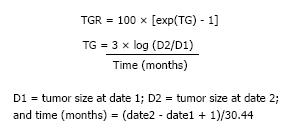Published online Apr 10, 2017. doi: 10.5306/wjco.v8.i2.100
Peer-review started: December 7, 2016
First decision: February 15, 2017
Revised: February 26, 2017
Accepted: March 12, 2017
Article in press: March 13, 2017
Published online: April 10, 2017
Processing time: 121 Days and 12.7 Hours
The therapeutic options for patients with metastatic renal cell carcinoma (mRCC) have completely changed during the last ten years. With the sequential use of targeted therapies, median overall survival has increased in daily practice and now it is not uncommon to see patients surviving kidney cancer for more than four to five years. Once treatment fails with the first line targeted therapy, head to head comparisons have shown that cabozantinib, nivolumab and the combination of lenvatinib plus everolimus are more effective than everolimus alone and that axitinib is more active than sorafenib. Unfortunately, it is very unlikely that we will ever have prospective data comparing the activity of axitinib, cabozantinib, lenvatinib or nivolumab. It is frustrating to observe the lack of biomarkers that we have in this field, thus there is no firm recommendation about the optimal sequence of treatment in the second line. In the absence of reliable biomarkers, there are several clinical endpoints that can help physicians to make decisions for an individual patient, such as the tumor burden, the expected response rate and the time to achieve the response to each agent, the prior response to the agent administered, the toxicity profile of the different compounds and patient preference. Here, we propose the introduction of the tumor-growth rate (TGR) during first-line treatment as a new tool to be used to select the second line strategy in mRCC. The rapidness of TGR before the onset of the treatment reflects the variability between patients in terms of tumor growth kinetics and it could be a surrogate marker of tumor aggressiveness that may guide treatment decisions.
Core tip: The landscape of renal cell carcinoma has dramatically changed in the last decade. Today, at least 6 agents are approved after failure with cytokines, sunitinib or pazopanib in first line treatment. Lack of reliable biomarkers to select the best treatment in daily practice is somewhat frustrating. Therefore, our decisions in real practice are based on safety profiles, patient’ co-morbidities and physician experience or preference. Here we debate the pros and cons of the tumor-growth rate as a tool to select second line systemic treatment after failure to a prior tyrosine kinase-inhibitor in patients with advanced renal cell carcinoma.
- Citation: Grande E, Martínez-Sáez O, Gajate-Borau P, Alonso-Gordoa T. Translating new data to the daily practice in second line treatment of renal cell carcinoma: The role of tumor growth rate. World J Clin Oncol 2017; 8(2): 100-105
- URL: https://www.wjgnet.com/2218-4333/full/v8/i2/100.htm
- DOI: https://dx.doi.org/10.5306/wjco.v8.i2.100
The increased knowledge about the underlying pathogenesis of the metastatic renal cell carcinoma (mRCC) has led to the development of new therapeutic drugs that have completely changed patient prognosis. These drugs are targeting the vascular endothelial growth factor receptor (VEGFR) axis, the mammalian target of rapamycin (mTOR) pathway or the immune system and tumor cell interactions (PD1/PDL1). The number of patients that are candidates for a second line therapy after progressing on a first line varies from 43% to 79%[1]. The second line treatment is determinant in mRCC as patients can also benefit from an improvement in overall survival (OS) already achieved with first line choice and expand their chances for a longer therapeutic sequence. In this regard, a large registry-based experience in the United Kingdom has shown that those patients who received a second line treatment lived longer (33 mo; ranging from 30.8-35.2) than those who did not receive further treatment after first line (20.9 mo; ranging from 16.4-25.3)[2]. Fortunately, options for second line therapy have multiplied with the recent approval of nivolumab, cabozantinib and the combination of everolimus with lenvatinib[3-6]. However, there are no head-to-head comparisons between them and no predictive biomarker has been validated for the second line treatment decision making[7]. Besides, the uncertainty regarding the optimal therapeutic sequence, there is an urgent need for developing prognostic and predictive variables, in order to select patients who will benefit from a specific second line treatment[8].
There are some clinical and economic-derived factors coming from the pivotal trials of each agent that could be considered at the time of second line treatment decisions (Table 1). The patient’s tumor burden has been suggested from retrospective data as being strongly correlated with the progression free survival (PFS) and OS in patients with mRCC[9-12]. The expected response rate from the approved drugs has been reported to be different between cabozantinib, nivolumab and axitinib that achieve an overall response rate (ORR) of 17% to 22%, unlike the combination of everolimus with lenvatinib that has been reported to be of 35% in the phase II pivotal trial[3-6]. Moreover, the time required to achieve a tumor response is a major concern for heavily symptomatic patients that need an early tumor control. Prior tolerance and duration of response to first line treatment may identify those patients harboring a kidney tumor that greatly benefits from the angiogenic blockade (angiogenesis addiction), but may limit the decision in primary refractory patients[13,14]. Finally, we also propose the assessment of the tumor-growth rate (TGR), as a novel outcome measure that could help in the therapeutic sequence decision in the mRCC setting.
| Axitinib | Cabozantinib | Lenvatinib + Everolimus | Nivolumab | |
| Trial design | Phase III | Phase III | Phase II | Phase III |
| Size | 361 | 330 | 51 | 410 |
| Patient population | 2nd Line (100%) | 2L- 71% | 2nd Line (100%) | 2L- 72% |
| 3L- 29% | 3L- 28% | |||
| MSKCC risk % (Good/int/poor) | 28/37/33 | 45/42/12 | 24/37/39 | 35/49/16 |
| Comparator | Sorafenib | Everolimus | Everolimus | Everolimus |
| ORR% (ICR) | 19% | 17% | 35% | 22% |
| Progression disease (%) | 22% | 12% | 4% | 35% |
| PFS (m) | 6.7 (HR 0.66) | 7.4 (HR 0.51) | 12.8 (HR 0.40) | 4.6 (HR 0.88) |
| PFS (m) in pts with bone mets | NR | 7.4 (HR 0.33) | NR | NR |
| OS (m) | 20.1 (HR 0.96) | 21.4 (HR 0.66) | 25.5 (HR 0.59) | 25.0 (HR 0.73) |
| Dose reductions | 30% | 60% | 71% | N/A |
| Discontinuations due to AEs | 7% | 9% | 25% | 8% |
| Toxicity G3/4 (%) | 56% | 68% | 71% | 19% |
| Average monthly cost (US basis) | 9580$ | 10229$ | 22461$ | 12435$ |
Several authors have discussed that the Response Evaluation Criteria in Solid Tumors (RECIST) may be inadequate to completely evaluate the response of targeted therapies in mRCC as often induce long-lasting stable disease rather than tumor shrinkage[15-18]. In addition, these criteria do not take into account tumor growth kinetics, and might not be relevant in slow-growing diseases[19,20]. Therefore, alternate modalities to assess the drug response have been proposed to overcome the limitations of the RECIST criteria, such as Choi, SACT, MASS, ETPIC or iRECIST. These approaches include the tumor perfusion evaluation, via the use of CT response assessment combining reduction in both, size and arterial phase density, changes in tumor CT texture or metabolism or the immune component evaluation. However, none of them appear to be an adequate surrogate of response or clinical outcome for its application in routine clinical practice[16,18,21,22].
TGR provides a dynamic and quantitative evaluation of tumor kinetics; it estimates the percentage of change in the tumor volume over one month. TGR is usually defined as the ratio between the slope of tumor growth before the initiation of treatment and the slope of tumor growth during treatment, and between the nadir and disease progression[9,23]. We can calculate TGR according to the formula shown in Figure 1[24]. The tumor size is defined using the sum of the longest diameters (SLD) of target lesions only, without considering non-target and new lesions. However, the assessment of the TGR in clinical practice is easier as there are internet tools available (http://ec2-54-218-32-173.us-west-2.compute.amazonaws.com:3838/tgrShiny/ or http://www.gustaveroussy.fr/doc/tgr_calculator/index_en.html).
Current evidence from phase I studies in solid tumors and from phase III studies in mRCC (TARGET and RECORD trials) and metastatic neuroendocrine tumors (NETs) (CLARINET trial), although retrospective, show a significant association between prior TGR before the onset of the second line approach with the expected PFS and OS with the later systemic treatment administered[9,24-28]. Moreover, TGR could be an important tool in the evaluation of prognosis during treatment and after the discontinuation of VEGFR targeted agents. Iacovelli et al[29] showed that those patients with a higher than median TGR during treatment had a significantly shorter OS and, indeed, those patients with lower than the median TGR after discontinuation had longer OS, as compared to TGR after discontinuation greater than or equal to the median. Therefore, it would be possible to use TGR as a possible surrogate for tumor aggressiveness and survival in mRCC patients while on VEGFR-directed TKI in the first line. In the post hoc analysis from the CLARINET trial, TGR seemed to provide more precise information to predict pretreatment progression regarding actively growing tumors, but considered as stable disease by RECIST criteria, and more sensitive to detect early antitumor activity from treatment compared with RECIST criteria[28]. We consider that the addition of TGR in the assessment of individual patients undergoing targeted therapies may help clinicians to know if a given agent is modifying or not the course of the disease and guide the decision of which agent would be preferred in the subsequent line. However, for the use of TGR in the clinical setting, a prospective clinical trial for its validation would be needed[23].
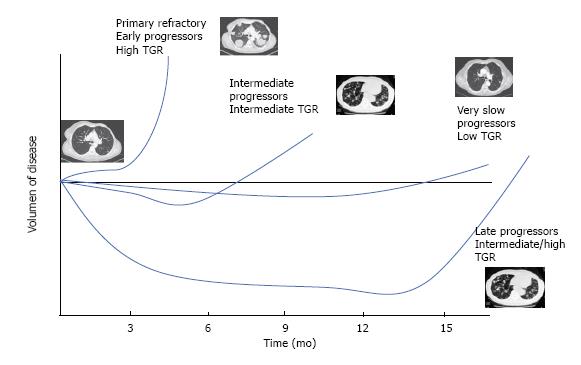
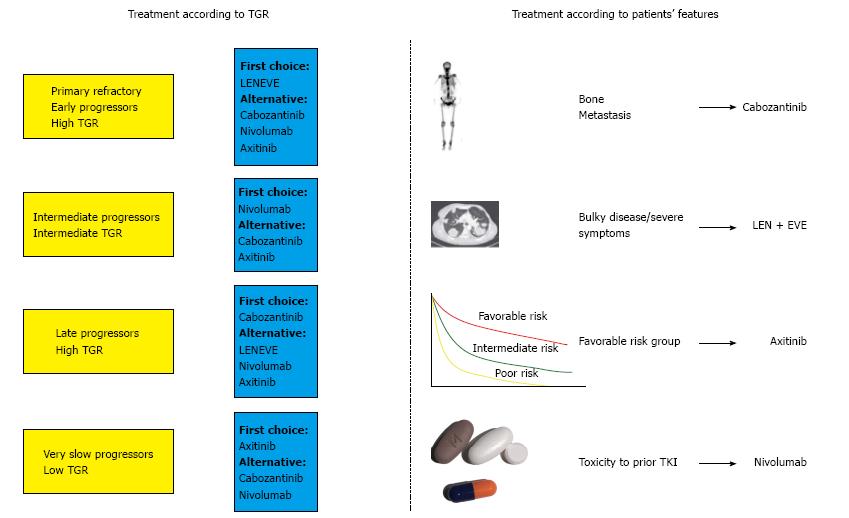
Considering all aspects previously discussed, patients with mRCC that are candidate for a second line treatment could be differentiated into four main subgroups (Figure 2). Patients with florid symptoms, high tumor burden, short time to response to the first line (PFS less than 6 mo, so called, early progressors) and high TGR, in which we would need an early and high response, the combination of everolimus with lenvatinib should be considered, as we will target several mechanisms of action (VEGFR, fibroblast growing factor receptor, FGFR, and m-TOR pathways). In such patients, the expected benefit outweighs the increased toxicity of the combination therapy. In those patients with a long response to first antiangiogenic drug (PFS more than 18 mo, so called angiogenesis addicts) and low or intermediate TGR, the use of cabozantinib may be considered. Regarding those patients that are not responding radiographically but are stable for the advanced disease for a long period with a very low TGR (increase of less than 4% in the sum of the longest diameters per month) and have an adequate tolerability, we propose that axitinib could be a reliable option to prolong the clinical benefit. Finally, for patients with an interval free of progression with first line treatment between 6 and 18 mo, as considered intermediate-progressors, nivolumab may be the treatment of choice as an inhibitor of an actionable immune target by introducing a different mechanism of action against tumor growth.
Lastly, we highlight the upcoming availability of novel immune agents such as ipilimumab, atezolizumab, pembrolizumab either as single agent or in combination that might impact in the first line setting of patients with advanced RCC. Therefore, it is very likely that second line landscape of metastatic RCC may change shortly. Adaptation to the clinic of the amount of new data that are expected in a short term promises to be challenge.
In conclusion, patients with mRCC receiving a second line treatment achieve a median OS of more than 2 years with novel agents. Thus, the optimal treatment selection in this setting allows us to provide the maximal clinical benefit to our patients, but with no definitive biomarker to guide our decision. In this setting, we have considered some relevant clinical parameters before choosing a certain agent such as the patient’s tumor burden, the expected response rate to the different drugs and the time to achieve this response, the prior response to previous VEGFR-TKIs, the toxicity profile of each agent and the patient preference. Thus, we propose the employment of the TGR as a new tool that could provide useful information in the management of mRCC patients in addition to clinical features that could better fit with one of the therapeutic alternatives (Figure 3). TGR may represent a surrogate of tumor aggressiveness, a relevant parameter before choosing a treatment and an early biomarker for treatment response and evaluation of the ability to interfere in the natural history of the tumor growth. TGR could be a valuable endpoint for clinical use in treatment decision-making favoring patients with mRCC, with more reliable information about prognosis and evaluation of response to molecular targeted agents.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Desai DJ, Iqbal M S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Levy A, Menard J, Albiges L, Loriot Y, Di Palma M, Fizazi K, Escudier B. Second line treatment of metastatic renal cell carcinoma: The Institut Gustave Roussy experience with targeted therapies in 251 consecutive patients. Eur J Cancer. 2013;49:1898-1904. [PubMed] |
| 2. | Wagstaff J, Jones R, Hawkins R, Porfiri E, Pickering L, Bahl A, Brown J, Buchan S. Treatment patterns and clinical outcomes in patients with renal cell carcinoma in the UK: insights from the RECCORD registry. Ann Oncol. 2016;27:159-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4375] [Cited by in RCA: 4591] [Article Influence: 459.1] [Reference Citation Analysis (0)] |
| 4. | Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, Hammers H, Hutson TE, Lee JL, Peltola K. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1814-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 953] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 5. | Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, Jassem J, Zolnierek J, Maroto JP, Mellado B. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16:1473-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 697] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 6. | Escudier B, Michaelson MD, Motzer RJ, Hutson TE, Clark JI, Lim HY, Porfiri E, Zalewski P, Kannourakis G, Staehler M. Axitinib versus sorafenib in advanced renal cell carcinoma: subanalyses by prior therapy from a randomised phase III trial. Br J Cancer. 2014;110:2821-2828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Modi PK, Farber NJ, Singer EA. Precision Oncology: Identifying Predictive Biomarkers for the Treatment of Metastatic Renal Cell Carcinoma. Transl Cancer Res. 2016;5:S76-S80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Sacré A, Barthélémy P, Korenbaum C, Burgy M, Wolter P, Dumez H, Lerut E, Loyson T, Joniau S, Oyen R. Prognostic factors in second-line targeted therapy for metastatic clear-cell renal cell carcinoma after progression on an anti-vascular endothelial growth factor receptor tyrosine kinase inhibitor. Acta Oncol. 2016;55:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Staehler M, Haseke N, Stadler T, Zilinberg E, Nordhaus C, Nuhn P, Khoder WY, Karl A, Stief CG. The growth rate of large renal masses opposes active surveillance. BJU Int. 2010;105:928-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Iacovelli R, Lanoy E, Albiges L, Escudier B. Tumour burden is an independent prognostic factor in metastatic renal cell carcinoma. BJU Int. 2012;110:1747-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Basappa NS, Elson P, Golshayan AR, Wood L, Garcia JA, Dreicer R, Rini BI. The impact of tumor burden characteristics in patients with metastatic renal cell carcinoma treated with sunitinib. Cancer. 2011;117:1183-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Stein WD, Huang H, Menefee M, Edgerly M, Kotz H, Dwyer A, Yang J, Bates SE. Other paradigms: growth rate constants and tumor burden determined using computed tomography data correlate strongly with the overall survival of patients with renal cell carcinoma. Cancer J. 2009;15:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Stukalin I, Alimohamed N, Heng DY. Contemporary Treatment of Metastatic Renal Cell Carcinoma. Oncol Rev. 2016;10:295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Malouf GG, Flippot R, Khayat D. Therapeutic Strategies for Patients With Metastatic Renal Cell Carcinoma in Whom First-Line Vascular Endothelial Growth Factor Receptor-Directed Therapies Fail. J Oncol Pract. 2016;12:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Ratain MJ, Eckhardt SG. Phase II studies of modern drugs directed against new targets: if you are fazed, too, then resist RECIST. J Clin Oncol. 2004;22:4442-4445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 183] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Charnsangavej C. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 410] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 17. | Maitland ML, Schwartz LH, Ratain MJ. Time to tumor growth: a model end point and new metric system for oncology clinical trials. J Clin Oncol. 2013;31:2070-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Nathan PD, Vinayan A, Stott D, Juttla J, Goh V. CT response assessment combining reduction in both size and arterial phase density correlates with time to progression in metastatic renal cancer patients treated with targeted therapies. Cancer Biol Ther. 2010;9:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Le Tourneau C, Servois V, Diéras V, Ollivier L, Tresca P, Paoletti X. Tumour growth kinetics assessment: added value to RECIST in cancer patients treated with molecularly targeted agents. Br J Cancer. 2012;106:854-857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Gomez-Roca C, Koscielny S, Ribrag V, Dromain C, Marzouk I, Bidault F, Bahleda R, Ferté C, Massard C, Soria JC. Tumour growth rates and RECIST criteria in early drug development. Eur J Cancer. 2011;47:2512-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Goh V, Ganeshan B, Nathan P, Juttla JK, Vinayan A, Miles KA. Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology. 2011;261:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 291] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 22. | Frampas E, Lassau N, Zappa M, Vullierme MP, Koscielny S, Vilgrain V. Advanced Hepatocellular Carcinoma: early evaluation of response to targeted therapy and prognostic value of Perfusion CT and Dynamic Contrast Enhanced-Ultrasound. Preliminary results. Eur J Radiol. 2013;82:e205-e211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Milella M. Optimizing clinical benefit with targeted treatment in mRCC: “Tumor growth rate” as an alternative clinical endpoint. Crit Rev Oncol Hematol. 2016;102:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Tumor Growth Rate (TGR) as an Indicator of Antitumor Activity With Lanreotide Autogel/Depot (LAN) Versus Placebo (Pbo) in Intestinal/Pancreatic NET: Post Hoc Analysis of CLARINET Data. Clin Adv Hematol Oncol. 2016;14:6-7. [PubMed] |
| 25. | Ferté C, Koscielny S, Albiges L, Rocher L, Soria JC, Iacovelli R, Loriot Y, Fizazi K, Escudier B. Tumor growth rate provides useful information to evaluate sorafenib and everolimus treatment in metastatic renal cell carcinoma patients: an integrated analysis of the TARGET and RECORD phase 3 trial data. Eur Urol. 2014;65:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Burotto M, Wilkerson J, Stein W, Motzer R, Bates S, Fojo T. Continuing a cancer treatment despite tumor growth may be valuable: sunitinib in renal cell carcinoma as example. PLoS One. 2014;9:e96316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Stein WD, Yang J, Bates SE, Fojo T. Bevacizumab reduces the growth rate constants of renal carcinomas: a novel algorithm suggests early discontinuation of bevacizumab resulted in a lack of survival advantage. Oncologist. 2008;13:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Zhang J, Kang SK, Wang L, Touijer A, Hricak H. Distribution of renal tumor growth rates determined by using serial volumetric CT measurements. Radiology. 2009;250:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Iacovelli R, Massari F, Albiges L, Loriot Y, Massard C, Fizazi K, Escudier B. Evidence and Clinical Relevance of Tumor Flare in Patients Who Discontinue Tyrosine Kinase Inhibitors for Treatment of Metastatic Renal Cell Carcinoma. Eur Urol. 2015;68:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |