Published online Jun 10, 2017. doi: 10.5306/wjco.v8.i3.249
Peer-review started: February 10, 2017
First decision: March 28, 2017
Revised: April 12, 2017
Accepted: May 12, 2017
Article in press: May 14, 2017
Published online: June 10, 2017
Processing time: 125 Days and 2.1 Hours
Extralevator abdominoperineal excision and pelvic exenteration are mutilating operations that leave wide perineal wounds. Such large wounds are prone to infection and perineal herniation, and their closure is a major concern to most surgeons. Different approaches to the perineal repair exist, varying from primary or mesh closure to myocutaneous flaps. Each technique has its own associated advantages and potential complications and the ideal approach is still debated. In the present study, we reviewed the current literature and our own local data regarding the use of biological mesh for perineal wound closure. Current evidence suggests that the use of biological mesh carries an acceptable risk of wound complications compared to primary closure and is similar to flap reconstruction. In addition, the rate of perineal hernia is lower in early follow-up, while long-term hernia occurrence appears to be similar between the different techniques. Finally, it is an easy and quick reconstruction method. Although more expensive than primary closure, the cost associated with the use of a biological mesh is at least equal, if not less, than flap reconstruction.
Core tip: Current literature regarding the use of biological mesh reconstruction after pelvic exenteration and extralevator abdominoperineal excision is scarce. However, it does suggest that the use of biological mesh has a lower short-term perineal hernia rate, but is probably not superior to other approaches with regards to perineal wound complications.
- Citation: Schiltz B, Buchs NC, Penna M, Scarpa CR, Liot E, Morel P, Ris F. Biological mesh reconstruction of the pelvic floor following abdominoperineal excision for cancer: A review. World J Clin Oncol 2017; 8(3): 249-254
- URL: https://www.wjgnet.com/2218-4333/full/v8/i3/249.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i3.249
Pelvic exenteration (PE) and extralevator abdominoperineal excision (ELAPE) are mutilating operations, leaving a large perineal incision. ELAPE for low rectal cancer was introduced to decrease the rate of positive resection margins and specimen perforation occurring during conventional abdominoperineal resection (cAPR)[1,2]. In a recent retrospective study, Stelzner et al[3] showed that the 5-year recurrence rate was 5.9% in the ELAPE group vs 18.2% in the cAPR group (P = 0.153). However, other units have not been able to reproduce such results[4], nor could they demonstrate a statistically significant superiority of ELAPE in terms of CRM positivity and bowel perforation. Furthermore, they reported comparable perineal complication rates for the two APR approaches.
Vivid discussions continue to fuel the debate regarding the pros and cons of ELAPE. Overall, it is well accepted that larger wounds are independent risk factors for perineal wound complications. The combination of neoadjuvant chemoradiotherapy and ELAPE almost doubles the rate of perineal wound complications (31% for ELAPE vs 18% for cAPR)[5]. While new techniques and approaches have attempted to reduce the size of the perineal incision (and therefore reduce the risk of wound complications)[6], optimal management of perineal defects is still under investigation. The options include primary closure, myocutaneous flaps, and mesh reconstruction, including the use of a biological mesh.
We aimed to evaluate the outcomes of perineal reconstruction with biological mesh following ELAPE and PE in our center and to review the current literature.
Perineal wound complications are a major concern following PE and ELAPE leading to increased morbidity, longer hospital stay, and delayed chemotherapy. Different reconstruction methods are currently used in practice with the aim of reducing the rates of wound complications and avoiding perineal herniation.
Risk factors for major perineal wound complications following APR are well known: Preoperative radiotherapy, patients with anal cancer, flap reconstruction, tumor size, obesity, and diabetes[7]. Minor wound complications appear more commonly in patients with inflammatory bowel disease or anal cancer than in those with rectal cancer[8].
Most patients with locally advanced rectal cancer, recurrent rectal cancer, and recurrent or persistent squamous cell carcinoma receive neoadjuvant radiochemotherapy or radiotherapy alone[9,10]. The poor healing ability of irradiated wounds has been attributed to local endarteritis and damaged fibroblasts[11]. It has been clearly demonstrated that preoperative radiotherapy increases the rate of major wound complications[5,12]. For example, Aldulaymi et al[13] reported a significantly increased risk of major perineal wound complications in patients undergoing APR for rectal cancer with primary closure of the perineum (26% in non-irradiated vs 71% in irradiated patients). Chadwick et al[14] found that the risk of developing a wound complication was 10 times higher after previous irradiation. This substantial problem with wound healing calls for the need to consider alternative closure techniques of the perineum.
Different methods have been described ranging from direct/primary closure to mesh reconstruction, gluteal and rectus abdominis flaps or combinations of these techniques. Currently, there is no consensus on which is the most ideal technique[15]. The vertical rectus abdominis flap (VRAM) is indicated to bring non-irradiated tissue into the perineal defect[16]. After VRAM, perineal wound complications have been reported to range from 0% to 28%[17-20]. The use of laparoscopy for the abdominal part of the resection is almost impossible because of the donor site. In addition, in cases of PE (with a right sided urostomy and left sided end colostomy), VRAM is often contra-indicated. A potential solution is the use of a wet double-barreled colostomy[21].
Other myocutaneous flaps can potentially be used, such as the gracilis flap and the gluteus maximus flap, which have a perineal wound complication rate of 12%[22] and 10%[2] respectively. However, these flaps are typically smaller than the VRAM flap and unlikely to provide adequate cover of large defects.
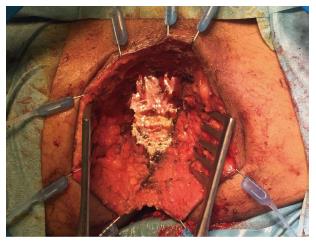
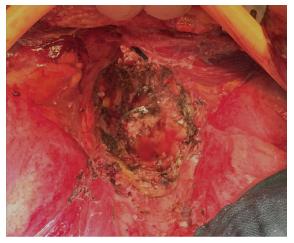
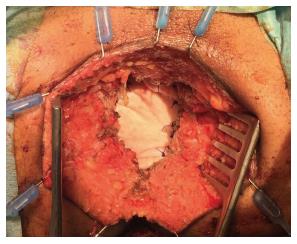
In addition, authors argue that myocutaneous flaps carry significant risks of donor site morbidity, flap necrosis, prolonged operative time, and usually require co-ordination with plastic surgeons[2,23,24]. Mesh reconstruction is another technique, which has attracted a lot of interest in the last few years, especially with the adoption of ELAPE. Briefly, the biological mesh is sutured directly to the pelvic side wall (Figures 1-3). The size of the mesh is adapted to the size of the defect. A perineal drain is routinely left at the end of the procedure, in order to avoid a perineal collection.
Both allogenic and xenogenic biological meshes are available for the reconstruction of the perineum. These types of meshes were initially used for abdominal wall reconstructions[25,26]. The allogenic mesh is predominantly made of human acellular dermis (e.g., HADM® Ruinuo, Qingyuanweiye Bio-Tissue Engineering Ltd, Beijing, China) as used by Han et al[27,28]. The xenogenic mesh consists of bovine pericardium or porcine dermis and intestinal mucosa. Similar to Musters et al[29] in the BIOPEX-study, we used the Strattice® mesh (LifeCell, Acelity Company, Branchburg, NJ) which is composed of non-reticulated porcine dermis. Jensen et al[30] and Christensen et al[24] used the Permacol® mesh (Tissue Science Laboratories plc, Covington, GA, United States) derived from reticulated porcine dermis[24,30]. Surgisis® Biodesign™ (Cook Medical, Bloomington, IN, United States) created using porcine intestinal mucosa was used by Peacock et al[31] for their pelvic reconstruction.
Reconstruction using a mesh is relatively simpler and faster compared to flap reconstruction[24]. When considering cost, meshes are expensive, especially if biological. However, with a potentially shorter operative time and length of hospital stay, overall costs can be controlled and even reduced in comparison to VRAM-flaps[32]. Biological meshes also have the advantage of being absorbable and can be used in infected environments[33].
On the other hand, perineal mesh reconstruction is not without its risks. Internal hernias following mesh repair have been reported. Melich et al[34] described resecting ischemic small bowel loops incarcerated in a pelvic hernia along the mesh in three patients. Jensen et al[30] reported a hole in the biological mesh in a patient with an infected perineal wound, who subsequently required mesh removal. These reports clearly raise concerns and highlight the risk of small bowel incarceration and necrosis associated with the use of a perineal mesh.
Table 1 summarizes the largest studies focusing on the use of biological mesh for perineal reconstruction. Interestingly, only one mesh was removed[30]. The overall safety profile appears to be good.
| Ref. | Study type | Operation | No.of patients | Averageage (median years) | Perineal complications (%) | Surgical perineal debridementn | Perineal hernias | Follow up | Comments |
| Musters BIOPEX-study 2016[29] | RCT | ELAPE | 50 | 65 | 37% overall perineal wound complications | 4% surgical drainage of perineal abscess, 6% percutaneous drainage of perineal abscess | 13% at 1 yr | 12 mo | |
| Jensen et al[30], 2014 | Cohort, prospective | ELAPE | 53 | NR | 21% perineal fistula, 7.5% superficial perineal abscess, 7.5% deep perineal abscess | 5 (9%) fistulectomy, 8 (15%) surgical debridements | 5.60% | Median 36 mo | 1 mesh removed (infection), 1 mesh failure (hole) replacement of a new mesh |
| Christensen et al[24], 2011 | Cohort, retrospective | ELAPE | 24 | 69.7 | 17%, with one fistula after 3 mo | 0 | 0 | Median 1.7 yr | - |
| Han et al[28], 2010 | Cohort, retrospective | ELAPE | 12 | 68 | 16% infection, 8% seroma | 0 | NR | Median 8 mo | - |
| Han et al[27], 2012 | Derived from RCT | ELAPE | 32 | 68 | 11.4% wound infections 11% seroma | NR | 14% | NR | - |
| Peacock et al[31], 2014 | Cohort, prospective | ELAPE | 34 | 62 | 32% overall; 9% superficial wound infections, 14% perineal fistula; 9% perineal abscess | 3 (9%) surgical debridement/VAC therapy | 0 | Median 21 mo | - |
| Schiltz present study | Cohort, retrospective | ELAPE + PE | 11 | 63 | Overall 27% wound infections with 1 superficial | 2 (18%) surgical debridement | 0 | Mean 18 mo | - |
The clinical consequences of perineal wound complications are wide and range from a simple redness of the skin to a persistent perineal fistula, and perineal sepsis. Perineal wound complications are often subdivided into two subgroups: Early and delayed wound dehiscence. The delayed (> 4 wk) perineal healing can occur in approximately 25% of cases. Importantly, up to 50% of these cases will develop long-term and persistent perineal symptoms such as pain, chronic sinus, sitting disability or tension between buttocks. All of which can seriously impact the patient’s quality of life. Delayed perineal healing may therefore be a risk factor for persistent symptoms providing yet another reason why surgeons must strive to identify the best repair method possible[35,36].
Primary closure leads to perineal wound complications in 18%-34%[27,29,37]. Moreover, one third of patients after PE will develop perineal wound dehiscence[38]. As a corollary, persistent presacral sinus was found in 10% of the patients following APR[39].
As mentioned in Table 1, 17%-37% of patients with biological mesh presented some degree of perineal wound dehiscence/infection. A Danish retrospective study reported that 15% of patients with biological mesh had a surgical re-intervention for perineal infection. In addition, 21% of the patients had a perineal fistula with 9% requiring surgical excision[30]. Similarly, Peacock et al[31] reported an overall perineal wound complication rate of 32%. Vacuum assisted wound therapy and surgical debridement were needed in up to 9% of cases.
Christensen et al[24] compared gluteal flap reconstruction with biological mesh repair. Seventeen percent of patients in the mesh group had a wound infection compared to 6% in the flap group (P = 0.26). At 3 mo, all wounds healed with one persistent sinus in each group[24].
Han et al[28] found similar results and subsequently conducted a randomized controlled trial evaluating ELAPE vs cAPR. Interestingly, in the ELAPE group, patients had biological mesh reconstruction. Overall, the perineal wound infection rate (11.4%) after ELAPE was lower than in the cAPR group where 18.8% of patients developed a perineal complication. However, seromas were more frequent in the mesh group (11.4% vs 0%)[27]. Seroma formation can be problematic, pushing most of the authors to recommend the routine use of a perineal drain.
Adding to the present literature, we conducted a retrospective study of our local data. From January 2012 to December 2015, all patients undergoing ELAPE or PE with biological mesh reconstruction were analyzed. Eleven patients were found; all of whom had preoperative radiochemotherapy. Overall, perineal complications were found in 3 (27%) of the patients. In 2 (18%) patients, perineal abscesses were surgically drained and treated with a vacuum assisted wound closure system. One superficial wound infection was treated conservatively. No meshes were removed.
The relatively poor quality of the available studies in the literature remains an issue. These are mainly retrospective or simple cohort studies designed to analyze oncological outcomes. Very few of them focus specifically on perineal complications. Additionally, the severity and grading system of wound complications can differ between reports, and thus it is difficult to draw definitive conclusions.
The only multicenter randomized controlled trial focusing on perineal reconstruction using biological mesh after ELAPE, the BIOPEX study[29], was recently published. Patients were randomized into two groups, one with perineal mesh reconstruction and the other with primary closure only (control group). Regular blinded wound follow-up, using the Southampton wound healing score, did not show a significant difference between the two groups at 30 d. In the control group, 34% of perineal wound complications occurred vs 37% in the mesh group (P = 0.7177). At 12-mo follow-up, the healing rates did not differ between groups (52% vs 54%). Omentoplasty or use of perineal drains did not affect the results in this study[29].
In summary, current evidence suggests that biological mesh reconstruction does not appear to reduce the risk of perineal wound complications. Results are similar between primary closure, flap and biological mesh.
The incidence of perineal hernia after APR ranges from 0.6% to 27% in the literature[5,29,40], occurring on average 8 to 22 mo after surgery[41,42] (Table 1). Such a wide range can partly be explained by the definition of a perineal hernia itself. Indeed, a clinical hernia is quite different from an asymptomatic radiologically identified perineal hernia. Smoking and chemoradiotherapy are well reported risk factors[42].
Given that recurrence rates following perineal hernia repair are high (up to 37%), prevention is certainly the best strategy[15]. Perineal hernia occurs significantly less often after biological mesh reconstruction (0%) than following gluteal flap surgery (21%) (P < 0.01)[24]. Thus suggesting that biological mesh repair can be a good option in order to avoid herniation.
The BIOPEX-study showed that 13% of perineal hernias (diagnosed on CT scan) occurred after biological mesh repair vs 27% in the primary closure group at one-year follow-up (P = 0.036)[29]. The hernias occurred nearer the end of the 12-mo follow-up in the mesh group. The long-term follow-up results are still pending. Interestingly, this delay in the hernia presentation is also described in patients without mesh reconstruction. However, this seems to occur after a median of 8 mo[41]. A possible explanation is that perineal hernias occur later in the mesh group due to the slower degradation of the biological mesh[43].
In our own data, no perineal hernia was found, neither clinically or radiologically, even after a mean follow-up of 18 mo.
Overall, biological mesh seems to protect, at least in early follow up, from the occurrence of perineal hernias in comparison to flap reconstruction or primary closure.
Perineal reconstruction following ELAPE, APE or PE remains a major problem and challenge. No ideal solution currently exists but various approaches have been attempted with more or less success. Primary closure remains the most frequent technique, carrying a significant risk of perineal hernia formation. On the other hand, the use of flap or mesh reconstruction could help reduce the risk of herniation. Biological mesh appears to be a valid option, at least in terms of hernia prevention, which can be reduced by up to 50%.
Yet, the role of mesh reconstruction in reducing wound infections is less clear. Whilst perineal infection is frequent in irradiated patients, the use of biological mesh seems logical, even if the evidence is scarce to draw definitive recommendations. On the other hand, perineal wound infection remains frequent and a perineal drain should be routinely used.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Switzerland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Niu ZS, Tomizawa M S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Nagtegaal ID, van de Velde CJ, Marijnen CA, van Krieken JH, Quirke P. Low rectal cancer: a call for a change of approach in abdominoperineal resection. J Clin Oncol. 2005;23:9257-9264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 416] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 2. | Holm T, Ljung A, Häggmark T, Jurell G, Lagergren J. Extended abdominoperineal resection with gluteus maximus flap reconstruction of the pelvic floor for rectal cancer. Br J Surg. 2007;94:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 443] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 3. | Stelzner S, Hellmich G, Sims A, Kittner T, Puffer E, Zimmer J, Bleyl D, Witzigmann H. Long-term outcome of extralevator abdominoperineal excision (ELAPE) for low rectal cancer. Int J Colorectal Dis. 2016;31:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Zhou X, Sun T, Xie H, Zhang Y, Zeng H, Fu W. Extralevator abdominoperineal excision for low rectal cancer: a systematic review and meta-analysis of the short-term outcome. Colorectal Dis. 2015;17:474-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Musters GD, Buskens CJ, Bemelman WA, Tanis PJ. Perineal wound healing after abdominoperineal resection for rectal cancer: a systematic review and meta-analysis. Dis Colon Rectum. 2014;57:1129-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 6. | Buchs NC, Kraus R, Mortensen NJ, Cunningham C, George B, Jones O, Guy R, Ashraf S, Lindsey I, Hompes R. Endoscopically assisted extralevator abdominoperineal excision. Colorectal Dis. 2015;17:O277-O280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Matsuda K, Hotta T, Takifuji K, Yokoyama S, Higashiguchi T, Tominaga T, Oku Y, Nasu T, Tamura K, Yamaue H. Long-term comorbidity of diabetes mellitus is a risk factor for perineal wound complications after an abdominoperineal resection. Langenbecks Arch Surg. 2009;394:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Christian CK, Kwaan MR, Betensky RA, Breen EM, Zinner MJ, Bleday R. Risk factors for perineal wound complications following abdominoperineal resection. Dis Colon Rectum. 2005;48:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | De Caluwé L, Van Nieuwenhove Y, Ceelen WP. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev. 2013;CD006041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Steele SR, Varma MG, Melton GB, Ross HM, Rafferty JF, Buie WD. Practice parameters for anal squamous neoplasms. Dis Colon Rectum. 2012;55:735-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Miller SH, Rudolph R. Healing in the irradiated wound. Clin Plast Surg. 1990;17:503-508. [PubMed] |
| 12. | Bullard KM, Trudel JL, Baxter NN, Rothenberger DA. Primary perineal wound closure after preoperative radiotherapy and abdominoperineal resection has a high incidence of wound failure. Dis Colon Rectum. 2005;48:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 272] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | Aldulaymi BH, Mohammad WA, Jess P. [Perineal wound complications following preoperative radiotherapy for rectal cancer]. Ugeskr Laeger. 2008;170:1225-1227. [PubMed] |
| 14. | Chadwick MA, Vieten D, Pettitt E, Dixon AR, Roe AM. Short course preoperative radiotherapy is the single most important risk factor for perineal wound complications after abdominoperineal excision of the rectum. Colorectal Dis. 2006;8:756-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Butt HZ, Salem MK, Vijaynagar B, Chaudhri S, Singh B. Perineal reconstruction after extra-levator abdominoperineal excision (eLAPE): a systematic review. Int J Colorectal Dis. 2013;28:1459-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Buchel EW, Finical S, Johnson C. Pelvic reconstruction using vertical rectus abdominis musculocutaneous flaps. Ann Plast Surg. 2004;52:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Butler CE, Gündeslioglu AO, Rodriguez-Bigas MA. Outcomes of immediate vertical rectus abdominis myocutaneous flap reconstruction for irradiated abdominoperineal resection defects. J Am Coll Surg. 2008;206:694-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 18. | Tei TM, Stolzenburg T, Buntzen S, Laurberg S, Kjeldsen H. Use of transpelvic rectus abdominis musculocutaneous flap for anal cancer salvage surgery. Br J Surg. 2003;90:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Barker T, Branagan G, Wright E, Crick A, McGuiness C, Chave H. Vertical rectus abdominis myocutaneous flap reconstruction of the perineal defect after abdominoperineal excision is associated with low morbidity. Colorectal Dis. 2013;15:1177-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Lefevre JH, Parc Y, Kernéis S, Shields C, Touboul E, Chaouat M, Tiret E. Abdomino-perineal resection for anal cancer: impact of a vertical rectus abdominis myocutaneus flap on survival, recurrence, morbidity, and wound healing. Ann Surg. 2009;250:707-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Bloemendaal AL, Kraus R, Buchs NC, Hamdy FC, Hompes R, Cogswell L, Guy RJ. Double-barrelled wet colostomy formation after pelvic exenteration for locally advanced or recurrent rectal cancer. Colorectal Dis. 2016;18:O427-O431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Shibata D, Hyland W, Busse P, Kim HK, Sentovich SM, Steele G, Bleday R. Immediate reconstruction of the perineal wound with gracilis muscle flaps following abdominoperineal resection and intraoperative radiation therapy for recurrent carcinoma of the rectum. Ann Surg Oncol. 1999;6:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 181] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Singh B, Lloyd G, Nilsson PJ, Chaudhri S. Laparoscopic extralevator abdominal perineal excision of the rectum: the best of both worlds. Tech Coloproctol. 2012;16:73-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Christensen HK, Nerstrøm P, Tei T, Laurberg S. Perineal repair after extralevator abdominoperineal excision for low rectal cancer. Dis Colon Rectum. 2011;54:711-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Cavallaro A, Lo Menzo E, Di Vita M, Zanghì A, Cavallaro V, Veroux PF, Cappellani A. Use of biological meshes for abdominal wall reconstruction in highly contaminated fields. World J Gastroenterol. 2010;16:1928-1933. [PubMed] |
| 26. | Poussier M, Denève E, Blanc P, Boulay E, Bertrand M, Nedelcu M, Herrero A, Fabre JM, Nocca D. A review of available prosthetic material for abdominal wall repair. J Visc Surg. 2013;150:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Han JG, Wang ZJ, Wei GH, Gao ZG, Yang Y, Zhao BC. Randomized clinical trial of conventional versus cylindrical abdominoperineal resection for locally advanced lower rectal cancer. Am J Surg. 2012;204:274-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Han JG, Wang ZJ, Gao ZG, Xu HM, Yang ZH, Jin ML. Pelvic floor reconstruction using human acellular dermal matrix after cylindrical abdominoperineal resection. Dis Colon Rectum. 2010;53:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Musters GD, Klaver CE, Bosker RJ, Burger JW, van Duijvendijk P, van Etten B, van Geloven AA, de Graaf EJ, Hoff C, Leijtens JW. Biological Mesh Closure of the Pelvic Floor After Extralevator Abdominoperineal Resection for Rectal Cancer: A Multicenter Randomized Controlled Trial (the BIOPEX-study). Ann Surg. 2017;265:1074-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 30. | Jensen KK, Rashid L, Pilsgaard B, Møller P, Wille-Jørgensen P. Pelvic floor reconstruction with a biological mesh after extralevator abdominoperineal excision leads to few perineal hernias and acceptable wound complication rates with minor movement limitations: single-centre experience including clinical examination and interview. Colorectal Dis. 2014;16:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Peacock O, Simpson JA, Tou SI, Hurst NG, Speake WJ, Tierney GM, Lund JN. Outcomes after biological mesh reconstruction of the pelvic floor following extra-levator abdominoperineal excision of rectum (APER). Tech Coloproctol. 2014;18:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Peacock O, Pandya H, Sharp T, Hurst NG, Speake WJ, Tierney GM, Lund JN. Biological mesh reconstruction of perineal wounds following enhanced abdominoperineal excision of rectum (APER). Int J Colorectal Dis. 2012;27:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | FitzGerald JF, Kumar AS. Biologic versus Synthetic Mesh Reinforcement: What are the Pros and Cons? Clin Colon Rectal Surg. 2014;27:140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 34. | Melich G, Lim DR, Hur H, Min BS, Baik SH, Arena GO, Gordon PH, Kim NK. Prevention of perineal hernia after laparoscopic and robotic abdominoperineal resection: review with illustrative case series of internal hernia through pelvic mesh. Can J Surg. 2016;59:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Asplund D, Prytz M, Bock D, Haglind E, Angenete E. Persistent perineal morbidity is common following abdominoperineal excision for rectal cancer. Int J Colorectal Dis. 2015;30:1563-1570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Welsch T, Mategakis V, Contin P, Kulu Y, Büchler MW, Ulrich A. Results of extralevator abdominoperineal resection for low rectal cancer including quality of life and long-term wound complications. Int J Colorectal Dis. 2013;28:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Rothenberger DA, Wong WD. Abdominoperineal resection for adenocarcinoma of the low rectum. World J Surg. 1992;16:478-485. [PubMed] |
| 38. | Wydra D, Emerich J, Sawicki S, Ciach K, Marciniak A. Major complications following exenteration in cases of pelvic malignancy: a 10-year experience. World J Gastroenterol. 2006;12:1115-1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Musters GD, Sloothaak DA, Roodbeen S, van Geloven AA, Bemelman WA, Tanis PJ. Perineal wound healing after abdominoperineal resection for rectal cancer: a two-centre experience in the era of intensified oncological treatment. Int J Colorectal Dis. 2014;29:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Martijnse IS, Holman F, Nieuwenhuijzen GA, Rutten HJ, Nienhuijs SW. Perineal hernia repair after abdominoperineal rectal excision. Dis Colon Rectum. 2012;55:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Mjoli M, Sloothaak DA, Buskens CJ, Bemelman WA, Tanis PJ. Perineal hernia repair after abdominoperineal resection: a pooled analysis. Colorectal Dis. 2012;14:e400-e406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 42. | Aboian E, Winter DC, Metcalf DR, Wolff BG. Perineal hernia after proctectomy: prevalence, risks, and management. Dis Colon Rectum. 2006;49:1564-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Pascual G, Sotomayor S, Pérez-López P, Buján J, Bellón JM. Long term behavior of biological prostheses used as abdominal wall substitutes. Histol Histopathol. 2014;29:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |