Published online Apr 10, 2017. doi: 10.5306/wjco.v8.i2.120
Peer-review started: July 21, 2016
First decision: September 5, 2016
Revised: September 16, 2016
Accepted: October 17, 2016
Article in press: October 18, 2016
Published online: April 10, 2017
Processing time: 261 Days and 14.4 Hours
Breast cancer is the most common type of cancer found in women and today represents a significant challenge to public health. With the latest breakthroughs in molecular biology and immunotherapy, very specific targeted therapies have been tailored to the specific pathophysiology of different types of breast cancers. These recent developments have contributed to a more efficient and specific treatment protocol in breast cancer patients. However, the main challenge to be further investigated still remains the emergence of therapeutic resistance mechanisms, which develop soon after the onset of therapy and need urgent attention and further elucidation. What are the recent emerging molecular resistance mechanisms in breast cancer targeted therapy and what are the best strategies to apply in order to circumvent this important obstacle? The main scope of this review is to provide a thorough update of recent developments in the field and discuss future prospects for preventing resistance mechanisms in the quest to increase overall survival of patients suffering from the disease.
Core tip: There are several reviews in the literature dedicated to breasts cancers. However, our manuscript is an updated review on the current knowledge and particularly on the molecular mechanisms involved in the relapse of patients on the current treatments. A summary of ongoing clinical trials gives a perspective for future therapeutic strategies. Our manuscript represents a working document for researchers/oncologists in the field of breast cancers.
- Citation: Masoud V, Pagès G. Targeted therapies in breast cancer: New challenges to fight against resistance. World J Clin Oncol 2017; 8(2): 120-134
- URL: https://www.wjgnet.com/2218-4333/full/v8/i2/120.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i2.120
Breast cancer targeted therapies involve substances or drugs which block the growth of cancer by interfering with the function of specific molecules responsible for tumor cell proliferation and survival[1-21]. Breast cancer cells may overexpress specific receptors which, when activated can initiate downstream signaling resulting in the expression of genes for cancer cell proliferation, growth, survival, migration, angiogenesis and other vital cell cycle pathways[22,23].
There are various types of breast cancer, some have hormone receptors like estrogen or progesterone (some have both) and are called ER+ or PR+ breast cancer respectively.
The estrogen receptor ER is a major driver of the majority of breast cancers as it is expressed in 75% of breast cancers overall. It is more frequently related with postmenopausal women and there is a 99% survival rate at ten years. Hormone sensitive breast cancer has a strong correlation with lower tumor grade; lower levels of amplification of the human epidermal growth factor receptor 2 (HER2) oncogene and concomitant loss of p53 tumor suppressor gene; expression of progesterone receptor (PR), soft tissue and bone metastases and slower rates of disease recurrence. In cases of hormone positive breast cancer along with the expression of ER, multigene tests may be carried out to make treatment decisions particularly for adjuvant therapy and screen those patients who would benefit more from combination of endocrine plus chemotherapy[24-26].
The most common receptors that are overexpressed in breast cancer cells are part of the epidermal growth factor receptor (EGFR) family of receptor tyrosine kinases: EGFR and HER2 are overexpressed in approximately 40% and 25% of breast cancers respectively and are believed to be responsible for more aggressive tumor behavior and poor prognosis[27].
Triple negative breast cancer (TNBC) is defined by the lack of expression of both estrogen and progesterone as well as the HER2 protein and is often associated with an unfavorable prognosis as no treatment is yet available for this particular breast cancer subtype[28].
The rapid acquisition of resistance in breast cancer targeted therapies seems to limit the effectiveness of treatment and even though some of the genetic mutations and epigenetic changes in molecular pathways have been understood, it is sometimes necessary to combine several pathway blockades in order to achieve successful treatment results[29-35].
The identification of new target molecules in breast cancer and the use of combination therapies may have improved the understanding of compensatory pathways which lead to the emergence of resistance mechanisms, nevertheless, breast cancer subtypes like TNBCs seem to exploit alternative proliferative pathways which are not yet fully understood and need urgent attention and elucidation[11] (Figure 1).
Estrogen and estrogen receptors are key drivers in breast cancer progression. This is the reason why targeting estrogen has been used for many years to inhibit the estrogen signaling pathway in women with estrogen positive breast cancer. Selective estrogen receptor modulators or SERM have been used to suppress tumor growth in estrogen dependent breast cancers and tamoxifen was the first drug to be approved for estrogen positive metastatic breast cancer reducing recurrences by approximately 40%-50%[36].
Aromatase inhibitors (anastrozole, letrozole, exemestane) are also used as an alternative therapy to treat estrogen dependent breast cancers as they block the biosynthesis of androgens through inhibition of the aromatase enzyme resulting in reduction of estrogen levels in tumor cells[36].
Other therapies are available for other forms of breast cancer that are not hormone dependent. The HER2 protein represents the most common overexpressed receptor signature in breast cancer and is considered a relevant biomarker for treatment.
The recombinant antibody trastuzumab ( Herceptin) targets HER2 and is the first drug that was approved by the FDA in 1998 for the treatment of HER2 positive breast cancers[37,38].
Other agents that followed such as pertuzumab and lapatinib have not shown immunity to the development of resistance mechanisms with significant side effects for the patients[7,39,40].
The conjugated monoclonal antibody TDM1 (trastuzumab emtansine) may be used in HER2 positive breast cancers as trastuzumab efficiently transports the DM1 drug, a microtubule inhibitor, directly into the breast cancer cells to inhibit growth.
Triple negative cancers lacking hormone receptors and HER2 may respond to agents like PARP1 inhibitors and may have HER1 as a potential target. The monoclonal antibody cetuximab combined with cisplatin chemotherapy has shown promising results in a Phase II study, suggesting some subtypes of TNBC may be EGFR inhibition sensitive[41].
The conventional route to treat TNBC patients by taxol derivatives and anthracycline chemotherapy is still widely used until more “druggable” targets are identified[41]. Recent studies suggest that the microtubule-stabilizing agent ixabepilone in combination with capecitabine may be effective in TNBC that are resistant to anthracycline and taxane drugs and the PACS08 Phase III trial is evaluating this possible treatment strategy[28].
Targeted therapies have also been approved against the vascular endothelial growth factor (VEGF) and the drug bevacizumab has proven effective in the treatment of advanced metastatic breast cancer when used in association with paclitaxel or docetaxel[42,43].
Inhibitors of downstream pathways like PI3K/AKT/ mTOR and RAS/MEK/ERK are also available for therapeutic purpose as well as agents directed against other tyrosine kinases like SRC, insulin-like-growth-factor [IGF/IGF-receptor (IGFR)], poly-ADP ribose polymerase (PARP) Inhibitors and also matrix metalloproteases (MMPs) which are involved in cancer cell invasion and metastasis[8,29,31,44-48].
Compensatory survival pathways, increased phosphatydil-inositol-3-kinase (PI3K)[49-52] signaling, receptor tyrosine kinase signaling outside the ErbB/HER family and involvement of other HER receptors[53], may all play a key role in the development of alternative molecular pathways responsible for the development of therapeutic resistance in breast cancer cells.
Breast cancer targeted therapies are used to treat patients whose breast cancer cells overexpress certain characteristic proteins on their surface allowing an abnormal growth pattern. Antibodies are sometimes used as they work in a similar way as the human immune system.
The most efficient breast cancer targeted therapy today is the one targeting the HER2 protein overexpression on the surface of breast cancer cells. At present, there are seven widely used breast cancer targeted therapies which are effective in blocking several molecular pathways: Afinitor or everolimus, an m-TOR inhibitor, stops cancer cells from getting energy supplies[54-57]; Avastin or bevacizumab inhibits the growth of new blood vessels which supply oxygen and nutrients to cancer cells for growth and function[14,58]; Herceptin or trastuzumab blocks the ability of cancer cells to receive signals which tell them to grow[12,59]; Kadcyla or T-DM1 is a combination of Herceptin and emtansine[7,60]. In this case Herceptin is used as a transport method to deliver the emtansine chemotherapy to cancer cells; Perjita or pertuzumab works by stopping cancer cells from receiving growth signals[12,61]; Tykerb or lapatinib is a HER2 inhibitor that blocks signals of cell growth[4,42].
The HER2 proto-oncogene is overexpressed in 10%-12% of over 2500 cases of human breast cancers and this is associated with malignant transformation and poorer overall survival rates particularly in breast tumors with lymph node metastasis[62].
The HER2 or neu oncogene (erbB2) is part of the EGFR family of tyrosine kinases and is located on chromosome 17 (17q12). It represents the most common overexpressed receptor in breast cancers and is considered a relevant therapeutic target[59,63-69].
The EGFR family is composed of four receptors: EGFR/HER1, ErbB2/HER2, ErbB3/HER3 and ErbB4/HER4. These receptors share common domains: an extracellular region characterized by leucine-rich repeats; cysteine rich repeats in the intracellular domain; a single transmembrane spanning region; a short juxtamembrane region; a kinase region and a cytoplasmic tail with various tyrosine phosphorylation sites[5,10]. Binding of ligands to the extracellular domain of EGFR, HER3 and HER4 allows for the formation of kinase active homo- and hetero-dimers to which HER2 is recruited as a preferential partner. Heterodimer formation between HER2/HER3 is the most common occurrence in these receptors’ preferences. HER3 is often responsible for the activation of the PI3K/AKT signaling pathway via six docking sites for the p85 adaptor subunit of PI3K. The HER3/PI3K axis plays a key role in the survival of HER2-dependent cells as the loss of HER3 inhibits the survival of HER2-overexpressing breast cancer cells[70,71].
The first recombinant antibody approved by the FDA to target HER2-positive breast cancers was trastuzumab or Herceptin followed by other agents like pertuzumab and lapatinib.
Trastuzumab binds to the juxtamembrane region of the HER2 receptor tyrosine kinase resulting in the uncoupling of the HER2/HER3 heterodimers and consequent inhibition of downstream signaling and cytotoxicity.
Resistance mechanisms to trastuzumab develop often as a result of HER2 gene amplification and RNA/protein overexpression. HER2 overexpressing tumor cells continue to depend on the HER2 oncogene even after bypassing trastuzumab action possibly due to signaling from receptor tyrosine kinases (RTK) outside the ErbB family, increased PI3K signaling and the presence of alternative forms of HER2 which are not detected by trastuzumab. Also, the modulation of Cdk inhibitor p27 by IGF-1 may be a key player in resistance to trastuzumab as overexpressed IGF-1 is responsible for the activation of the PI3K downstream signaling pathway and further effects on Akt[72,73]. One of the key players in trastuzumab-resistance in HER2 positive breast cancer was the inhibition of expression of miR-375, a tumor suppressor gene which targets IGF1R[74]. Also, molecular pathway crosstalk may have resulted in increased cell survival and division by interference with HER2 accessibility, independent downstream signaling activation as well as HER2 mutations, particularly the expression of p95HER2, an active truncated form of HER2. Blocking IGFR1 completely resulted in restored sensitivity of HER2 positive cancer cells to trastuzumab in vitro. The loss of miR-375 with consequent epigenetic changes such as DNA methylation and histone deacetylation may drive the upregulation of IGFR1 and hence the development of trastuzumab-resistant cancer cells; in this case, targeting miR-375 may prove to be worthy of further investigation as a potential therapeutic target to restore trastuzumab sensitivity in HER2 positive breast cancer cells[74].
The new antibody-drug conjugate trastuzumab-DM1 (TDM1) which has been recently developed for the treatment of HER2 positive cancer has proved to be effective in inhibiting trastuzumab sensitive and resistant HER2 positive breast cancer cell lines in vitro. TDM1 drives both apoptosis and mitotic catastrophe in the trastuzumab resistant breast cancer cell line Jimt-1, acting as a potent inhibitor of microtubule assembly. These cells are characterized for having several co-existing trastuzumab resistance mechanisms like a mutation in the PIK3CA gene, low PTEN expression, overexpression of NRG1 and a moderate expression of the HER2 receptor. Interestingly, in the T-DM1 treated Jimt-1 cell line model, an accumulation of HER2 was observed in organelles which resembled enlarged lysosomes, suggesting sequestration of the protein in these intracellular granules[75].
The integrin αvβ6, involved in promoting migration, invasion and cancer cell survival, seems to play a significant role in the development of trastuzumab resistance mechanisms. Targeting αvβ6 with the 264RAD antibody in HER2 positive breast cancer cell lines expressing both HER2 and the integrin seems to slow down the growth of trastuzumab resistant tumors[62].
Resistance of breast cancer cells to trastuzumab mediated cytotoxicity has been implicated in the secretion of soluble factors by adipocytes and preadipocytes in adipose tissue proximal to breast cancer cells. The development of resistance mechanisms in this case occurs by inhibition of trastuzumab-mediated tumor lysis by natural killer cells in vitro and by adipose tissue in vivo. A reduced antitumor effect was observed in mice which had tumors in close proximity to a lipoma, while in another group of mice which had tumors located distant to the lipoma, the trastuzumab anti-tumor effects were enhanced. The inhibition of antitumor activity was enhanced when the adipocytes were in hypoxic conditions, these factors might suggest a link between patient obesity and development of trastuzumab resistance mechanisms[76].
The dual targeting of HER family receptors with antibody therapy may prove to be a strategy to overcome acquired resistance mechanisms by cancer cells to cetuximab. When both HER3 and EGFR were neutralized by cetuximab and the anti HER3 monoclonal antibody U3-1287, cetuximab sensitive tumor cells showed a significant decrease in proliferation possibly due to inhibition of both MAPK and AKT pathways and a diminished signaling from all three HER family receptors[77].
The efficacy of trastuzumab in inhibiting proliferation of breast cancer cells might be dependent on the presence of endogenous HER-receptor activating ligands EGF and heregulin-β1; the receptor density of HER-family members and growth ligands are key players in the development of resistance mechanisms to trastuzumab therapy, which interfers with cell cycle kinetics by inducing a G1 accumulation in HER-2 positive breast adenocarcinomas[78].
An unexpected mechanism of resistance is associated with down-stream mutations especially those targeting the mRNA binding protein tristetraprolin (TTP). ttp gene germinal mutation generates a form of TTP mRNA which is inefficiently translated in protein. The lack of TTP and the general increase of the TTP competitor the ELAV-like protein 1 (HuR) results in the increase of the half-life of mRNAs encoding oncogenes, inflammatory and angiogenic factors. The mutation of TTP is predictive of trastuzumab resistance[79]. Hence TTP is considered as a tumor suppressor for breast cancers[80-82]. TTP and HuR are phosphorylated by the same kinases and phosphorylation has antagonistic effects on both proteins (inactivation/degradation for TTP and activation/stabilization for HuR). Hence, activation of intracellular signaling pathways results in a general increase of proteins associated with oncogenic properties[83] (Figure 1).
The main drawback in trastuzumab therapy is represented by the emergence of serious cardiac side effects resulting from administration of this monoclonal antibody. Analysis of HER2 specific mutation may predict cardiac toxic effect[84].
HER-2 is expressed in the adult human myocardium and trastuzumab therapy unfortunately carries the risk of inducing cardiac dysfunction and congestive heart failure. When adjacent chemotherapy is applied in addition to trastuzumab, one has to take into consideration anthracyclin-associated cardiotoxicity following the inhibition of the HER-2/erbB2 receptor to ensure safety for patients. Some of the cardiotoxicity side effects of trastuzumab may be reversible over time and in some cases, administering the monoclonal antibody after chemotherapy or radiotherapy may decrease the risk of potential cardiac side effects. Trastuzumab therapy seems to represent clear overall benefits for patients in the long run, therefore, should be still considered as an appropriate standard choice of treatment as a HER-2/erbB2 inhibitor as long as care is taken to minimize its side effects[85].
Resistance to hormone therapy is a major challenge within hormone sensitive breast cancers even though ER and PR targeted therapy has proven to be very effective, improving the quality of life of hormone sensitive breast cancer patients. The major pathways responsible for endocrine resistance mechanisms might be several: The HER tyrosine kinase receptor family; receptors for insulin/IGF1, FGF and VEGF, Src, AKT, stress related kinases, might each play a pivotal role in contributing to endocrine therapy resistance when their cognate ligands are amplified or overexpressed.
Cross-talk between the estrogen receptor (ER) and growth factor receptor signaling with hyperactivation of the PI3K pathway have also been associated the development of endocrine resistance[86].
Nuclear receptors and the androgen receptors may also act as alternative growth stimulators by post translational modification, enabling the bypass of ER inhibition. Co-targeting the EGFR and HER2 pathway simultaneously seems to be the most promising way forward in circumventing endocrine resistance as these two seem to be the most important factors responsible in this particular resistance scenario[26].
The mTOR pathway seems to be a master regulator of cell physiology and may be a key player in the targeted therapy of cancer[87]. When the natural product rapamycin was discovered in the early 1970’s as an antifungal agent, it emerged in later studies that the molecule could halt growth in many types of eukaryotic cells and have a powerful immunosuppressive function. In 1999 the FDA approved sirolimus as a drug used against rejection of transplanted organs particularly the kidneys. Rapamycin binds to another molecule, FKBP12 and once this complex is formed it associates with a protein called mTOR[88]; a serine/threonine kinase, resembling the kinase domain of PI3 kinase and its related enzymes. The circuitry of the mTOR pathway is of interest as it represents a key element of the mammalian cell cycle integrating incoming signals and vital mechanisms such as glucose import and protein synthesis, as well as phosphorylating two kinases involved in translation: S6 kinase (S6KI) and 4E-BP1[89,90]. The activation of S6KI is followed by the activation of the small 40-S ribosomal subunit which can initiate protein synthesis after associating to the large ribosomal subunit. mTOR is also a key upstream regulator which controls the AKT signaling pathway for the regulation of apoptosis and proliferation; inhibiting the mTOR complex results in a shutdown of the AKT signaling stream resulting in an hyperactivated PI3K/loss of PTEN expression[91,92].
The PI3K/AKT/mTOR pathway is overactivated in 70% of breast cancers and the protein kinases found along these pathways may be potential drug targets for breast cancer therapy. Due to the large scale involvement of this pathway the cell cycle regulation, selectively silencing of the PI3K/AKT/mTOR pathway represents an attractive approach for patients who might have shown resistance mechanisms to previous types of therapy. The combination of mTOR inhibitors with other targeted therapies might be a winning formula to circumvent resistance mechanisms of breast cancer patients.
Inhibition of the mTOR pathway by the drug everolimus in combination with HER-2 or estrogen receptor inhibitors may be a promising future strategy to apply, in order to reinstate sensitivity of breast cancer cells to traditional therapies and overcome resistance mechanisms which seem to emerge when the mTOR pathway is functioning in hyperactive mode[93]. Molecular alterations like mutations in EGFR, BRAF, AKT, or PI3K are associated with activation of downstream signaling pathways resulting in unrestricted proliferation in cancer cells.
Glaysher et al[94] have shown that targeting a breast epithelial cell line after having knocked-in mutations and using EGFR and mTOR inhibitors, there was an increased sensitivity to therapeutic drugs. As development of resistance in breast cancer cells may be a result of the activation of the PI3K/AKT/mTOR pathway, Glaysher et al[94] studied the effects of inhibiting both mTOR and EGFR by combined drug action of ZSTK474/sirolimus and erlotinib/gefitinib, observing a more effective signaling blockade, as opposed to use of single agents on the parental cell line and irrespective of the knocked-in mutations in EGFR,KRAS,PI3K,BRAF or AKT[94].
Lapatinib is a dual EGFR/HER2 tyrosine kinase inhibitor which acts as an ATP competitor. It is used as a first line monotherapy in patients with HER2 positive metastatic breast cancer in addition to conventional chemotherapy like paclitaxel.
Unfortunately, the activation of compensatory pathways after onset of therapy with lapatinib seems to be responsible for the emergence of resistance mechanisms, particularly when inhibition of AKT phosphorylation leads to increased estrogen receptor-α transcription and estrogen receptor signaling. This mechanism of resistance can be circumvented by administering an ER-down-regulator fulvestrant, which can prevent the proliferation of lapatinib resistant cells. In addition, mutations in the HER2 protein, particularly a YVMA insertion at G776 in exon 20, seems to be responsible for mechanisms of de novo resistance to lapatinib as well as trastuzumab[73].
The inhibitory effects of lapatinib may be bypassed as downstream signaling is amplified and upregulation of activated HER3 becomes responsible for compromising the inhibitory effects of tyrosine kinases.
Activation of the PI3K/AKT pathway results from HER3 upregulation with a subsequent nuclear increase in FoxO3A family of transcription factors responsible for control of cell cycle, neoplastic transformation and epithelial-to-mesenchymal transition[30].
Targeting erb-B3 (HER3) with an antibody has proven to be quite effective in both preclinical and clinical studies although tumor cells eventually develop resistance as the antibody is only active in inhibiting signaling without altering the actual expression of the erb-B3 receptors. New strategies which aim at reducing erb-B3 levels are being investigated such as the HDAC inhibitor entinostat and the antisense oligonucleotide EZN-3920[95].
The hepatocyte growth factor receptor HGFR/c-Met tyrosine kinase responsible for cell proliferation, protection from apoptosis and cell invasion, seems to be implicated in the emergence of resistance to targeted therapies particularly lapatinib and trastuzumab and recent preclinical studies suggested that inhibition of c-MET in gastric cancer cell lines circumvented resistance mechanisms as well as restored growth inhibition[96].
The overexpression of the receptor tyrosine kinase AXL is associated with poor prognosis and a more aggressive phenotype in ovarian, breast colon, esophageal, thyroid and lung cancers and may be implicated in the emergence of lapatinib acquired resistance in in vitro models of preclinical breast cancer studies.
Lapatinib resistance has been also associated with SRC tyrosine kinase activity; overexpression of SRC in breast cancer cell lines seems to result in an increased interaction with EGFR rather than HER2. According to Formisano et al[97], when EGFR was inhibited with the monoclonal antibody cetuximab and SRC was inhibited by the small molecule saracatinib, lapatinib resistant breast cancer cells would not survive and sensitivity was restored. The combined treatment of lapatinib with cetuximab both in vitro and in vivo resulted in the reduction of EGFR/HER2 signaling and proved to be effective[97].
As observed by Wilson et al[98], autocrine tumor cell production might be responsible for increased levels of receptor tyrosine kinase-ligand levels and in breast cancer cell lines the HER3 ligand neuregulin-1 seems to induce complete rescue from lapatinib.
An additional mechanism of resistance to lapatinib may occur as a result of crosstalk between the estrogen receptor and the HER2 pathway. Lapatinib induced upregulation of ER by inhibition of the PI3K/AKT signaling pathway results in overexpression of the anti-apoptotic protein Bcl-2 leading to the emergence of lapatinib resistance and cell death escape[99].
The VEGF and its cell surface receptors represent the main modulators in the emergence of tumor angiogenesis. Avastin or bevacizumab, a humanized anti-VEGF antibody, has played a key role in anti-angiogenic therapy for cancer treatment in concomitance with small molecule VEGF receptor kinase inhibitors[43].
The VEGF ligand presents itself as an antiparallel homodimeric structure in which each monomer is made mostly of β strands stabilized by a disulfide knot and two symmetrically disposed intermolecular disulfide bridges that are responsible for linking the monomers together. On the extracellular domain of each of the three VEGF receptors (VEGF-1, VEGF-2, VEGF-3) there are seven immunoglobulin-like structures (Ig domain)[100,101].
All four members of the VEGF family and the placenta growth factor bind to three endothelial cell tyrosine kinase receptors which have each different functions. VEGFR1 is responsible for promoting differentiation and vascular maintenance, VEGFR2 induction of endothelial cell proliferation and vascular permeability, VEGFR3 stimulation of lynphangiogenesis. Isoform specific receptors neuropilin-1 and neuropilin-2 may bind to class 3 semaphorins involved in axonal growth and also to some isoforms of VEGF1 as co-receptors which results in additional VEGF binding to VEGFR2[102].
Several other pathways are implicated by the function of VEGF as proteolytic and heparin activation further modulates receptor sites resulting in various cellular effects like the increase of vascular permeability, endothelial cell proliferation, survival and tubular formation. The VEGFR are usually endothelial in origin but in some instances they may be located in the stroma as macrophages and tumor cells themselves. Under abnormally low oxygen conditions (hypoxia), the hypoxia-inducible factor (HIF) plays a central role in transcription of genes like VEGF.
In normoxic conditions, the alpha-subunit of the HIF heterodimer (alpha, beta) is degraded by ubiquitylation as HIF-alpha binds to the von Hippel-Lindau tumor suppressor protein (p-VHL) forming the E3 ubiquitin ligase complex, a recognition component leading to proteasome-dependent degradation. In hypoxic conditions, as the HIF-alpha subunit is stabilized by heterodimerization with HIF-beta and hypoxia response elements (HRE), regulatory elements of HIF target genes are activated including VEGF, genes controlling cell proliferation and cell metabolism[103,104].
VEGF is one of the genes that is upregulated in hypoxia microenvironments eliciting a particular vascular phenotype; the high expression of VEGF is a common prognostic factor in human breast cancer malignancies representing an important therapeutic target. Other family members though play a role in angiogenesis even when VEGF is not expressed, in addition to the function of these homologues, the switching of angiogenic pathways may represent an area for further investigation to be possibly circumvented by multiple pathway inhibition[105].
Emerging patient data suggests that the combination of the anti-angiogenic drug bevacizumab with chemotherapy agents such as paclitaxel has proven to be a very dangerous therapeutic choice in terms of fatal side effects including hemorrhage, neutropenia, perforations of the gastrointestinal tract, blockage of arteries and stroke[106].
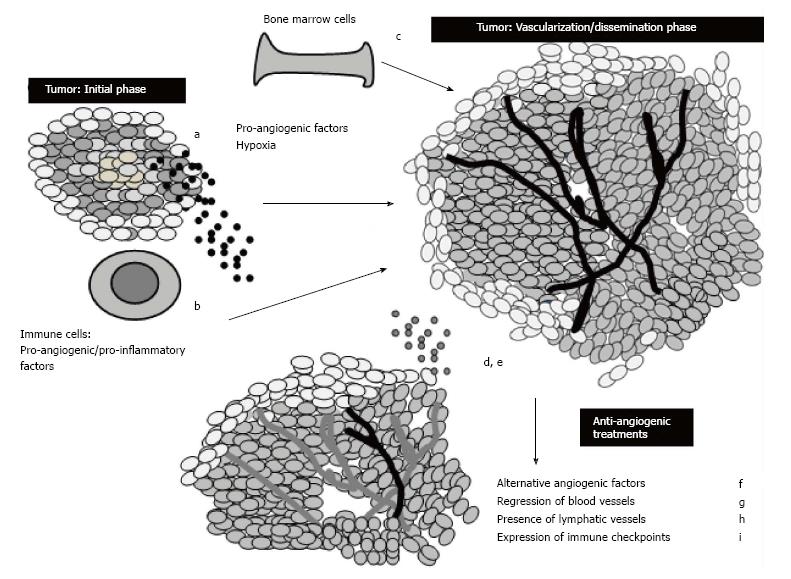
Several mechanisms are implicated in the emergence of resistance mechanisms to anti-angiogenic therapy (Figure 2). The most prominent one relates to the promiscuity of cancer cells to produce many types of alternative angiogenic signals which limit drug efficacy. The rescue of tumor vascularization may occur as escape mechanisms are induced by anti-angiogenic therapy and hypoxia of tumor tissue.
Cancer cells may amplify angiogenic genes which in return do not respond to low doses of anti-angiogenic drugs; they may switch from vessel sprouting to vessel co-option, vasculogenesis or vascular mimicry in order to ensure tumor nutrients. The recruitment of bone-marrow derived cells by cancer cells may result in the secretion of pro-angiogenic factors like angiopoietin, fibroblast growth factor or ephrins. The VEGF receptors may induce the release of a cytokine cascade which results in an inflamed microenvironment allowing for the emergence of tumor extravasation and metastasis.
Some of the alternative targets to overcome drug resistance to anti-angiogenesis therapies might be to target the placental growth factor and Bv8 (Bombina variegata) to reduce tumor inflammation, reduce leakiness of vessels, moderate hypoxia and reduce angiogenesis; the Notch pathway by anti-delta like ligands 4 (DII4) and secretase inhibitors to reduce excessive sprouting and reduce leaky dysfunctional vessels. Vessel normalization may be achieved by PHD2 inhibition improving vessel function and reducing metastasis and hypoxia. Lymphangiogenesis may be targeted by inhibiting neuropilin-2 (Npn2) and by targeting neuropilin-1, tumor growth and angiogenesis can be significantly reduced[107].
Several alternative pathways may take over as resistance develops to anti-angiogenic therapy through intrinsic tumor resistance or acquired resistance: angiogenic redundancy involves the production of redundant pro-angiogenic factors like the fibroblast growth factors (FGFs), platelet derived growth factors (PDGFs), placenta growth factor (PlGF), tumor necrosis factor-α (TNF-α). As these pro-angiogenic factors allow for the growth of tumor vasculature despite the VEGF pathway being inhibited it would be appropriate to target several of them synergistically.
The increase of tumor hypoxia as a result of anti-angiogenic therapy is often implicated in angiogenic redundancy: The overexpression of the hypoxia-induced factor-1 (HIF-1) is correlated with chemotherapy resistance and selection of aggressive cancer cells as it is directly involved in the induction of transcription of genes involved in angiogenesis. The important role of activating the membrane tyrosine kinase receptor c-MET by the hepatocyte growth factor during angiogenesis, allows for downstream activation of SRC, AKT, MEK, STAT3 with an increased expression of VEGF and its receptor by endothelial cells. The HGF/c-MET collaboration is often associated with invasive cancer phenotypes and increased metastasis. In these cases, the selection of more invasive tumor cells may occur as hypoxic environments pressure cancer cells to move rapidly toward normoxic locations. The recruitment of bone marrow derived pro-angiogenic cells and inflammatory cell invasion may contribute to adaptive mechanisms of resistance as low oxygen concentrations induce these cells to release large amounts of pro-angiogenic factors. As alterations in endothelial cells and pericytes may be responsible for crosstalk between angiogenic pathways resulting in the emergence of anti-angiogenesis therapy resistance, inhibiting the VEGF pathway and the platelet derived growth factor receptor with a tyrosine kinase inhibitor simultaneously might be a promising strategy to enhance treatment efficacy. The process of vessel co-option may result in cancer cells displaying a normal looking vasculature which is less sensitive to anti-angiogenic therapy and early stage tumors may escape inhibition as they grow in an angiogenesis independent fashion[108].
The future of anti-angiogenic therapy seems to depend on how different tumors become vascularized and by what alternative pathways these manage to escape therapeutic effects. Elucidation of the complexity of the biology of angiogenesis, coupled with the function of key biomarkers, may prove to be a promising way forward to enhance the function of anti-angiogenic therapy to achieve vascular normalization and increase the effects of complementary chemotherapy.
TNBC represent 10%-20% of invasive breast cancers in the general population and have been associated with the African-American ethnic group where a clear prevalence of the disease affects up to 28% of all patients within that group[109].
About 80% of breast tumors which lack the overexpression of the HER-2/erbB2 protein, the estrogen receptor (ER) and the progesterone receptor (PgR) fall under the category of TNBC. They may be characterized by elevated levels of PARP enzymes and often originate from basal-like cell types. TNBC represent the most aggressive phenotype of the disease with no specific targeted therapies available for treatment. Twelve percent of TNBC are characterized by a claudin-low subtype; these can be identified by DNA microarray expression profiling, a method slowly emerging in clinical practice for the detection of this rare form of breast cancer. These tumors seem to respond to molecules which target DNA repair systems to induce synthetic lethality if used in combination with other drugs. PARP inhibitors are an example of therapeutic choice when one of the genes in a synthetic lethal pair, with one gene already defective, is targeted resulting in cell death. PARP iso-enzymes include a group of 18 molecules which are central to base-excision repair pathways of single strand DNA breaks. An example is the BRCA1-2 mutation in breast cancer, this scenario allows for PARP inhibitors to target and block the only functioning DNA repair system, hence, the selective killing of tumor cells while sparing healthy ones and limiting toxicity for the patient[110]. Nuclear basic fibroblast growth factor (bFGF) is a protein found in a subset of TNBC which contributes to the emergence of resistance following chemotherapy[111]. In vitro studies have shown that a residual TNBC subpopulation remains after short-term chemotherapy and this resumes proliferation over time. When bFGF was knocked down in these residual cancer cells using short hairpin RNA, the number of residual TNBC cells decreased. This phenomenon is linked to a down-regulation of DNA-dependent protein kinase (DNA-PK) responsible for accelerated DNA repair. This study might suggest that expression of bFGF in TNBC cells could be a prognostic predictor of incomplete chemotherapy response and future tumor recurrence in TNBC patients[111].
The main challenge of circumventing treatment induced resistance mechanisms and the emergence of alternative escape pathways, significantly lowers the overall survival rate of breast cancer patients belonging to this particular subtype as they often exhibit an incomplete pathological response[93].
Sunitinib seems to suppress angiogenesis, tumor proliferation, migration and growth of basal like breast cancer cells; xenograft models indicate that tumor volumes decrease under sunitinib action but due to its effects on the Notch-1 protein expression and hypoxia through HIF-1, there was an increase in proliferation of breast cancer stem cells. The use of a γ-secretase inhibitor in addition to sunitinib may represent a promising treatment option for TNBC while simultaneously targeting cancer stem cells and angiogenesis[112].
Sunitinib may prove to be an effective treatment choice for patients with TNBC as this breast cancer subtype may express increased levels of VEGF. High levels of VEGF may act as a potential prognostic factor in TNBC as the vascular pathway is a key component when targeting this particularly rare subtype of breast cancer[113].
As targeted therapies have not yet been discovered for TNBC, the conventional route is to treat patients with chemotherapy particularly anthracycline and taxane. The multitude of pathways which drive proliferation of this particular breast cancer subtype need to be further investigated in order to isolate potential therapeutic targets. Patients with BRCA1 and BRCA2 gene mutations which are present in 20% of TNBC, may be sensitive to the function of PARP inhibitors in addition to chemotherapy[6].
In a Phase II clinical trial carried out to evaluate the combined administration of the PARP1 inhibitor iniparib with cisplatin and gemcitabine on patients with TNBC, iniparib seemed to show significant anticancer activity enhancing the antiproliferative and cytotoxic effects of cisplatin and gemcitabine[114]. Combination therapy of cisplatin, gemcitabine and iniparib is currently under Phase III clinical trial to see if this association could represent the new standard of care for the treatment of TNBC (ClinicalTrials.gov No.NCT00938652).
Breast cancer has been considered non-immunogenic for quite a long time and only recently, data has suggested that TNBC and HER2 positive types are characterized by an immune infiltrate, which might prove to be a promising target to complement the function of other synergistic drugs. Solid tumors like melanoma and lung cancer have already responded to immunotherapeutic agents like ipilimumab and sipuleucel-T has proven a successful vaccine against castration-resistant prostate cancers. Ongoing studies are also evaluating to what extent immune response is correlated to prognosis in breast cancer (Table 1).
| Target pathway | Current therapy | Combination therapy |
| HER2 (HER2-positive breast cancer) | Trastuzumab/herceptin Pertuzumab lapatinib | Combination trastuzumab/lapatinib (EPHOS-B trial) trastuzumab/264RAD |
| m-TOR pathway | Everolimus | Possible combination everolimus/HER2 inhibitor |
| Angiogenesis (VEGF) | Bevacizumab paclitaxel Docetaxel | Targeting the placental growth factor and Bv8/Targeting the Notch pathway by anti-delta like ligands 4 and secretase inhibitors inhibiting simultaneously the VEGF pathway and the platelet derived growth factor receptor with a TK inhibitor |
| DNA repair mechanisms (TNBC) Notch-1 protein over-expression/breast cancer stem cells proliferation (TNBC) | Parp inhibitors/anthracyclins and taxanes | Possible combination cisplatin/gemcitabine/iniparib Possible combination of g-secretase inhibitor in addition to sunitinib |
| Immune system response Cell cycle checkpoints | Immunotherapeutic agents Antibodies against PD-1 T-cell inhibitory molecule or its ligand PD-L1 | Nelipepimut-S(human leukocyte antigen)/GM-CSF Pembrolizumab in TNBC/PD-L1 positive (KEYNOTE-086 trial) |
The aim of immunotherapy is that of activating the human immune response to recognize tumors as a foreign entity and eventually kill the tumor cells. The tumor microenvironment (TME) including T-regulatory cells (T-Reg) involves a complex structure of intercellular communication which represent a very promising area of research aiming at the isolation of key immunogenic targets which may enhance the function of existing therapies[115].
The immune-checkpoint receptor PD-1 is expressed on tumor-infiltrating lymphocytes (TILs) with the role of inhibiting the activity of effector T-cells, preventing autoimmunity and inflammatory response; it is often upregulated on tumor cell surface in many types of solid tumors. The PD-1 ligand PDL1 engages with T-cells resulting in upregulation of the receptor followed by an immunosuppressive signal, which inhibits kinases involved in the activation of the immune response[116]. Clinical blockade of the PD-1/PDL1 axis should enhance antibody function in cancer patients underlining the importance of further investigation in this particular area of breast cancer research (Table 2). Pro-inflammatory cytokines and the overexpression of PDL1 inhibitory ligand may play a key role in the development of cancer immune resistance mechanisms, resulting in a state of exhausted or tolerant immune T-cell response hence the importance of studying the possible role of PDL1 expression as a resistance biomarker. Overall, main role of PD-1 blockade results in the reversal of chronic antigen response which is often found in cancer and viral infection scenarios[117]. The anti PD-1 antibody nivolumab has shown successful activity in melanoma and lung cancer patients targeting these immunoregulatory proteins and enhancing tumor response. There are several other ligands being investigated at present which might be potential targets like: CD80, CD86, PDL2, ICOS-L, B7-H3, B7-H4 and B7-H6 and future directions in cancer immunotherapy research point towards the effects of combined checkpoint blockade to maximize clinical response[118].
| Title of clinical trial | Type of breast cancer |
| Phase III clinical trial: NEUVAX: nelipepimut-S or E75NCT01479244 | HER1+ HER2+ |
| Phase II clinical trial: NEUVAX NCTO1570036 | Node positive or TNBC |
| Phase I clinical trial: Pembrolizumab PD1 antibody + dendritic cell vaccine NCTO2479230 | Metastatic breast cancer |
| Phase II trial: Pembrolizumab PD1 antibody + HDAC inhibitor and anti-estrogen therapy NCT02395627 | Breast cancer |
| Phase II first line neo adjuvant trial: Atezolizumab + chemotherapy NCTO2530489 | TNBC |
| Phase I clinical trial: Atezolizumab and HER2 inhibitors NCTO2605915 | HER2+ |
| Phase I/II clinical trial: PDR001(PD1 antibody) | Advanced breast cancer, TNBC |
| Phase I/II clinical trial: MEDI6469 anti OX40 antibody NCTO1642290 | Stage 4 breast cancer (patients with prior failure of hormone or chemotherapy) |
| Pilot study of QBX258 targeting IL-4 and IL-13 NTCO2494206 | Advanced TNBC whose cancer cells make a protein called glycoprotein NMB to which CDX-011 binds |
Over the last few years, new agents have been introduced in breast cancer targeted therapy resulting in overall improved treatments and greater patient overall survival rates. Some of the most widely used combination therapies involve the use of agents which target the PI3K/AKT/mTOR pathways such as everolimus combined to exemestane. The everolimus-FKBP12 complex that forms when the m-TOR inhibitor binds with high affinity to the intracellular receptor FKBP12, is very effective in inhibiting down strem signaling in cancer cells. The BOLERO study has demonstrated the efficacy of the m-TOR inhibitor everolimus used in combination with exemestane (endocrine therapy) to restore hormonal sensitivity in breast cancer patients[6]. Palbocib has been combined with letrozole in treating women with ER positive (estrogen positive), HER2 negative, advanced breast cancers as a first line endocrine therapy in metastatic cases. Trastuzumab and lapatinib have been used successfully in combination to treat metastatic breast cancers that overexpress HER2[6]. Trastuzumab and pertuzumab have been used in combination for the treatment of HER2 positive metastatic breast cancers and have shown a statistically significant increase in overall survival of patients[6]. The trastuzumab/lapatinib/hormonal therapy combination has proven to be effective in cases of hormonal receptor positive and overexpressed HER2 protein breast cancers like the luminal B/HER2 enriched type. Iniparib, a PARP1 inhibitor, in combination with gemcitabine and carboplatin chemotherapy have been evaluated in a Phase I clinical trial for the treatment of metastatic TNBCs and a clinical benefit of 56% was observed in the combined therapy arm, compared to the gemcitabine/carboplatin arm which had a 34% clinical benefit[114].
A promising area of clinical research for breast cancer targeted therapy involves the use of immune checkpoints inhibitors or immune checkpoint stimulatory molecules. In order to unleash anti-cancer immune responses, inhibitory molecules are blocked or stimulatory molecules are activated to allow the immune system to attack directly cancer cells as foreign invaders. An example would be the anti PD1 antibody pembrolizumab (Keytruda), anti CTLA antibodies, the anti PD-L1 antibody atezolizumab, anti CD73 antibodies or anti OX40 antibodies being tested currently in Phase I/II clinical trials (Table 2).
As the importance of the TME is being discovered with its potential contribution to cancer therapy, novel agents are being developed to target the non-malignant tumor stroma like trabectedin which inhibits macrophage differentiation; other drugs target the tumor necrosis factor-related apoptosis inducing ligand (TRAIL) pathway such as mapatumumab and dulanermin; immunomodulators used alone or in combination to cytotoxic agents should be also investigated as a strategy to decrease the immunosuppression caused by T-effector cell upregulation in the quest to increase the innate immune response against cancer cells, keeping the right balance as immune over-stimulation could be potentially harmful to patients[86].
The main future challenge for breast cancer combination therapy is to design a winning formula that is simultaneously effective against the many subtypes of breast cancers like luminal A, luminal B, basal-like and overexpressing HER2. This approach would represent a hopeful avenue to explore in the quest to inhibit the multitude of pathways being exploited by the various breast cancer subtypes. The phenotype of each breast cancer subtype should be thoroughly investigated as well, to allow researchers to gather a general picture describing in detail the different mechanisms of action for cell survival. Only then, more precise targets can be identified allowing for the discovery of more inclusive breast cancer combination therapies. A more precise and personalized characterization of each cancer as well as the identification of factors involved in resistance for each patient may provide useful improvements in current therapeutic approaches.
Decoding of the human genome has allowed for the isolation of key gene signatures for many types of known cancers; unfortunately, targeted therapies to inhibit the function of these genes have proven quite elusive as the quest to circumvent the emergence of resistance mechanisms continues. Breast cancer subtypes, particularly TNBCs, still represent a major challenge; future studies should revolve around the discovery of new prognostic biomarkers as no targets for these rare types of breast cancer have yet been identified.
The EPHOS-B trial carried out by researchers in The Institute of Cancer Research, London, the University of Manchester and University Hospital of South Manchester NHS Foundation Trust investigating the response of HER2 positive breast cancer to dual lapatinib and trastuzumab therapy shortly after diagnosis and surgery to remove the tumors, has released very promising data in which of 257 women who were administered the two drugs synergistically 11 d before surgery, 17% had only minimal residual disease with invasive tumor smaller than 5 mm in size, 11% had a pathological complete response with no biological invasive tumor present in the breast and 3% had a complete response. This dramatic response after only 11 d suggests that combination anti-HER2 targeted therapy prior surgery may reduce the number of breast cancer patients requiring chemotherapy in the future and significantly eliminate long term chemotherapy associated side effects[4,119].
Resistance mechanisms in breast cancer targeted therapies represent the main challenge to current research; the combination of different molecules used to target different levels of signaling pathways by synergistically blocking cancer cell escape routes and minimizing the emergence of survival mechanisms, could prove to be a promising way forward, keeping in mind that specific molecular profiling particularly for metastatic relapses should be carried out to elucidate further resistance phenotypes and allow for the design of specific new targets. Several clinical trials are underway to try to improve survival of the worse cases (Table 3).
| Title of clinical trial | Type of cancer |
| Randomized open label Phase II trial: Kadcyla, tykerb and abraxane vs herceptin, tykerb and taxol before Surgery for HER2+ tumors NCT02073487 | HER2+ |
| Phase III randomised, placebo controlled clinical trial: Chemotherapy and a PARP-inhibitor for BRCA1/2+, HER2- advanced breast cancer NCTO2163694 | HER2-, BRCA1/2+ metastatic or locally advanced unresectable breast cancer |
| Phase II, multicenter, randomized clinical trial: Alisertib with taxol for advanced ER+/HER2- or TNBC NCTO2187991 | ER+/HER2- TNBC |
| Phase II Clinical trial: Gemzar, herceptin and perjeta for HER2+ metastatic breast cancer NCTO2252887 | HER2+ metastatic breast cancer |
| Phase I clinical trial: CD-839 for advanced breast tumors NCTO2071862 | Advanced breast cancer and solid tumors |
| Phase I clinical trial: Saracatinib and anastrozole for ER-positive disease NCTO1216176 | ER+ |
| Randomised Phase III clinical trial: Hormone therapy with or without ibrance for HR+, HER2- stage II-III breast cancer NCTO2513394 | HR+, HER2- |
| Phase II clinical trial: CDK-inhibitor for previously treated metastatic disease NCTO1037790 | Previously treated metastatic breast cancer |
| Phase I clinical trial: GS-5745 in metastatic HER2- breast cancer and other solid tumors NCTO1813282 | Metastatic HER2- breast cancer not responding to other treatments |
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: France
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Song J, Shao R, Wei JF, Wang L S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Rugo HS. Dosing and Safety Implications for Oncologists When Administering Everolimus to Patients With Hormone Receptor-Positive Breast Cancer. Clin Breast Cancer. 2016;16:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Mittendorf EA, Vila J, Tucker SL, Chavez-MacGregor M, Smith BD, Symmans WF, Sahin AA, Hortobagyi GN, Hunt KK. The Neo-Bioscore Update for Staging Breast Cancer Treated With Neoadjuvant Chemotherapy: Incorporation of Prognostic Biologic Factors Into Staging After Treatment. JAMA Oncol. 2016;2:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 3. | Mittendorf EA, Ardavanis A, Symanowski J, Murray JL, Shumway NM, Litton JK, Hale DF, Perez SA, Anastasopoulou EA, Pistamaltzian NF. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence. Ann Oncol. 2016;27:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | Clavarezza M, Puntoni M, Gennari A, Paleari L, Provinciali N, D’Amico M, DeCensi A. Dual Block with Lapatinib and Trastuzumab Versus Single-Agent Trastuzumab Combined with Chemotherapy as Neoadjuvant Treatment of HER2-Positive Breast Cancer: A Meta-analysis of Randomized Trials. Clin Cancer Res. 2016;22:4594-4603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Rimawi MF, Schiff R, Osborne CK. Targeting HER2 for the treatment of breast cancer. Annu Rev Med. 2015;66:111-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 205] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 6. | Zanardi E, Bregni G, de Braud F, Di Cosimo S. Better Together: Targeted Combination Therapies in Breast Cancer. Semin Oncol. 2015;42:887-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Moasser MM, Krop IE. The Evolving Landscape of HER2 Targeting in Breast Cancer. JAMA Oncol. 2015;1:1154-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 8. | Kalimutho M, Parsons K, Mittal D, López JA, Srihari S, Khanna KK. Targeted Therapies for Triple-Negative Breast Cancer: Combating a Stubborn Disease. Trends Pharmacol Sci. 2015;36:822-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 218] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 9. | Yamamoto-Ibusuki M, Arnedos M, André F. Targeted therapies for ER+/HER2- metastatic breast cancer. BMC Med. 2015;13:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Elster N, Collins DM, Toomey S, Crown J, Eustace AJ, Hennessy BT. HER2-family signalling mechanisms, clinical implications and targeting in breast cancer. Breast Cancer Res Treat. 2015;149:5-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Marmé F, Schneeweiss A. Targeted Therapies in Triple-Negative Breast Cancer. Breast Care (Basel). 2015;10:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Giordano SH, Temin S, Kirshner JJ, Chandarlapaty S, Crews JR, Davidson NE, Esteva FJ, Gonzalez-Angulo AM, Krop I, Levinson J. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:2078-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 259] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 13. | Akin S, Babacan T, Sarici F, Altundag K. A novel targeted therapy in breast cancer: cyclin dependent kinase inhibitors. J BUON. 2014;19:42-46. [PubMed] |
| 14. | Gianni L, Romieu GH, Lichinitser M, Serrano SV, Mansutti M, Pivot X, Mariani P, Andre F, Chan A, Lipatov O. AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol. 2013;31:1719-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 199] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 15. | Kaumaya PT, Foy KC. Peptide vaccines and targeting HER and VEGF proteins may offer a potentially new paradigm in cancer immunotherapy. Future Oncol. 2012;8:961-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Miles D, Zielinski C, Martin M, Vrdoljak E, Robert N. Combining capecitabine and bevacizumab in metastatic breast cancer: a comprehensive review. Eur J Cancer. 2012;48:482-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Duffy MJ, O’Donovan N, Crown J. Use of molecular markers for predicting therapy response in cancer patients. Cancer Treat Rev. 2011;37:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Palmieri C, Krell J, James CR, Harper-Wynne C, Misra V, Cleator S, Miles D. Rechallenging with anthracyclines and taxanes in metastatic breast cancer. Nat Rev Clin Oncol. 2010;7:561-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Kerbel RS. Issues regarding improving the impact of antiangiogenic drugs for the treatment of breast cancer. Breast. 2009;18 Suppl 3:S41-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Ito Y, Tokudome N, Sugihara T, Takahashi S, Hatake K. Does lapatinib, a small-molecule tyrosine kinase inhibitor, constitute a breakthrough in the treatment of breast cancer? Breast Cancer. 2007;14:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005;23:7350-7360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 686] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 22. | Pal SK, Childs BH, Pegram M. Triple negative breast cancer: unmet medical needs. Breast Cancer Res Treat. 2011;125:627-636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 23. | Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4920] [Cited by in RCA: 5102] [Article Influence: 212.6] [Reference Citation Analysis (1)] |
| 24. | Murphy CG, Dickler MN. Endocrine resistance in hormone-responsive breast cancer: mechanisms and therapeutic strategies. Endocr Relat Cancer. 2016;23:R337-R352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 25. | Fan W, Chang J, Fu P. Endocrine therapy resistance in breast cancer: current status, possible mechanisms and overcoming strategies. Future Med Chem. 2015;7:1511-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 26. | Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 866] [Cited by in RCA: 910] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 27. | Nuciforo P, Radosevic-Robin N, Ng T, Scaltriti M. Quantification of HER family receptors in breast cancer. Breast Cancer Res. 2015;17:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N. Triple-negative breast cancer--current status and future directions. Ann Oncol. 2009;20:1913-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 470] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 29. | Wei W, Lewis MT. Identifying and targeting tumor-initiating cells in the treatment of breast cancer. Endocr Relat Cancer. 2015;22:R135-R155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | D'Amato V, Raimondo L, Formisano L, Giuliano M, De Placido S, Rosa R, Bianco R. Mechanisms of lapatinib resistance in HER2-driven breast cancer. Cancer Treat Rev. 2015;41:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 31. | Wilks ST. Potential of overcoming resistance to HER2-targeted therapies through the PI3K/Akt/mTOR pathway. Breast. 2015;24:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 32. | Furth PA. STAT signaling in different breast cancer sub-types. Mol Cell Endocrinol. 2014;382:612-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Duru N, Candas D, Jiang G, Li JJ. Breast cancer adaptive resistance: HER2 and cancer stem cell repopulation in a heterogeneous tumor society. J Cancer Res Clin Oncol. 2014;140:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Lackner MR, Wilson TR, Settleman J. Mechanisms of acquired resistance to targeted cancer therapies. Future Oncol. 2012;8:999-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 35. | Rexer BN, Arteaga CL. Intrinsic and acquired resistance to HER2-targeted therapies in HER2 gene-amplified breast cancer: mechanisms and clinical implications. Crit Rev Oncog. 2012;17:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 263] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 36. | den Hollander P, Savage MI, Brown PH. Targeted therapy for breast cancer prevention. Front Oncol. 2013;3:250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 37. | Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8204] [Cited by in RCA: 8120] [Article Influence: 338.3] [Reference Citation Analysis (0)] |
| 38. | Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, Baly D, Baughman SA, Twaddell T, Glaspy JA. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16:2659-2671. [PubMed] |
| 39. | Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1303] [Cited by in RCA: 1504] [Article Influence: 150.4] [Reference Citation Analysis (0)] |
| 40. | Maximiano S, Magalhães P, Guerreiro MP, Morgado M. Trastuzumab in the Treatment of Breast Cancer. BioDrugs. 2016;30:75-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 41. | Higgins MJ, Baselga J. Targeted therapies for breast cancer. J Clin Invest. 2011;121:3797-3803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 42. | Kawalec P, Łopuch S, Mikrut A. Effectiveness of targeted therapy in patients with previously untreated metastatic breast cancer: a systematic review and meta-analysis. Clin Breast Cancer. 2015;15:90-100.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666-2676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2393] [Cited by in RCA: 2300] [Article Influence: 127.8] [Reference Citation Analysis (0)] |
| 44. | Almstedt K, Schmidt M. Targeted Therapies Overcoming Endocrine Resistance in Hormone Receptor-Positive Breast Cancer. Breast Care (Basel). 2015;10:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Dominguez-Brauer C, Thu KL, Mason JM, Blaser H, Bray MR, Mak TW. Targeting Mitosis in Cancer: Emerging Strategies. Mol Cell. 2015;60:524-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 355] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 46. | Jamdade VS, Sethi N, Mundhe NA, Kumar P, Lahkar M, Sinha N. Therapeutic targets of triple-negative breast cancer: a review. Br J Pharmacol. 2015;172:4228-4237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 47. | Redmond KL, Papafili A, Lawler M, Van Schaeybroeck S. Overcoming Resistance to Targeted Therapies in Cancer. Semin Oncol. 2015;42:896-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 48. | Munagala R, Aqil F, Gupta RC. Promising molecular targeted therapies in breast cancer. Indian J Pharmacol. 2011;43:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 49. | Azim HA, Kassem L, Treilleux I, Wang Q, El Enein MA, Anis SE, Bachelot T. Analysis of PI3K/mTOR Pathway Biomarkers and Their Prognostic Value in Women with Hormone Receptor-Positive, HER2-Negative Early Breast Cancer. Transl Oncol. 2016;9:114-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Chamberlin MD, Bernhardt EB, Miller TW. Clinical Implementation of Novel Targeted Therapeutics in Advanced Breast Cancer. J Cell Biochem. 2016;117:2454-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Weinberg MA. RES-529: a PI3K/AKT/mTOR pathway inhibitor that dissociates the mTORC1 and mTORC2 complexes. Anticancer Drugs. 2016;27:475-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 52. | Yang F, Lyu S, Dong S, Liu Y, Zhang X, Wang O. Expression profile analysis of long noncoding RNA in HER-2-enriched subtype breast cancer by next-generation sequencing and bioinformatics. Onco Targets Ther. 2016;9:761-772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 53. | Dey N, Williams C, Leyland-Jones B, De P. A critical role for HER3 in HER2-amplified and non-amplified breast cancers: function of a kinase-dead RTK. Am J Transl Res. 2015;7:733-750. [PubMed] |
| 54. | Steelman LS, Martelli AM, Cocco L, Libra M, Nicoletti F, Abrams SL, McCubrey JA. The Therapeutic Potential of mTOR Inhibitors in Breast Cancer. Br J Clin Pharmacol. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 55. | Nicolini A, Ferrari P, Kotlarova L, Rossi G, Biava PM. The PI3K-AKt-mTOR Pathway and New Tools to Prevent Acquired Hormone Resistance in Breast Cancer. Curr Pharm Biotechnol. 2015;16:804-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Lee JJ, Loh K, Yap YS. PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol Med. 2015;12:342-354. [PubMed] |
| 57. | Johnston SR. Enhancing Endocrine Therapy for Hormone Receptor-Positive Advanced Breast Cancer: Cotargeting Signaling Pathways. J Natl Cancer Inst. 2015;107:pii: djv212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 58. | Chan A, Miles DW, Pivot X. Bevacizumab in combination with taxanes for the first-line treatment of metastatic breast cancer. Ann Oncol. 2010;21:2305-2315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Denduluri N, Somerfield MR, Eisen A, Holloway JN, Hurria A, King TA, Lyman GH, Partridge AH, Telli ML, Trudeau ME. Selection of Optimal Adjuvant Chemotherapy Regimens for Human Epidermal Growth Factor Receptor 2 (HER2) -Negative and Adjuvant Targeted Therapy for HER2-Positive Breast Cancers: An American Society of Clinical Oncology Guideline Adaptation of the Cancer Care Ontario Clinical Practice Guideline. J Clin Oncol. 2016;34:2416-2427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 60. | Boyraz B, Sendur MA, Aksoy S, Babacan T, Roach EC, Kizilarslanoglu MC, Petekkaya I, Altundag K. Trastuzumab emtansine (T-DM1) for HER2-positive breast cancer. Curr Med Res Opin. 2013;29:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 61. | Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1741] [Cited by in RCA: 1833] [Article Influence: 141.0] [Reference Citation Analysis (0)] |
| 62. | Moore KM, Thomas GJ, Duffy SW, Warwick J, Gabe R, Chou P, Ellis IO, Green AR, Haider S, Brouilette K. Therapeutic targeting of integrin αvβ6 in breast cancer. J Natl Cancer Inst. 2014;106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 63. | Asif HM, Sultana S, Ahmed S, Akhtar N, Tariq M. HER-2 Positive Breast Cancer - a Mini-Review. Asian Pac J Cancer Prev. 2016;17:1609-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 64. | De Mattos-Arruda L, Shen R, Reis-Filho JS, Cortés J. Translating neoadjuvant therapy into survival benefits: one size does not fit all. Nat Rev Clin Oncol. 2016;13:566-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 65. | Esparis-Ogando A, Montero JC, Arribas J, Ocana A, Pandiella A. Targeting the EGF/HER Ligand-Receptor System in Cancer. Curr Pharm Des. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 66. | Kanaya N, Somlo G, Wu J, Frankel P, Kai M, Liu X, Wu SV, Nguyen D, Chan N, Hsieh MY. Characterization of patient-derived tumor xenografts (PDXs) as models for estrogen receptor positive (ER+HER2- and ER+HER2+) breast cancers. J Steroid Biochem Mol Biol. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Sapino A. Predictive Pathology in the Target Therapy Era in Breast Cancer. Curr Drug Targets. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 68. | Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I, Martinelli E. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 733] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 69. | Tsiambas E, Ragos V, Lefas AY, Georgiannos SN, Rigopoulos DN, Georgakopoulos G, Stamatelopoulos A, Grapsa D, Syrigos K. Molecular assays in detecting EGFR gene aberrations: an updated HER2-dependent algorithm for interpreting gene signals; a short technical report. J BUON. 2016;21:512-515. [PubMed] |
| 70. | Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci USA. 2003;100:8933-8938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 762] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 71. | Holbro T, Civenni G, Hynes NE. The ErbB receptors and their role in cancer progression. Exp Cell Res. 2003;284:99-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 461] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 72. | Ahmed S, Sami A, Xiang J. HER2-directed therapy: current treatment options for HER2-positive breast cancer. Breast Cancer. 2015;22:101-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 73. | Garrett JT, Arteaga CL. Resistance to HER2-directed antibodies and tyrosine kinase inhibitors: mechanisms and clinical implications. Cancer Biol Ther. 2011;11:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 74. | Ye XM, Zhu HY, Bai WD, Wang T, Wang L, Chen Y, Yang AG, Jia LT. Epigenetic silencing of miR-375 induces trastuzumab resistance in HER2-positive breast cancer by targeting IGF1R. BMC Cancer. 2014;14:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 75. | Barok M, Tanner M, Köninki K, Isola J. Trastuzumab-DM1 causes tumour growth inhibition by mitotic catastrophe in trastuzumab-resistant breast cancer cells in vivo. Breast Cancer Res. 2011;13:R46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 76. | Duong MN, Cleret A, Matera EL, Chettab K, Mathé D, Valsesia-Wittmann S, Clémenceau B, Dumontet C. Adipose cells promote resistance of breast cancer cells to trastuzumab-mediated antibody-dependent cellular cytotoxicity. Breast Cancer Res. 2015;17:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 77. | Iida M, Brand TM, Starr MM, Huppert EJ, Luthar N, Bahrar H, Coan JP, Pearson HE, Salgia R, Wheeler DL. Overcoming acquired resistance to cetuximab by dual targeting HER family receptors with antibody-based therapy. Mol Cancer. 2014;13:242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 78. | Hurrell T, Outhoff K. The in vitro influences of epidermal growth factor and heregulin-β1 on the efficacy of trastuzumab used in Her-2 positive breast adenocarcinoma. Cancer Cell Int. 2013;13:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 79. | Griseri P, Bourcier C, Hieblot C, Essafi-Benkhadir K, Chamorey E, Touriol C, Pagès G. A synonymous polymorphism of the Tristetraprolin (TTP) gene, an AU-rich mRNA-binding protein, affects translation efficiency and response to Herceptin treatment in breast cancer patients. Hum Mol Genet. 2011;20:4556-4568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 80. | Al-Souhibani N, Al-Ahmadi W, Hesketh JE, Blackshear PJ, Khabar KS. The RNA-binding zinc-finger protein tristetraprolin regulates AU-rich mRNAs involved in breast cancer-related processes. Oncogene. 2010;29:4205-4215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 81. | Brennan SE, Kuwano Y, Alkharouf N, Blackshear PJ, Gorospe M, Wilson GM. The mRNA-destabilizing protein tristetraprolin is suppressed in many cancers, altering tumorigenic phenotypes and patient prognosis. Cancer Res. 2009;69:5168-5176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 82. | Griseri P, Pagès G. Regulation of the mRNA half-life in breast cancer. World J Clin Oncol. 2014;5:323-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 83. | Griseri P, Pagès G. Control of pro-angiogenic cytokine mRNA half-life in cancer: the role of AU-rich elements and associated proteins. J Interferon Cytokine Res. 2014;34:242-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 84. | Beauclair S, Formento P, Fischel JL, Lescaut W, Largillier R, Chamorey E, Hofman P, Ferrero JM, Pagès G, Milano G. Role of the HER2 [Ile655Val] genetic polymorphism in tumorogenesis and in the risk of trastuzumab-related cardiotoxicity. Ann Oncol. 2007;18:1335-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 85. | Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, Perren T, Passalacqua R, Bighin C, Klijn JG. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol. 2007;25:3859-3865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 407] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 86. | Di Leo A, Curigliano G, Diéras V, Malorni L, Sotiriou C, Swanton C, Thompson A, Tutt A, Piccart M. New approaches for improving outcomes in breast cancer in Europe. Breast. 2015;24:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 87. | Hatem R, El Botty R, Chateau-Joubert S, Servely JL, Labiod D, de Plater L, Assayag F, Coussy F, Callens C, Vacher S. Targeting mTOR pathway inhibits tumor growth in different molecular subtypes of triple-negative breast cancers. Oncotarget. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 88. | De P, Miskimins K, Dey N, Leyland-Jones B. Promise of rapalogues versus mTOR kinase inhibitors in subset specific breast cancer: old targets new hope. Cancer Treat Rev. 2013;39:403-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 89. | Ni J, Ramkissoon SH, Xie S, Goel S, Stover DG, Guo H, Luu V, Marco E, Ramkissoon LA, Kang YJ. Combination inhibition of PI3K and mTORC1 yields durable remissions in mice bearing orthotopic patient-derived xenografts of HER2-positive breast cancer brain metastases. Nat Med. 2016;22:723-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 90. | Rojo F, Najera L, Lirola J, Jiménez J, Guzmán M, Sabadell MD, Baselga J, Ramon y Cajal S. 4E-binding protein 1, a cell signaling hallmark in breast cancer that correlates with pathologic grade and prognosis. Clin Cancer Res. 2007;13:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 91. | Chaffer CL, Weinberg RA. How does multistep tumorigenesis really proceed? Cancer Discov. 2015;5:22-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 92. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [PubMed] [DOI] [Full Text] |
| 93. | Grunt TW, Mariani GL. Novel approaches for molecular targeted therapy of breast cancer: interfering with PI3K/AKT/mTOR signaling. Curr Cancer Drug Targets. 2013;13:188-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 94. | Glaysher S, Bolton LM, Johnson P, Torrance C, Cree IA. Activity of EGFR, mTOR and PI3K inhibitors in an isogenic breast cell line model. BMC Res Notes. 2014;7:397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 95. | Ma J, Lyu H, Huang J, Liu B. Targeting of erbB3 receptor to overcome resistance in cancer treatment. Mol Cancer. 2014;13:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 96. | Chen CT, Kim H, Liska D, Gao S, Christensen JG, Weiser MR. MET activation mediates resistance to lapatinib inhibition of HER2-amplified gastric cancer cells. Mol Cancer Ther. 2012;11:660-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 97. | Formisano L, Nappi L, Rosa R, Marciano R, D’Amato C, D’Amato V, Damiano V, Raimondo L, Iommelli F, Scorziello A. Epidermal growth factor-receptor activation modulates Src-dependent resistance to lapatinib in breast cancer models. Breast Cancer Res. 2014;16:R45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 98. | Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 972] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 99. | Giuliano M, Hu H, Wang YC, Fu X, Nardone A, Herrera S, Mao S, Contreras A, Gutierrez C, Wang T. Upregulation of ER Signaling as an Adaptive Mechanism of Cell Survival in HER2-Positive Breast Tumors Treated with Anti-HER2 Therapy. Clin Cancer Res. 2015;21:3995-4003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 100. | Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 772] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 101. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6747] [Cited by in RCA: 6925] [Article Influence: 314.8] [Reference Citation Analysis (0)] |
| 102. | Bielenberg DR, Pettaway CA, Takashima S, Klagsbrun M. Neuropilins in neoplasms: expression, regulation, and function. Exp Cell Res. 2006;312:584-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 103. | Kaelin WG, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2073] [Cited by in RCA: 2445] [Article Influence: 143.8] [Reference Citation Analysis (0)] |
| 104. | Mole DR, Maxwell PH, Pugh CW, Ratcliffe PJ. Regulation of HIF by the von Hippel-Lindau tumour suppressor: implications for cellular oxygen sensing. IUBMB Life. 2001;52:43-47. [PubMed] |
| 105. | Fox SB, Generali DG, Harris AL. Breast tumour angiogenesis. Breast Cancer Res. 2007;9:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 106. | Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA. 2011;305:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 325] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 107. | Loges S, Schmidt T, Carmeliet P. Mechanisms of resistance to anti-angiogenic therapy and development of third-generation anti-angiogenic drug candidates. Genes Cancer. 2010;1:12-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 108. | Giuliano S, Pagès G. Mechanisms of resistance to anti-angiogenesis therapies. Biochimie. 2013;95:1110-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 109. | Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol. 2012;23 Suppl 6:vi7-v12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 411] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 110. | Banerjee S, Kaye SB. New strategies in the treatment of ovarian cancer: current clinical perspectives and future potential. Clin Cancer Res. 2013;19:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 111. | Li S, Payne S, Wang F, Claus P, Su Z, Groth J, Geradts J, de Ridder G, Alvarez R, Marcom PK. Nuclear basic fibroblast growth factor regulates triple-negative breast cancer chemo-resistance. Breast Cancer Res. 2015;17:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 112. | Chinchar E, Makey KL, Gibson J, Chen F, Cole SA, Megason GC, Vijayakumar S, Miele L, Gu JW. Sunitinib significantly suppresses the proliferation, migration, apoptosis resistance, tumor angiogenesis and growth of triple-negative breast cancers but increases breast cancer stem cells. Vasc Cell. 2014;6:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 113. | Linderholm BK, Hellborg H, Johansson U, Elmberger G, Skoog L, Lehtiö J, Lewensohn R. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann Oncol. 2009;20:1639-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 114. | O'Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, Koo IC, Sherman BM, Bradley C. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364:205-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 606] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 115. | Mittendorf EA, Peoples GE. Injecting Hope--A Review of Breast Cancer Vaccines. Oncology (Williston Park). 2016;30:475-481, 485. [PubMed] |
| 116. | Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM, Akcakanat A. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 1036] [Article Influence: 94.2] [Reference Citation Analysis (0)] |
| 117. | Ramsay AG. Immune checkpoint blockade immunotherapy to activate anti-tumour T-cell immunity. Br J Haematol. 2013;162:313-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 118. | Callahan MK, Postow MA, Wolchok JD. CTLA-4 and PD-1 Pathway Blockade: Combinations in the Clinic. Front Oncol. 2014;4:385. [PubMed] |
| 119. | Bundred N, Cameron D, Kalaitzaki E, Morley R, Cramer A, Webster-Smith M, Narayanan S, Brunt M, Horgan K, Hanby A. Effects of perioperative lapatinib and trastuzumab, alone and in combination, in early HER2 breast cancer - the UK EPHOS-B trial (CRUK/08/002). Abstract congress ECCO. 2016;2016:6LBA. [DOI] [Full Text] |