Published online Oct 10, 2016. doi: 10.5306/wjco.v7.i5.420
Peer-review started: June 3, 2016
First decision: July 5, 2016
Revised: July 21, 2016
Accepted: September 13, 2016
Article in press: September 15, 2016
Published online: October 10, 2016
Processing time: 128 Days and 20.1 Hours
Breast cancer (BC) in men represents between 0.5% and 1% of all BC diagnosed each year. We report a case of advanced BC in a 62-year-old male treated at our interdisciplinary Breast Cancer Center. The patient presented with a newly diagnosed large, symptomatic mass in his left breast. Clinical examination showed a not movable mass of 16 cm diameter, deforming the whole breast; the overlying skin was livid and hypervascularized. Enlarged lymph nodes were palpable in the axillary pit. He had no concomitant diseases at time of presentation. He denied any first- or second degree family medical history of cancer of any type and he never received radiotherapy. Ultrasound guided minimal-invasive 14-gauge core biopsy revealed a moderately differentiated encapsulated papillary carcinoma with high expression of estrogen and progesterone receptors (both > 80%, IRS 12) and HER2-negative. Because of the tumor size a mastectomy with axillary dissection and chest wall reconstruction using a latissimus dorsi flap was performed. Histological analysis showed invasive growth besides typical (non-invasive) papillary carcinoma and was classified as invasive solid papillary carcinoma; pT3 (10 cm), pN0 (0/15), M0, R0; OncotypeDX Recurrence Score indicated low risk (RS: 2). After discussion in the interdisciplinary tumor board meeting, radiation therapy and tamoxifen were recommended. The patient had an uneventful recovery and is disease-free after two years of follow-up. Male BC is typically diagnosed at an advanced stage, most likely due to a lack of awareness that men can develop BC. Therefore, in case of a large tumor, a flap-based thoracic reconstruction may be required.
Core tip: Male breast cancer (BC) is typically diagnosed at an advanced stage, most likely due to a lack of awareness that men can develop BC. Therefore, in case of a large tumor, a flap-based thoracic reconstruction may be required.
- Citation: Banys-Paluchowski M, Burandt E, Banys J, Geist S, Sauter G, Krawczyk N, Paluchowski P. Male papillary breast cancer treated by wide resection and latissimus dorsi flap reconstruction: A case report and review of the literature. World J Clin Oncol 2016; 7(5): 420-424
- URL: https://www.wjgnet.com/2218-4333/full/v7/i5/420.htm
- DOI: https://dx.doi.org/10.5306/wjco.v7.i5.420
Breast cancer (BC) in men represents between 0.5% and 1% of all breast cancers diagnosed each year[1]. Few epidemiological or clinical trial data on male BC are available. The disease appears to share similar risk factors and characteristics with postmenopausal BC in women[2]. Favorable parameters, such as low nuclear grade and positive hormone receptor status, are more common for men and postmenopausal women than for premenopausal women. As in women, the most common subtype is invasive ductal carcinoma, while lobular tumor types are rarely diagnosed in men. In contrast to female BC, men are significantly more likely to present with a more advanced stage at diagnosis and with lymph node involvement[3]. We report an interesting case of a large papillary invasive breast cancer in a 62-year-old man.
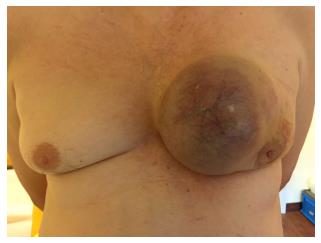
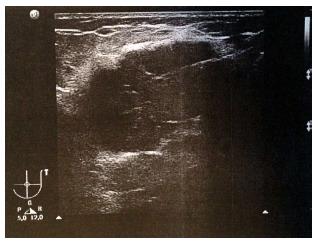
A 62-year-old Caucasian male presented at the certified Breast Cancer Center, Klinikum Pinneberg, Germany, with a newly diagnosed large, symptomatic mass in the left breast. Clinical examination showed a not movable mass of 16 cm diameter, deforming the whole breast (Figure 1); the overlying skin was livid and hypervascularized. Enlarged lymph nodes were palpable in the axillary pit. He had no concomitant diseases at time of presentation; his previous surgeries included circumcision as a child and he was a nonsmoker. He denied any first- or second degree family medical history of cancer of any type and he never received radiotherapy. His serum estradiol levels were normal. The patient reported that he had noticed the tumor one year prior to presentation; the reason for eventually seeing a doctor was an increasing pain, most likely due to the pressure on the skin by the growing tumor. There was no nipple discharge. At ultrasound, the lesion was scored BI-RADS 5 (Figure 2). Axillary lymph nodes were suspicious on sonography. Mammography was not possible due to tenderness on palpation.
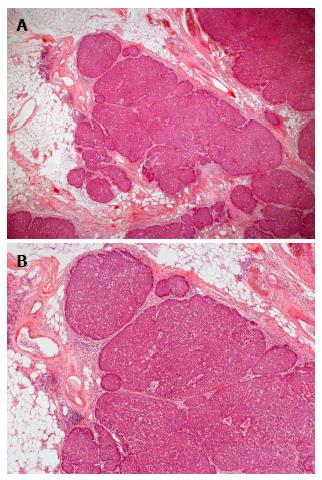
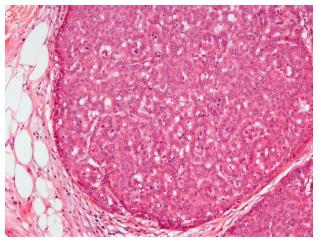
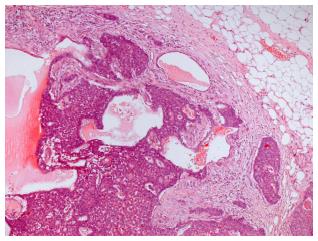
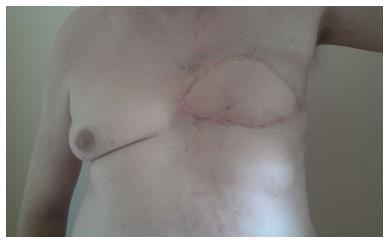
Ultrasound guided minimal-invasive 14-gauge core biopsy revealed a moderately differentiated non-invasive encapsulated papillary carcinoma with high expression of estrogen and progesterone receptors (both > 80%, IRS 12) and HER2-negative (Figure 3). Because of the tumor size a mastectomy with axillary dissection and chest wall reconstruction using a latissimus dorsi (LADO) flap was performed. Histological analysis showed invasive growth besides typical non-invasive papillary carcinoma and was classified as invasive solid papillary carcinoma (Figures 4 and 5). The TNM stage was pT3 (10 cm), pN0 (0/15), M0 and the resection margins were at least 5 mm in all directions. After discussion in our interdisciplinary tumor board we conducted the OncotypeDX test which indicated low risk (Recurrence Score: 2). Radiation therapy and tamoxifen were recommended. The patient was referred for genetic counseling but decided against genetic testing. He had an uneventful recovery and is disease-free after two years of follow-up (Figure 6).
To our knowledge, this is the first case report on a giant invasive papillary carcinoma in a man treated by LADO flap-based reconstruction of the thoracic wall. Papillary breast cancer is a very rare type of BC with an estimated incidence of approximately 1% of all breast cancer cases[4]. In terms of histopathology, papillary carcinoma refers to a morphologically heterogeneous group of lesions, all of which are characterized by arborescent fibrovascular stalks lined by epithelial cells. In most cases of invasive papillary carcinoma, ductal carcinoma in situ is also present. Available epidemiological data suggest an improved survival of patients with papillary carcinoma in comparison to the more common invasive ductal cancer[4]. In men, papillary tumor type is more common than in women: In a large series of 778 men with invasive BC, 34 (4.4%) were diagnosed with this tumor subtype[5]. In case of our patient, the minimally invasive core biopsy showed in situ papillary cancer, while the examination of the whole surgical specimen revealed invasive growth as well.
According to the epidemiological data from the Surveillance, Epidemiology, and End Results Program of the American National Cancer Institute, men tend to be older than women at the time of diagnosis, with a median age of 67 years compared with 62 years for women[3]. Further, men are more likely to be diagnosed with advanced disease: At time of diagnosis, 20% of women had tumors smaller than 1 cm compared with only 9.8% of men, 38% of men had regional lymph node involvement compared with 29% of women, and more men had distant metastasis at time of diagnosis than women. On the other hand, tumor biology appeared to be more favourable in men than in women: Men have a significantly higher proportion of hormone receptor positive tumors than women (91% of men and 76% of women present with ER-positive disease)[3]. With regard to molecular gene expression assays, there is limited evidence available related specifically to men, although tumors in men display very similar gene signatures to those in women[6] and OncotypeDX-based clinical trials such as the Ontario trial include male BC patients as well[7]. With respect to risk factors, genetic factors including BRCA mutations, family history, age, androgen/estrogen imbalance, radiation therapy and environmental exposures seem to predispose to male breast cancer[8]. In context of genetic counseling, BRCA2 germline mutation leads to a 100-fold increase in breast cancer risk in male carriers while this association is less established for the BRCA1 mutation. The cumulative risk of BC for male BRCA1 mutation carriers at age 70 years is 1.2% compared to 6.8% for BRCA2 mutation carriers[9]. Another risk factor, the excessive estrogen stimulation, may be due to exogenous hormonal exposure (i.e., hormonal treatment or estrogen-containing compounds/testosterone), overweight, chronic liver diseases and thyroid disease[10]. Men with Klinefelter syndrome are more at risk for developing breast cancer as well[11].
The approach to men with a suspicious breast mass is similar to that of women and includes mammography, ultrasound and biopsy (Table 1). As in women, male breast cancer is classified according to the TNM staging system and tumor biology (estrogen receptor, progesterone receptor and HER2 status) is crucial for choosing adequate systemic therapy. Most men with early stage disease undergo a simple mastectomy. Flap-based reconstruction of the thoracic wall may be necessary in case of a very large tumor[12]. Both the LADO flap and the abdominal tissue transfer, such as transverse rectus abdominis muscle flap or deep inferior epigastric perforator flap, are reliable techniques. The use of LADO flap in a variety of reconstructive settings has been described since early 20th century[13]. In this surgical technique, the pedicled musculocutaneous flap is dissected along with the fat and skin overlying the vascularized muscle and tunneled subcutaneously under the axilla to be transposed into the wound in the thoracic wall. Beyond breast or thoracic wall reconstruction, the LADO flap may be used in men for phalloplasty after penile trauma or in case of congenital anomalies of the penis; in this setting, the flap is not pedicled but is transferred as a “free flap” to another body site and the circulation is re-established via microsurgical anastomosis between the flap and the femoral vessels[14].
| Female breast cancer | Male breast cancer | |
| Epidemiology | Very common (125 new cases per 100000 women per year) | Very rare (1.2 new cases per 100000 men per year) |
| Average age at diagnosis | 62 yr | 67 yr |
| Diagnostics | Mammography, sonography; in selected cases MRI | Sonography; mammography if possible; in selected cases MRI |
| Association with BRCA mutation | 5%-10% of all cases are BRCA-positive | 10%-20% of all cases are BRCA-positive |
| Surgery | Breast-conserving surgery (70%-80% patients) or mastectomy; sentinel node biopsy in cN0 | Mastectomy; sentinel node biopsy in cN0 |
| Reconstruction techniques | Implant or flap-based reconstruction after mastectomy | Flap-based reconstruction of thoracic wall in case of a large tumor |
| Chemotherapy | Recommendation depends on tumor biology and tumor load; adjuvant or neoadjuvant use; usually anthracycline- and taxane-based | |
| Endocrine therapy | Recommended in hormone receptor positive tumors; tamoxifen or aromatase inhibitor | Recommended in hormone receptor positive tumors; tamoxifen |
| HER2-targeted treatment | Trastuzumab recommended in HER2-positive tumors | |
| Radiation therapy | Thoracic wall or lymph node radiation in case of higher tumor load | |
| Recommended after breast-conserving surgery; thoracic wall or lymph node radiation in case of higher tumor load | ||
Most experts agree that sentinel node biopsy should be performed in early male breast cancer in absence of clinically or sonographically suspicious nodes; this approach is in accordance with the ASCO clinical guidelines[15]. Although large clinical trials on sentinel node biopsy in men have not been carried out, smaller studies confirm this technique to be as feasible as in women[16,17].
As in women, adjuvant therapy of male breast cancer may include radiation therapy, endocrine therapy, chemotherapy, and HER2-targeted treatment. In the absence of large clinical trials focusing on male BC, therapy recommendations radiation therapy, chemo- and HER2-therapy mirror those for women. In the context of endocrine therapy, the use of tamoxifen rather than an aromatase inhibitor is recommended[18].
A 62-year-old male with a newly diagnosed large, symptomatic mass in the left breast.
A not movable mass of 16 cm diameter, deforming the whole breast; the overlying skin livid and hypervascularized; enlarged lymph nodes in the axillary pit.
Benign breast tumor.
Ultrasound: Large irregular structure of low echogenicity with spiculae (BI-RADS 5), suspicious axillary lymph nodes.
Core biopsy: Moderately differentiated non-invasive encapsulated papillary carcinoma with high expression of estrogen and progesterone receptors (both > 80%, IRS 12) and HER2-negative. Surgical specimen: Invasive solid papillary carcinoma, pT3, pN0 (0/15), M0, OncotypeDX low risk (Recurrence Score: 2).
Mastectomy with axillary dissection and chest wall reconstruction using a latissimus dorsi flap.
This case report describes a rare case of male papillary breast cancer and emphasizes the importance of flap-based reconstruction in case of a large tumor.
This is a case report of rare men breast cancer and then the author gave a short literature review. The review provides some useful information.
Manuscript source: Invited manuscript
Specialty Type: Oncology
Country of Origin: Germany
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Castaneda CA, Hosseini N, Lyakhovich A, Pan F, Vetvicka V S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | White J, Kearins O, Dodwell D, Horgan K, Hanby AM, Speirs V. Male breast carcinoma: increased awareness needed. Breast Cancer Res. 2011;13:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Anderson WF, Althuis MD, Brinton LA, Devesa SS. Is male breast cancer similar or different than female breast cancer? Breast Cancer Res Treat. 2004;83:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004;101:51-57. [PubMed] |
| 4. | Pal SK, Lau SK, Kruper L, Nwoye U, Garberoglio C, Gupta RK, Paz B, Vora L, Guzman E, Artinyan A. Papillary carcinoma of the breast: an overview. Breast Cancer Res Treat. 2010;122:637-645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Burga AM, Fadare O, Lininger RA, Tavassoli FA. Invasive carcinomas of the male breast: a morphologic study of the distribution of histologic subtypes and metastatic patterns in 778 cases. Virchows Arch. 2006;449:507-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Shak S, Palmer G, Baehner FL, Millward C, Watson D, Sledge GW. Molecular characterization of male breast cancer by standardized quantitative RT-PCR analysis: First large genomic study of 347 male breast cancers compared to 82,434 female breast cancers. J Clin Oncol. 2009;27:549. |
| 7. | Levine MN, Julian JA, Bedard PL, Eisen A, Trudeau ME, Higgins B, Bordeleau L, Pritchard KI. Prospective Evaluation of the 21-Gene Recurrence Score Assay for Breast Cancer Decision-Making in Ontario. J Clin Oncol. 2016;34:1065-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Ferzoco RM, Ruddy KJ. The Epidemiology of Male Breast Cancer. Curr Oncol Rep. 2016;18:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Tai YC, Domchek S, Parmigiani G, Chen S. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:1811-1814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 248] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 10. | Thomas DB. Breast cancer in men. Epidemiol Rev. 1993;15:220-231. [PubMed] |
| 11. | Hultborn R, Hanson C, Köpf I, Verbiené I, Warnhammar E, Weimarck A. Prevalence of Klinefelter’s syndrome in male breast cancer patients. Anticancer Res. 1997;17:4293-4297. [PubMed] |
| 12. | Spear SL, Bowen DG. Breast reconstruction in a male with a transverse rectus abdominis flap. Plast Reconstr Surg. 1998;102:1615-1617. [PubMed] |
| 13. | Smith SL. Functional morbidity following latissimus dorsi flap breast reconstruction. J Adv Pract Oncol. 2014;5:181-187. [PubMed] |
| 14. | Perovic SV, Djinovic R, Bumbasirevic M, Djordjevic M, Vukovic P. Total phalloplasty using a musculocutaneous latissimus dorsi flap. BJU Int. 2007;100:899-905; discussion 905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Lyman GH, Giuliano AE, Somerfield MR, Benson AB, Bodurka DC, Burstein HJ, Cochran AJ, Cody HS, Edge SB, Galper S. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703-7720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 514] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 16. | Gentilini O, Chagas E, Zurrida S, Intra M, De Cicco C, Gatti G, Silva L, Renne G, Cassano E, Veronesi U. Sentinel lymph node biopsy in male patients with early breast cancer. Oncologist. 2007;12:512-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Goyal A, Horgan K, Kissin M, Yiangou C, Sibbering M, Lansdown M, Newcombe RG, Mansel RE, Chetty U, Ell P. Sentinel lymph node biopsy in male breast cancer patients. Eur J Surg Oncol. 2004;30:480-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Eggemann H, Ignatov A, Smith BJ, Altmann U, von Minckwitz G, Röhl FW, Jahn M, Costa SD. Adjuvant therapy with tamoxifen compared to aromatase inhibitors for 257 male breast cancer patients. Breast Cancer Res Treat. 2013;137:465-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |