this variant compared to the traditionally established varieties of acinar-type prostate adenocarcinoma. Accordingly, there is a critical need for more cases of the oncocytic variant to be reported, for it to be added to a future WHO classification, and to identify a variable clinical behavior from the usual variant, if that is indeed the case.
The 64-year-old Caucasian male with past medical history of hypertension, hyperlipidemia, type-2 diabetes, and otherwise asymptomatic, was also found at routine screening to have an elevated total serum prostate-specific antigen (PSA) of 7.33 ng/mL (range
0-4.0 ng/mL). Review of systems was unremarkable. He denied tobacco use and reported occasional alcohol use. Family history was unremarkable for genitourinary malignancy. Digital rectal exam indicated irregular prostate borders with a single indurated nodule on the right. The patient subsequently underwent transrectal prostate biopsy which revealed a right-sided prostate adenocarcinoma, the left-side being unremarkable. Patient was discharged after biopsy and elected to undergo targeted cryoablation of the prostate at an outside institution.
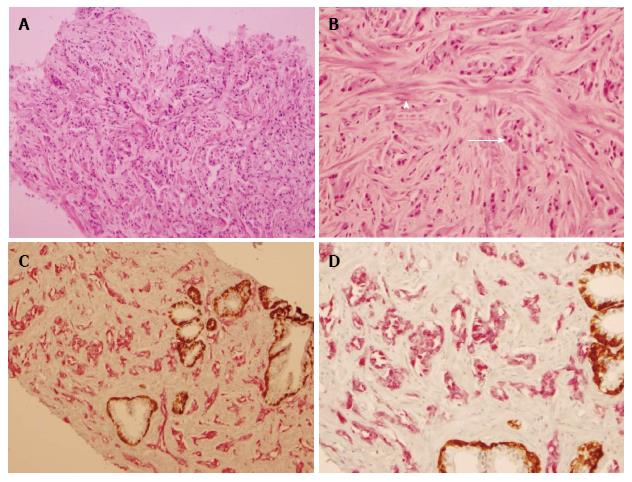
At routine hematoxylin and eosin (HE) histology, the right side of the prostate contained a poorly delineated malignancy with tumor cells arranged in vague glandular forms, as well as apparent cords, and possibly some single cells (Figure 1A). The cells had a low nuclear:cytoplasmic ratio, small to intermediate sized vesicular nuclei with only very rare prominent nucleoli, granular amphophilic to acidophilic cytoplasm, and sharp cell membranes (Figure 1B). It was difficult to reliably differentiate tumor cells from residual prostatic myocytes because of overlapping cytomorphology and staining quality (Figure 1B). Routine ABC immunohistochemistry with AMACR (P504S) and prostate basal cell markers (PIN4) was very helpful to confirm this cancer to be primary to prostate, and for accurate Gleason scoring of the tumor, as it clearly demonstrated the absence of single tumor cells and extensive gland formation, mostly discrete, with some tumor cells merging into more solid structures (1C and 1D). The tumor was assigned Gleason score 3 + 4 (20%) = 7, present in all 6/6 cores, 25% of total biopsy, with perineural invasion, but no vascular invasion. Biopsies of the left prostate were negative for malignancy.
Oncocytic tumors of prostate are exceedingly rare. The first reported case was an oncocytoma in an 87-year-old man who underwent transurethral resection for prostatic hypertrophy[2]. While the tumor cells were immunoreactive with cytochrome-oxidase, they were not reactive with PSA.
In 1992, Ordóñez et al[3] reported the first case of prostatic carcinoma with oncocytic features in a 63-year-old patient who presented with inguinal lymph node metastasis and an unknown primary, with a normal serum PSA. The tumor cells had finely granular cytoplasm, which ultrastructural examination showed to contain numerous mitochondria. The cells were immunoreactive with PSA and prostatic acid phosphatase. Subsequent prostatectomy confirmed primary oncocytic adenocarcinoma of the prostate. The authors postulated that the reason for oncocytic transformation may involve possible mitochondrial dysfunction in the cancer cell of origin, resulting in the proliferation of an oncocytic cancer cell type.
Pinto et al[4] reported the 2nd case of primary carcinoma of prostate with diffuse oncocytic changes in a 66-year-old patient, who presented with a retro-ocular tumor and a PSA level of 100 ng/mL. Digital rectal exam indicated prostatic enlargement, which was subsequently biopsied, and the retro-ocular metastasis was also resected. Both the tumor sites contained identical poorly differentiated oncocytic tumor cells with strong immunoreactivity for PSA. This patient also had hyperdense metastatic lesions in various bony sites. The authors postulated that the prognosis of these very rare oncocytic tumors is no different from the usual prostatic acinar carcinoma and is more related to the tumor differentiation (Gleason scoring). We agree with this opinion, though we feel this is still anecdotal because of insufficient experience with this tumor variant.
Fiandrino et al[5] reported the third case of prostatic adenocarcinoma with oncocytic features in a 72-year-old patient who presented with dysuria and prostate enlargement. The patient underwent prostatectomy which revealed prostatic adenocarcinoma with oncocytic features involving the entire tumor mass. Capsular infiltration and perineural invasion were also present. Based on extensive gland fusion, it was assigned a Gleason score of 8 (4 + 4) involving 60% of the prostate (both lobes). At immunohistochemistry, the oncocytic tumor cells were strongly positive for PSA and prostatic acid phosphatase (PSAP). Cells also stained positive for antimitochondrial antibody which demonstrated granular cytoplasmic reactivity in tumor cells but not normal glands. Ultrastructural evaluation was performed and similarly demonstrated a high mitochondrial density in tumor cells compared to the adjacent parenchyma.
Khadim et al[6] reported the most recent case of oncocytic variant of prostatic adenocarcinoma in a 57-year-old, who presented with urinary urgency, hesitancy, increased frequency, poor stream, enlarged firm prostate at digital rectal examination and a markedly elevated PSA level of 40 ng/mL. At transurethral resection, the entire prostatic tumor comprised of oncocytic cells, arranged in solid sheets, with round to ovoid hyperchromatic nuclei and granular eosinophic cytoplasm and PSA immunoreactivity. Gleason score of 5 + 4 = 9 was assigned, involving 80% of the tissue sampled and without perineural or lymphovascular invasion. No follow-up was provided.
Gilloteaux et al[7] have reported a peculiar, rare oncocyte-like cell in prostatic carcinoma (DU145) cell line, with a small nucleus and with cytoplasm almost entirely filled with often distorted mitochondria. It is enticing to speculate if this might be the cell-type which gives rise to the oncocytic variant of prostatic adenocarcinoma.
In summary, the reasons for presenting this case are multiple, foremost to add to the very limited literature on this variant of prostatic adenocarcinoma and to highlight the challenge of optimal Gleason scoring at HE assessment only. Our calculated Gleason score prior to AMACR (P504) staining was 4 + 5 = 9 because of the presence of poorly differentiated glands and perceived numerous single eosinophilic cells, with only rare well-formed glands. Our final Gleason score was 3 + 4 (20%) = 7, as AMACR (P504S) staining confirmed the absence of single tumor cells in the biopsy and the presence of numerous glands, mostly well-formed, with rare additional distorted and merged tumor glands. We believe the overestimation of Gleason score at HE is related to the difficulty of differentiating residual benign myocytes from tumor cells because of the overlapping cytomorphology and staining characteristics. We feel that AMACR (P504S) staining is critically important for optimal assessment of tumor differentiation and Gleason scoring of the oncocytic variant of prostatic adenocarcinoma. The non-recognition of this cancer variant in the 2016 WHO classification of tumors of prostate, among other known variants of classic acinar type prostatic adenocarcinoma[8], is likely related to the exceptionally low number of reported cases, most likely related to very low incidence. Variants of conventional prostate cancer (pseudohyperplastic, foamy gland, hypernephroid, atrophic, microcystic, with Paneth cell-like changes, with collagenous micronodules, with glomeruloid formations, and oncocytic) do not have any known prognostic significance and are graded according to the Gleason system. The prognosis and clinical behavior of the oncocytic variant, therefore, is also likely to be related to the degree of tumor differentiation and clinical staging, and not morphologic variation.
A 64-year-old Caucasian male with a serum prostate-specific antigen level of 7.33 ng/mL (range 0-4.0 ng/mL).
Prostatic enlargement.
Prostatic carcinoma, prostatitis, prostatic hypertrophy.
Primary adenocarcinoma of prostate, oncocytic variant, Gleason score 3 + 4 (20%) = 7, 6/6 cores, 20% of total tissue involved on the right, with peri-neural invasion.
The patient opted for cryoablation of the prostate at another facility. No further follow-up is available at this point.
This is an interesting case report of a very rare tumor.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Naspro R, Russo MA S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol. 2016;70:106-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 1206] [Article Influence: 134.0] [Reference Citation Analysis (0)] |
| 2. | Beer M, Occhionero F, Welsch U. Oncocytoma of the prostate: a case report with ultrastructural and immunohistochemical evaluation. Histopathology. 1990;17:370-372. [PubMed] |
| 3. | Ordóñez NG, Ro JY, Ayala AG. Metastatic prostatic carcinoma presenting as an oncocytic tumor. Am J Surg Pathol. 1992;16:1007-1012. [PubMed] |
| 4. | Pinto JA, González JE, Granadillo MA. Primary carcinoma of the prostate with diffuse oncocytic changes. Histopathology. 1994;25:286-288. [PubMed] |
| 5. | Fiandrino G, Lucioni M, Filippin F, Viglio A, Nicola M, Molo S, Necchi V, Zmerly H, Ravasi S, Paulli M. Prostatic adenocarcinoma with oncocytic features. J Clin Pathol. 2011;64:177-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Khadim MT, Hassan U, Ejaz A, Mamoon N. Oncocytic variant of prostatic adenocarcinoma- A case report. Pak Armed Forces Med J. 2012;62. |
| 7. | Gilloteaux J, Eze N, Jamison JM, McGuire K, Summers JL. A rare, human prostate oncocyte cell originates from the prostatic carcinoma (DU145) cell line. Ultrastruct Pathol. 2013;37:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Mikuz G. Histologic classification of prostate cancer. Anal Quant Cytopathol Histpathol. 2015;37:39-47. [PubMed] |