Published online Apr 10, 2017. doi: 10.5306/wjco.v8.i2.151
Peer-review started: November 6, 2016
First decision: November 30, 2016
Revised: December 8, 2016
Accepted: December 27, 2016
Article in press: December 29, 2016
Published online: April 10, 2017
Processing time: 154 Days and 11.1 Hours
To stratify the malignancy risks in thyroid nodules in a tertiary care referral center using the Bethesda system.
From January, 2012 to December, 2014, a retrospective analysis was performed among 1188 patients (15-90 years) who had 1433 thyroid nodules and fine-needle aspiration at Prince Sultan Military Medical City, Saudi Arabia. All thyroid cyto-pathological slides and ultra sound reports were reviewed and classified according to the Bethesda System for Reporting Thyroid Cytopathology. Age, gender, cytological features and histological types of the thyroid cancer were collected from patients’ medical chart and cytopathology reports.
There were 124 total cases of malignancy on resection, giving an overall surgical yield malignancy of 33.6%. Majority of the thyroid cancer nodules (n = 57, 46%) in Bethesda VI category followed by Bethesda IV (n = 25, 20.2%). Almost 40% of the cancer nodules in 31-45 age group in both sex. Papillary thyroid carcinoma (PTC) was the most common form of thyroid cancer among the study population (111, 89.6%) followed by 8.9% of follicular thyroid carcinoma (FTC), 0.8% of medullary carcinoma and 0.8% of anaplastic carcinoma. Among the Bethesda IV category 68% thyroid nodules were PTC and 32% FTC.
The malignancy values reported in our research were constant and comparable with the results of other published data with respect to the risk of malignancy. Patients with follicular neoplasm/suspicious for follicular neoplasm and suspicious of malignancy categories, total thyroidectomy is indicted because of the substantial risk of malignancy.
Core tip: The purpose of this study was to stratify the malignancy risks in thyroid nodules in a tertiary care referral center using the Bethesda system. The study found that there were 124 total cases of malignancy on resection, giving an overall surgical yield malignancy of 33.6%. Majority of the thyroid cancer nodules in Bethesda VI category followed by Bethesda IV. Almost 40% of the cancer nodules in 31-45 age group in both sex. Papillary Thyroid Carcinoma was the most common form of thyroid cancer among the study population followed by follicular thyroid carcinoma, medullary carcinoma and anaplastic carcinoma.
- Citation: Al Dawish MA, Robert AA, Muna A, Eyad A, Al Ghamdi A, Al Hajeri K, Thabet MA, Braham R. Bethesda System for Reporting Thyroid Cytopathology: A three-year study at a tertiary care referral center in Saudi Arabia. World J Clin Oncol 2017; 8(2): 151-157
- URL: https://www.wjgnet.com/2218-4333/full/v8/i2/151.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i2.151
According to epidemiological and clinical studies thyroid nodules are commonly encountered in clinical exams, palpable in 5% of the population on thyroid examination and detectable in nearly 60% of those subjected to thyroid ultrasound. While the majority of the nodules are benign (non-cancerous), they are normally the first indicators of thyroid cancer; therefore, further investigations are required to identify the cancerous nodule[1,2].
The last decades have revealed a constant and remarkable rise in the occurrence of thyroid cancer across the world, including Saudi Arabia[3-5]. The Saudi Cancer Registry (SCR) report has registered 890 thyroid cancer cases, in nearly 8.1% of all the newly diagnosed cases in 2012. However, studies revealed variations in the incidence of thyroid cancer globally. Thyroid cancer is the 5th most common cancer among females in the United States, whereas in Saudi Arabia it is the 2nd commonest identified cancer in females, and 8th among males[6]. However, compared with the developed countries, research regarding the malignancy risks in thyroid nodules is still insufficient due to lack of appropriate studies being conducted in these specified areas.
One of the most widely used diagnostic tools is fine-needle aspiration (FNA) cytology with ultrasound imaging to determine the necessity for the surgical excision of a thyroid nodule. Today, molecular genetic biomarker analyses are employed to increase the diagnostic accuracy of the FNA biopsies, and can at times drastically change clinical decision procedures as they become more commonly available and better assessed. FNA cytology (FNAC) continues to remain the initial investigation mode for malignancy in patients with thyroid nodules and the selection of patients for thyroid surgery[7]. This minimally invasive and useful method is highly effective in identifying a large percentage of thyroid nodules as benign and eliminating unnecessary surgery for patients with benign disease[8]. However, because a standardized reporting system is still unavailable, pathologists have been employing varying terminologies and diagnostic criteria, thus causing misunderstanding among the referring clinicians while interpreting cytopathology reports, resulting in non-definitive clinical management[9-11]. In 2007, the National Cancer Institute (NCI) established guidelines employing a standardized nomenclature to interpret thyroid FNAs called the Bethesda System for Reporting Thyroid Cytopathology (BSRTC) which is now accepted as the proposed diagnostic categories for thyroid cancer[12]. This study attempts to stratify the malignancy risks in thyroid nodules in a tertiary care referral center in Saudi Arabia utilizing the Bethesda system.
From January, 2012 to December, 2014 (36 mo), a retrospective analysis was performed among 1188 patients (15-90 years old) who had 1433 thyroid nodules and FNA at Prince Sultan Military Medical City (PSMMC), a 1200 bedded tertiary care center, Riyadh, Saudi Arabia. The PSMMC caters to the patients referred from different regions of Saudi Arabia and considered a worthy representative of Saudi Arabia in general. The study protocol was approved by the Research and Ethics Committee of PSMMC, Riyadh, Saudi Arabia.
All thyroid cytopathological slides and ultra sound reports were reviewed and classified according to the BSRTC system. Age, gender, cytological features and histological types of the study population were collected from patients’ medical chart and cyto-pathology reports.
Currently, the Bethesda system of reporting thyroid cytology (TBSRTC) is used for reporting FNAC specimens of thyroid. According to Cibas[13], this system was innovated in 2007 and consists of six categories: (1) Unsatisfactory (UNS) or nondiagnostic (ND); (2) Benign and nonneoplastic; (3) Atypia of undetermined significance or follicular lesion of undetermined significance (AUS/FLUS); (4) Follicular neoplasm or suspicious for follicular neoplasm (FNS/SFN); (5) Suspicious for, but not diagnostic of, malignancy; and (6) Malignant (Table 1).
| Diagnostic category | Cytological diagnosis | Risk of malignancy, % | Usual management |
| I | Nondiagnostic or unsatisfactory | 1-4 | Repeat FNA with ultrasound guidance |
| II | Benign | 0-3 | Clinical follow-up |
| III | AUS/FLUS | 5-15 | Repeat FNA |
| IV | FNS/SFN | 15-30 | Surgical lobectomy |
| V | Suspicious for malignancy | 60-75 | Near-total thyroidectomy or surgical |
| VI | Malignant | 97-99 | Near-total thyroidectomy |
All FNAs were performed by one of five interventional radiologists under ultrasound (US) guidance, performing 3-5 passes by using 25 gauge needles. On-site FNAs stained with the Diff-Quik stain and adequacy assessment was performed for all samples. All slides interpreted by among of five accredited cyto-pathologists.
The histological diagnoses of thyroid nodules were classified into two types: Benign and nonneoplastic and malignant. For papillary thyroid carcinoma (PTC), subtype variants were documented such as the follicular variant, classical variant, conventional variant and tall cell variant. Also were follicular thyroid carcinoma (FTC) subdivided to minimally invasive follicular thyroid carcinoma (MIFTC) and Widely Invasive follicular thyroid carcinoma (WIFTC).
All statistical calculations were performed using IBM SPSS Statistics (IBM SPSS Statistics for Windows, Version 22, SPSS Inc. an IBM Company) program and Microsoft Excel 2010 (Microsoft Corporation, Seattle, WA, United States). The descriptive analysis of the epidemiological data presented as frequencies, percentages and mean ± standard deviation (SD). χ2 test was performed to find out the variables associated with cancer among the surgical patients.
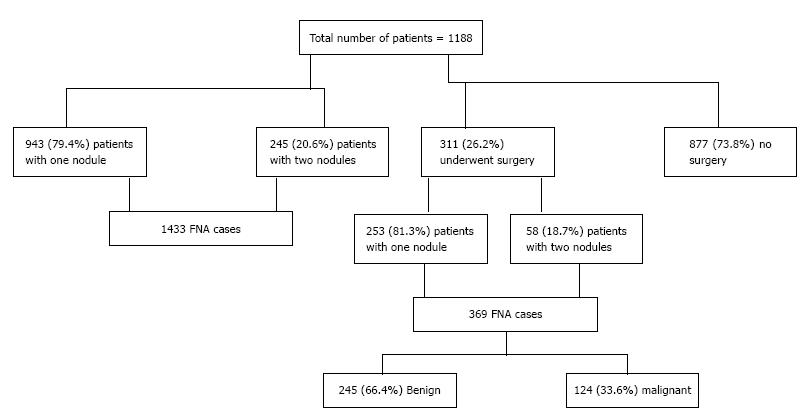
A total of 1188 patients (range 15-90 years) included in this study. The mean age of the study population was 46.3 ± 15.1 (SD), median 45 years, and mode 49 years. Of the 1188 (212 male; 976 female) patients, 245 patients had two thyroid nodules, which resulted in a total of 1433 FNA cases (nodules). Among the study population, a total of 311 patients underwent surgery and 877 patients did not undergo surgery. Of the 311 patients who underwent surgery, 58 patients had two thyroid nodules, which resulted in a total of 369 cases (245 benign and 124 malignant) (Figure 1). Among patients who underwent surgery, no statistically significant differences were observed on the presence of cancer among both gender (P = 0.463), and different age groups (P = 0.928).
As shown in Table 2, the distribution of all cases in the six Bethesda diagnostic categories were as follows: 46 cases (3.2%) of category I, 1080 cases (75.3%) of category II, 131 cases (9.1%) of category III, 71 cases (5%) of category IV, 32 cases (2.2%) of category V and 73 cases (5.1%) of category VI.
| Age (yr) | Total number of patients | Gender | All FNAs (n = 1433) n, % | ||||||
| F/M | Bethesda I | Bethesda II | Bethesda III | Bethesda IV | Bethesda V | Bethesda VI | Total | ||
| 15-30 | 176 (14.8) | 159/17 | 9 (4.5) | 149 (74.9) | 17 (8.5) | 12 (6) | 4 (2) | 8 (4) | 199 |
| 31-45 | 420 (35.4) | 362/58 | 12 (2.4) | 375 (74.7) | 41 (8.2) | 28 (5.6) | 14 (2.8) | 32 (6.4) | 502 |
| 46-60 | 374 (31.5) | 301/73 | 15 (3.3) | 347 (75.1) | 40 (8.8) | 22 (4.8) | 9 (2) | 23 (5) | 456 |
| 61-75 | 175 (14.7) | 126/49 | 10 (4.5) | 162 (72.3) | 33 (14.7) | 7 (3.1) | 4 (1.8) | 8 (3.6) | 224 |
| > 75 | 43 (3.6) | 28/15 | 0 | 47 (90.4) | 0 | 2 (3.8) | 1 (1.9) | 2 (3.8) | 52 |
| Total | 1188 | 976/212 | 46 (3.2) | 1080 (75.3) | 131 (9.1) | 71 (5) | 32 (2.2) | 73 (5.1) | 1433 |
The distributions of follow-up diagnoses for each initial Bethesda diagnostic classification are shown in Table 3. There were 124 total cases of malignancy on resection, giving an overall surgical yield of malignancy of 33.6%. Eight of (2.2%) 369 thyroid nodules were diagnosed as ND, 181 (49.1%) diagnosed as benign, 42 (11.4%) diagnosed as AUS/FLUS, 53 (14.4%) as FNS/SFN. Category V (SM) diagnoses (26 cases) reminded benign in 8 cases, but histologically confirmed as carcinoma in 18 case (69.2%). Finally, category VI diagnoses (59 cases) reminded benign in 2 cases, but histologically confirmed as carcinoma in 57 cases (96.7%).
| Cytopathology | Histopathological diagnosis | Total | |
| Benign | Malignant, n (%) | ||
| Bethesda I | 6 | 2 (25) | 8 |
| Bethesda II | 165 | 16 (8.9) | 181 |
| Bethesda III | 36 | 6 (14.3) | 42 |
| Bethesda IV | 28 | 25 (47.2) | 53 |
| Bethesda V | 8 | 18 (69.3) | 26 |
| Bethesda VI | 2 | 57 (96.7) | 59 |
| Total | 245 | 124 (33.6) | 369 |
Table 4 shows the comparison rates of malignancy on surgical resection for FNA diagnostic categories and malignancy risk of the present findings and previously published data. Table 5 shows the age and sex distribution of thyroid cancer. Majority of the thyroid cancer nodules (n = 57, 46%) in Bethesda VI category followed by Bethesda IV (n = 25, 20.2%) and Bethesda V (n = 18, 14.5%). Among the Bethesda IV category 17 (68%) were PTC and 8 (32%) were follicular carcinoma. Almost 40% of the cancer nodules in 31-45 age groups in both sex.
| Published year | Comparison of diagnostic categories | ||||||
| I (ND) | II (Benign) | III (AUS/FLUS) | IV (FN/SFN) | V (SM) | VI (malignant) | ||
| Recent studies | |||||||
| Park et al[22] | 2014 | 13.3 | 40.6 | 9.1 | 0.4 | 19.3 | 17.6 |
| Mondal et al[10] | 2013 | 1.2 | 87.5 | 1 | 4.2 | 1.4 | 4.7 |
| Mufti et al[29] | 2012 | 11.6 | 77.6 | 0.8 | 4 | 2.4 | 3.6 |
| Wu et al[30] | 2012 | 20.1 | 39 | 27.2 | 8.4 | 2.6 | 2.7 |
| Bongiovanni et al[31] | 2012 | 2 | 54.7 | 6.3 | 25.3 | 6.3 | 5.4 |
| Present study | 3.2 | 75.3 | 9.1 | 5 | 2.2 | 5.1 | |
| Comparison of malignancy risk | |||||||
| Haugen et al[32] (meta-analysis) | 2016 | 9-32 | 1-10 | 6-48 | 14-34 | 53-97 | 94-100 |
| Pantola et al[33] 2016 | 2016 | 0 | 0 | 8.3 | 10 | 100 | 100 |
| Park et al[22] | 2014 | 35.3 | 5.6 | 69 | 50 | 38.7 | 98.9 |
| Mondal et al[10] | 2013 | 0 | 4.5 | 20 | 30.6 | 75 | 97.8 |
| Mufti et al[29] | 2012 | 20 | 3.1 | 50 | 20 | 80 | 100 |
| Wu et al[30] | 2012 | 12 | 8 | 27 | 33 | 68 | 100 |
| Present study | 25 | 8.9 | 14.3 | 47.2 | 69.3 | 96.7 | |
| Age (yr) | Total number of nodules | Gender | All FNAs (n = 124) n, % | |||||
| F/M | Bethesda I | Bethesda II | Bethesda III | Bethesda IV | Bethesda V | Bethesda VI | ||
| 15-30 | 18 (14.5) | 3/15 | 0 | 3 | 1 | 3 | 4 | 7 |
| 31-45 | 49 (39.5) | 39/10 | 1 | 5 | 2 | 9 | 7 | 25 |
| 46-60 | 43 (34.7) | 35/8 | 1 | 7 | 3 | 9 | 5 | 18 |
| 61-75 | 12 (9.7) | 8/4 | 0 | 1 | 0 | 3 | 2 | 6 |
| > 75 | 2 (1.6) | 2/0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Total | 124 | 87/37 | 2 (1.6) | 16 (12.9) | 6 (4.8) | 25 (20.2) | 18 (14.5) | 57 (46) |
Type and variants of thyroid cancer among histopathological diagnosis are shown in Table 6. Papillary carcinoma was the most common form of thyroid cancer among the study population (111, 89.6%). Among PTC (n = 111), four histologic variants exist, with classic variant PTC accounting for 51.4% of PTC followed by follicular-variant PTC (30.6%). Furthermore, 8.9% of malignancies were FTC (including 0.8% of the highest risk widely invasive phenotype), 0.8% of medullary thyroid carcinoma (MTC) and 0.8% of anaplastic thyroid carcinoma (ATC). Among the Bethesda IV category 17 (68%) thyroid nodules were PTC and 8 (32%) were FTC.
| Type of cancer | Total = 124 (n, %) | BETHESDA (n, %) | |||||
| I | II | III | IV (n = 25) | V | VI | ||
| PTC | |||||||
| Classic variant | 57 | 1 | 5 | 1 | 3 | 8 | 39 |
| Follicular variant | 34 | 1 | 8 | 2 | 11 | 6 | 6 |
| Conventional | 19 | 0 | 2 | 2 | 3 | 3 | 9 |
| Tall-cell variant | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Total PTC | 111 (89.6) | 2 | 15 | 5 | 17 (68) | 17 | 55 |
| FTC | |||||||
| MIFTC | 10 | 0 | 1 | 1 | 7 | 1 | 0 |
| WIFTC | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Total FTC | 11 (8.9) | 0 | 1 | 1 | 8 (32) | 1 | 0 |
| MTC | 1 (0.8) | 0 | 0 | 0 | 0 | 0 | 1 |
| ATC | 1 (0.8) | 0 | 0 | 0 | 0 | 0 | 1 |
Over the last few decades thyroid cancer has been on the rise considerably, globally, while mortality has steadily dropped, including in Saudi Arabia[14]. This reduction in the mortality resulting from thyroid cancer reflects the variations in the exposure to risk factors and alters the diagnosis and treatment of the disease, while the rise in the incidence is probably due to the improvement in the identification of this neoplasm[14]. However, in comparison with the developed countries, research on the incidence, prevalence and type of thyroid cancer in Saudi Arabia is still inadequate due to the lack of suitable studies being done on this specific aspect. Therefore, the objective of the current study is to stratify the risk of malignancy in the thyroid nodules based on the Bethesda system, which enhances the interpretation of the FNAC reports and enables a more accurate study and diagnosis of such thyroid nodules[13,15]. In this study, the distribution of age and gender among the patients is almost similar to those recorded in identical studies[1,2,16]. Besides, the female/male ratio reported in this study for thyroid cancer (4.7:1) concurs with the concept that thyroid cancer occurs more commonly among women. In the present study we found that the overall malignant rate was 33.6% which exactly matches the percentage (33.8%) of 25445 thyroid FNAs used in the meta-analysis done by Bongiovanni et al[17], as well as Jo et al[18] who reported 30.9%. However, this high malignancy rate is not unusual if it is understood that the FNAC is consistently being performed today for most patients with thyroid nodules. This has resulted in a drop in the number of unwarranted surgeries and thereby to an increase in the percentage for reported malignancies[1]. It is noteworthy that the number of FNA cases in this study steadily rose from 2012 (n = 357) to 2014 (n = 449). From various studies it was evident that the percentage of cases that were subjected to surgery differed widely among different institutions, reporting a range from 11.8%[19] to 45.1%[20] with an average rate of 25%[17]; the current study identified 26.2% of the study population who had surgical outcome.
Each Bethesda category showed a malignancy rate ranging from 1%-10% (“benign category) to 94%-100% (“malignant” category). This comprehensive range highlights the ability of the Bethesda system to differentiate and determine the likelihood of malignancy. The results recorded in our research concurred closely with the results reported in the American Thyroid Association Management Guidelines and other studies: 25% vs 9%-32% (“non-diagnostic or unsatisfactory” category), 9.3% vs 1%-10% (“benign and non-neoplastic” category), 14.3% vs 6%-48% (AUS/FLUS), 69.2% vs 53%-97% (“suspicious for malignancy” category), and 96.7% vs 94%-100% (“malignant” category)[13,17]. Among Bethesda, category IV found 47.2% malignancy risk, a value higher than the meta-analysis results of 14%-34% (FNS/SFN), published recently by Bongiovanni et al[17]. However, many studies revealed the greatest variation in the risk of malignancy class IV, some of which are higher (malignancy rate 50%-67%) than the present values[21-23].
The current study reported PTC (89.6%) as the commonest type of thyroid cancer in the population under study. Studies also reported that overall PTC as the commonest kind of thyroid cancer represents 80% of all the thyroid malignancies and more than 90% of the differentiated thyroid cancers[13,24,25]. A spurt in the occurrence of PTC over the past decades has triggered greater interest in this disease. This is one of the fastest growing kinds of cancer recording over 20000 new cases annually. Although individuals are susceptible to papillary carcinoma irrespective of age, most patients will show the disease prior to 45 years of age[26], a fact corroborated by the current findings (42% PTC between 31-45 years of age). Unfortunately, FTC is not being diagnosed as often, although there is an increasing incidence of well-differentiated thyroid carcinomas everywhere else[27,28], concurring with the results of the current study.
There are a two limitations to this study, mainly the retrospective design and performance in a single center. As the PSMMC is a tertiary center for thyroid lesions, the data of this study may not precisely reflect the general population. More research is warranted to overcome the limitations of the study.
In conclusion, 33.6% of the cases overall among the surgically excised nodules, showed malignancy. The malignancy values reported in our research were constant and comparable with the results of other data with respect to the risk of malignancy. For the FN/SF patients and those with suspicions of malignancy, total thyroidectomy is indicated because of the substantial risk of malignancy. It is clear, that reviewing the thyroid FNAs with the Bethesda system allowed a more precise cytological diagnosis. However, the impact of Bethesda application may vary among different institutions. Clinicians are advised to be aware of the malignancy rate in the Bethesda categories in their respective institutions to improve the investigation and decision regarding patients with thyroid nodules.
The National Cancer Institute, United States, established guidelines employing a standardized nomenclature to interpret thyroid fine-needle aspirations (FNAs) called the Bethesda System for Reporting Thyroid Cytopathology (BSRTC) which is now accepted as the proposed diagnostic categories for thyroid cancer.
Compared with the developed countries, research regarding the malignancy risks in thyroid nodules is still inadequate due to lack of appropriate studies being conducted in these specified areas in Saudi Arabia. Hence, this present study attempts to stratify the malignancy risks in thyroid nodules in a tertiary care referral center in Saudi Arabia utilizing the Bethesda system.
The study found that there were 124 total cases of malignancy on resection, giving an overall surgical yield malignancy of 33.6%. Majority of the thyroid cancer nodules in Bethesda VI category followed by Bethesda IV. Almost 40% of the cancer nodules in 31-45 age group in both sex. Papillary thyroid carcinoma was the most common form of thyroid cancer among the study population followed by follicular thyroid carcinoma, medullary carcinoma and anaplastic carcinoma.
Reviewing the thyroid FNAs with the Bethesda system allowed a more precise cytological diagnosis. However, the impact of Bethesda application may vary among different institutions. Clinicians are advised to be aware of the malignancy rate in the Bethesda categories in their respective institutions to improve the investigation and decision regarding patients with thyroid nodules.
PTC: Papillary thyroid carcinoma; FTC: Follicular thyroid carcinoma; SCR: Saudi Cancer Registry; FNA: Fine-needle aspiration; FNAC: Fine-needle aspiration cytology; NCI: National Cancer Institute, United States; BSRTC: Bethesda System for Reporting Thyroid Cytopathology; PSMMC: Prince Sultan Military Medical City; TBSRTC: The Bethesda system of reporting thyroid cytology; UNS: Unsatisfactory; ND: Nondiagnostic; AUS/FLUS: Atypia of undetermined significance or follicular lesion of undetermined significance; US: Ultrasound; MIFTC: Minimally invasive follicular thyroid carcinoma; WIFTC: Widely Invasive follicular thyroid carcinoma; ATC: Anaplastic thyroid carcinoma.
The study shows a very exhaustive analysis of the throughput of thyroid cytopathology over a three-year period. The manuscript contains a detailed exposition of the results, including comprehensive tables and a comparison to other recent studies. In my opinion, this manuscript fulfills all the requirements to be published.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: Saudi Arabia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Peters GJ, Velasco I, Xu Z S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Hajmanoochehri F, Rabiee E. FNAC accuracy in diagnosis of thyroid neoplasms considering all diagnostic categories of the Bethesda reporting system: A single-institute experience. J Cytol. 2015;32:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Muratli A, Erdogan N, Sevim S, Unal I, Akyuz S. Diagnostic efficacy and importance of fine-needle aspiration cytology of thyroid nodules. J Cytol. 2014;31:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Alghamdi IG, Hussain II, Alghamdi MS, Dohal AA, Almalki SS, El-Sheemy MA. The incidence rate of thyroid cancer among women in Saudi Arabia: an observational descriptive epidemiological analysis of data from Saudi Cancer Registry 2001-2008. J Immigr Minor Health. 2015;17:638-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Al-Zahrani AS, Ravichandran K. Epidemiology of thyroid cancer: a review with special reference to Gulf Cooperation Council (GCC) states. Gulf J Oncolog. 2007;2:17-28. [PubMed] |
| 5. | Hussain F, Iqbal S, Mehmood A, Bazarbashi S, ElHassan T, Chaudhri N. Incidence of thyroid cancer in the Kingdom of Saudi Arabia, 2000-2010. Hematol Oncol Stem Cell Ther. 2013;6:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Cancer Incidence Report 2012. Saudi Cancer Registry, Saudi Arabia. Available from: http://www.iacr.com.fr/index.php?option=com_comprofiler&task=userprofile&user=1215&Itemid=498. |
| 7. | Mazzaferri EL. Thyroid cancer in thyroid nodules: finding a needle in the haystack. Am J Med. 1992;93:359-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 128] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Cibas ES. Fine-needle aspiration in the work-up of thyroid nodules. Otolaryngol Clin North Am. 2010;43:257-271, vii-viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Lewis CM, Chang KP, Pitman M, Faquin WC, Randolph GW. Thyroid fine-needle aspiration biopsy: variability in reporting. Thyroid. 2009;19:717-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Mondal SK, Sinha S, Basak B, Roy DN, Sinha SK. The Bethesda system for reporting thyroid fine needle aspirates: A cytologic study with histologic follow-up. J Cytol. 2013;30:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Redman R, Yoder BJ, Massoll NA. Perceptions of diagnostic terminology and cytopathologic reporting of fine-needle aspiration biopsies of thyroid nodules: a survey of clinicians and pathologists. Thyroid. 2006;16:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Baloch ZW, LiVolsi VA, Asa SL, Rosai J, Merino MJ, Randolph G, Vielh P, DeMay RM, Sidawy MK, Frable WJ. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 547] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 13. | Cibas ES, Ali SZ. The Bethesda System For Reporting Thyroid Cytopathology. Am J Clin Pathol. 2009;132:658-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1029] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 14. | La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, Negri E. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. 2015;136:2187-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 702] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 15. | Arul P, Masilamani S. A correlative study of solitary thyroid nodules using the bethesda system for reporting thyroid cytopathology. J Cancer Res Ther. 2015;11:617-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Sinna EA, Ezzat N. Diagnostic accuracy of fine needle aspiration cytology in thyroid lesions. J Egypt Natl Canc Inst. 2012;24:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis. Acta Cytol. 2012;56:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 658] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 18. | Jo VY, Stelow EB, Dustin SM, Hanley KZ. Malignancy risk for fine-needle aspiration of thyroid lesions according to the Bethesda System for Reporting Thyroid Cytopathology. Am J Clin Pathol. 2010;134:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 203] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 19. | Theoharis CG, Schofield KM, Hammers L, Udelsman R, Chhieng DC. The Bethesda thyroid fine-needle aspiration classification system: year 1 at an academic institution. Thyroid. 2009;19:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 20. | Bohacek L, Milas M, Mitchell J, Siperstein A, Berber E. Diagnostic accuracy of surgeon-performed ultrasound-guided fine-needle aspiration of thyroid nodules. Ann Surg Oncol. 2012;19:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Lee K, Jung CK, Lee KY, Bae JS, Lim DJ, Jung SL. Application of Bethesda System for Reporting Thyroid Aspiration Cytology. Korean J Patho. 2010;44:521–527. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Park JH, Yoon SO, Son EJ, Kim HM, Nahm JH, Hong S. Incidence and malignancy rates of diagnoses in the bethesda system for reporting thyroid aspiration cytology: an institutional experience. Korean J Pathol. 2014;48:133-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Stamataki M, Anninos D, Brountzos E, Georgoulakis J, Panayiotides J, Christoni Z, Peros G, Karakitsos P. The role of liquid-based cytology in the investigation of thyroid lesions. Cytopathology. 2008;19:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Hu MI, Vassilopoulou-Sellin R, Lustig R, Lamont JP. Thyroid and parathyroid cancers. Cancer Management: A Multidisciplinary Approach. 11th edition. Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ, editors. Louisville, KY, USA: Congregational Ministries Publishing 2008; . |
| 25. | Sosa JA, Udelsman R. Papillary thyroid cancer. Surg Oncol Clin N Am. 2006;15:585-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Preston-Martin S, Franceschi S, Ron E, Negri E. Thyroid cancer pooled analysis from 14 case-control studies: what have we learned? Cancer Causes Control. 2003;14:787-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Liu X, Medici M, Kwong N, Angell TE, Marqusee E, Kim MI, Larsen PR, Cho NL, Nehs MA, Ruan DT. Bethesda Categorization of Thyroid Nodule Cytology and Prediction of Thyroid Cancer Type and Prognosis. Thyroid. 2016;26:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Sobrinho-Simões M, Eloy C, Magalhães J, Lobo C, Amaro T. Follicular thyroid carcinoma. Mod Pathol. 2011;24 Suppl 2:S10-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Mufti ST, Molah R. The bethesda system for reporting thyroid cytopathology: a five-year retrospective review of one center experience. Int J Health Sci (Qassim). 2012;6:159-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Wu HH, Rose C, Elsheikh TM. The Bethesda system for reporting thyroid cytopathology: An experience of 1,382 cases in a community practice setting with the implication for risk of neoplasm and risk of malignancy. Diagn Cytopathol. 2012;40:399-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Bongiovanni M, Crippa S, Baloch Z, Piana S, Spitale A, Pagni F, Mazzucchelli L, Di Bella C, Faquin W. Comparison of 5-tiered and 6-tiered diagnostic systems for the reporting of thyroid cytopathology: a multi-institutional study. Cancer Cytopathol. 2012;120:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10769] [Cited by in RCA: 9624] [Article Influence: 1069.3] [Reference Citation Analysis (1)] |
| 33. | Pantola C, Kala S, Khan L, Pantola S, Singh M, Verma S. Cytological diagnosis of pediatric thyroid nodule in perspective of the Bethesda System for Reporting Thyroid Cytopathology. J Cytol. 2016;33:220-223. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |