Published online Oct 10, 2016. doi: 10.5306/wjco.v7.i5.340
Peer-review started: April 26, 2016
First decision: June 16, 2016
Revised: July 18, 2016
Accepted: August 6, 2016
Article in press: August 8, 2016
Published online: October 10, 2016
Processing time: 166 Days and 15.5 Hours
Epidermal growth factor receptor (EGFR) has been an attractive target for treatment of epithelial cancers, including colorectal cancer (CRC). Evidence from clinical trials indicates that cetuximab and panitumumab (anti-EGFR monoclonal antibodies) have clinical activity in patients with metastatic CRC. The discovery of intrinsic EGFR blockade resistance in Kirsten RAS (KRAS)-mutant patients led to the restriction of anti-EGFR antibodies to KRAS wild-type patients by Food and Drug Administration and European Medicine Agency. Studies have since focused on the evaluation of biomarkers to identify appropriate patient populations that may benefit from EGFR blockade. Accumulating evidence suggests that patients with mutations in EGFR downstream signaling pathways including KRAS, BRAF, PIK3CA and PTEN could be intrinsically resistant to EGFR blockade. Recent whole genome studies also suggest that dynamic alterations in signaling pathways downstream of EGFR leads to distinct oncogenic signatures and subclones which might have some impact on emerging resistance in KRAS wild-type patients. While anti-EGFR monoclonal antibodies have a clear potential in the management of a subset of patients with metastatic CRC, further studies are warranted to uncover exact mechanisms related to acquired resistance to EGFR blockade.
Core tip: Epidermal growth factor receptor (EGFR) blockade treatment is a well-established targeted therapy in metastatic colorectal cancer (CRC) patients. However, a limited number of patients benefit from EGFR inhibition, with limited time duration of response. This review article discusses the most recent updates from the current-state-of-the-science related to molecular pathways of EGFR signaling, the mechanism of action and efficacy of EGFR blockade treatment, and possible molecular pathways related to EGFR blockade resistance in CRC. We further discuss potential mechanisms contributing to targeted EGFR inhibition. Lastly, future perspectives are discussed to shed some light on efforts to overcome this potential challenge in the era of targeted treatment.
- Citation: Sobani ZA, Sawant A, Jafri M, Correa AK, Sahin IH. Oncogenic fingerprint of epidermal growth factor receptor pathway and emerging epidermal growth factor receptor blockade resistance in colorectal cancer. World J Clin Oncol 2016; 7(5): 340-351
- URL: https://www.wjgnet.com/2218-4333/full/v7/i5/340.htm
- DOI: https://dx.doi.org/10.5306/wjco.v7.i5.340
Colorectal cancer (CRC) with an incidence of 1.2 million cases per year is now the third most common cancer in males and second in females[1]. Although there is a minor trend "towards" a decrease in the incidence of the disease, it is yet one of the major health care burden in United States[2]. Despite extensive research, a limited number of targeted agents have been shown to be active in CRC.
Cetuximab and Panitumumab are two new generation monoclonal antibodies targeting epidermal growth factor receptor (EGFR) recently approved by the Food and Drug Administration (FDA) for the management of metastatic CRC in the United States. Although their exact mechanism of action is unknown it is hypothesized that the direct interaction of these antibodies with EGFR results in apoptosis. Herein, we discuss the molecular signature of the EGFR pathway, the possible mechanism of action of anti-EGFR monoclonal antibodies, the clinical consequences of these cell-based interactions in treatment of metastatic CRC, along with emerging resistance to these agents during the treatment of CRC.
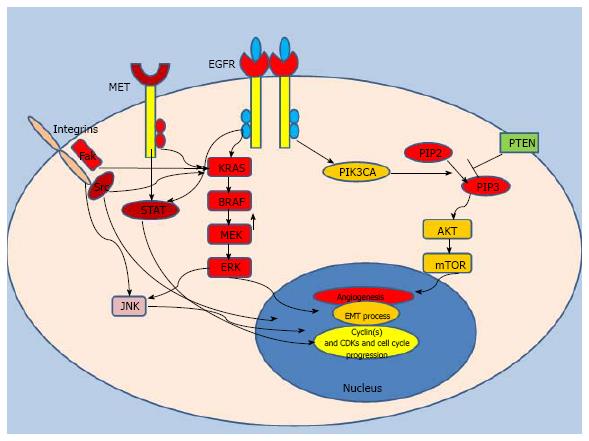
EGFR, also known as ErbB, is a member of the receptor tyrosine kinase family; it stimulates multiple intracellular proto-oncogenic signaling mediators including Kirsten RAS (KRAS)[3]. As a 170 kDa transmembrane glycoprotein expressed on cell surface, EGFR has physiological roles in many organs including the epithelium of the gastrointestinal system, bronchial tract and cutaneous tissue[4]. In normal homeostasis, EGFR is activated by binding with external ligands such as growth factors (EGF, epiregulin or amphiregulin). This interaction initiates homodimerization and heterodimerization processes via a diverse combination of identical and different members of the family such as ErbB2 (HER-2), ErbB3 (HER-3) and ErbB4 (HER-4)[5]. The ensuing phosphorylation of tyrosine kinase domain results in activation of oncogenic pathways including mitogen activated protein kinase (MAPK) and phosphotidylinositol-3-kinase (PI3KCA) pathways (Figure 1). These signaling axes have been shown to function in many critical pro-survival cellular reactions in cancer cells including protein synthesis, cell growth, cell cycle progression, transformation and invasion. KRAS, a critical growth signal response in cancer cells, is an upstream activator of the MAPK pathway[6] (Figure 1). KRAS-driven MAPK translocates into the cell nucleus, initiates a transcription cascade and promotes cell growth[7]. For example, KRAS activation leads to upregulation of c-myc which fuels proliferation of human colon cancer cells and provides a survival advantage[8]. Signal cascades of KRAS also induce cell cycle progression via activation of the transcription factor Elk-1, which ultimately increases the expression of cell cycle promoting proteins such Cyclin D1[9]. Moreover, as a part of the complex network of EGFR signaling, the KRAS driven MAPK pathway interacts with JNK signaling to modulate cellular stress responses which enhance cellular plasticity. This response helps malignant cells to adapt to dynamic microenvironmental changes[10]. In transformed cancer cells, KRAS mutations abolish regulation via the upstream EGFR loop; the MAPK and PI3KCA pathways, and other pro-survival cascades are continuously activated, leading to distinct cellular behavior[11,12].
Phosphatidylinositol 3-kinase (PIK3CA) is another well-studied signal transducer of the EGFR pathway. In normal homeostasis, activation of PIK3CA by EGFR leads to induction of Akt-mTOR pathway which has been shown to be crucial signal for protein synthesis and cell cycle progression[13]. Activation of PIK3CA also abrogates apoptosis and cellular senescence in cancer cells[14]. PIK3CA-driven mTOR activates Bcl-2 and ultimately inhibits apoptosis[15], indicating that PIK3CA signaling may have an important role in the immortality of transformed cells. PIK3CA activation has also been shown to be related to elevated expression of COX-2 which enhances angiogenesis in CRC[16]. Consistent with evidence from preclinical observations, mutant PIK3CA is associated with development of various cancers including CRC[17]. Current thinking suggests that the changes in the gene expression profile caused by activating mutations of PIK3CA may culminate in changes in the proteome of cancer cells and that this transformation enhances cellular growth and invasion by creating distinct oncogenic signatures[18].
BRAF, a member of the RAF kinase family, functions as a serine/threonine protein kinase, and gets activated by the upstream Ras oncogene (Figure 1)[19]. Activating mutations of the BRAF oncogene occur in the kinase domain and the V599E mutation accounts for the vast majority of point mutations (approximately 80%)[20]. Mutant BRAF propagates Raf-MAPK signaling in the absence of upstream stimulation and ultimately induces cell growth and proliferation in malignant clones[21]. Similar to PIK3CA mutations, BRAF mutations also transform the protein expression profiles of cancer cells and alter internal metabolism. For example, CRC cells with mutant BRAF were found to be more resistant to apoptosis compared to those carrying wild-type BRAF[22]. Moreover, BRAF may increase the expression of cell cycle promoting proteins which further enhance the expansion of selected clones[23]. BRAF mutations have also been shown to be associated with constitutively activated NF-κB[24], leading to tumor angiogenesis that optimizes the microenviroment for cancer cells[24]. All this evidence suggests that activation of the BRAF oncogene may add further distinct characteristics to the cancer cells’ genomic fingerprint.
Src and STAT, two other critical oncogenes, have been demonstrated to be involved in the development and progression of epithelial tumors along with cancer angiogenesis, and both mediators operate in the signaling cascade of the EGFR pathway[25]. A study showed that EGFR overexpressing cancer cells bear a 10-fold increase in Src activity compared to low EGFR expressing cancer cells[26], and that increased Src activity has been associated with highly aggressive tumor behavior and metastatic potential in CRC cells[27]. Oncogenic Src, as a non-receptor tyrosine kinase (nRTK), turns on many downstream survival pathways such Ras-MAPK pathway. It further activates receptor tyrosine kinases which create continuous growth signals for cancer cells[28]. Activating mutations of Src are related to adverse outcomes in CRC[29,30]. STATs are also activated by EGFR[25] and function as transcriptional factors in downstream pathways of receptor tyrosine kinases and cytokine receptors[31]. Induction of STATs through EGFR signaling[32] may also fuel angiogenesis in the tumor microenvironment[33]. Although activation of STATs has shown to be related to enhanced proliferation in CRC cancer cells[34], the exact role of STATs in development and progression of CRC remains to be elucidated.
Altogether, current evidence indicates intricate EGFR signaling. Variant alterations in the downstream signal transducers of EGFR are likely responsible for the change in expression profiles and molecular behavior of cancer cells.
Considering the diverse oncogenic pathways activated by EGFR, it has become a promising target for therapy in various epithelial tumors[35,36]. The aforementioned studies led to research targeting EGFR signaling via different approaches, including small molecule inhibitors of receptor tyrosine kinases such as erlotinib and monoclonal antibodies to neutralize the receptor via an internalization and degradation process.
The initial investigations in this field were conducted using murine anti-EGFR antibodies. In these studies, both agonist and antagonist antibodies were tested[37], and antagonist antibodies inhibited the proliferation of the malignant clones with high affinity binding[38]. Since murine antibodies are recognized as foreign antigens in humans, human-mouse chimeric antibodies were generated to further study this effect and they were shown to have superior biological efficacy compared to murine antibodies in human tumor xenograft models[39]. Cetuximab, a chimeric antibody against EGFR, was demonstrated to have 10 times higher affinity to EGFR compared the murine antibodies (M225)[40], and limited toxicity was observed in phase I clinical studies[41].
The mechanism of action of anti-EGFR antibodies was at first attributed to internalization of receptors bound by the anti-EGFR antibodies[42]. Further studies demonstrated that the EGFR blockading agents not only catalyze the removal of the receptors but also inhibit receptor tyrosine kinase activity, and induce apoptosis via cell cycle arrest in colonic adenocarcinoma cell lines[43]. Besides its-single agent activity, an enhanced anti-cancer effect of cetuximab was observed in combination with the topoisomerase inhibitor (irinotecan) in human CRC xenograft models[44]. These discoveries led to further studies to elucidate the role of EGFR blockade in modern cancer treatment, and clinical trials were opened for enrollment in different clinical settings.
To further investigate the promising results observed in preclinical studies, early phase and clinical efficacy trials were designed (Table 1). A phase I safety trial was conducted to determine the optimal biological dose, and the doses achieved at maximal systemic clearance were well tolerated in both monotherapy and combined treatment models[45]. In a phase II clinical trial of 120 irinotecan-resistant CRC patients with positive EGFR expression, cetuximab reversed the resistance to irinotecan with a 22.5% major objective response rate and 17% radiologic response rate in a combination treatment model[46]. The promising radiological response rate had already resulted in FDA approval for the cetuximab treatment for metastatic CRC patients. In order to solidify the response observed in the original study, cetuximab was investigated as a single agent treatment in patients with advanced stage refractory CRC[47]. In this study, 9% partial response rate along with a median survival of 6.4 mo was reported after cetuximab monotherapy in CRC patients expressing EGFR. To further investigate these findings and to test the superiority of combination treatment to monotherapy, a randomized phase II clinical trial was conducted[48]. Patients were randomized to receive cetuximab and irinotecan combination vs cetuximab alone. Patients in the combination arm demonstrated a 22.9% partial response compared to 10.8% in the cetuximab monotherapy arm (P = 0.007). While progression free survival (PFS) was significantly improved in patients receiving combination therapy there was no overall survival (OS) difference between the groups. A subsequent study demonstrated limited survival benefit in patients treated with cetuximab compared to best supportive care (6.1 mo vs 4.6 mo respectively)[49]. Although EGFR expression was considered as a predictor of EGFR blockade response in early clinical trials, this concept has since changed and EGFR expression is no longer considered a biomarker in CRC patients[50].
| Ref. | Year | Sample size | Mutation status | Treatment groups | Special considerations | Summarized findings |
| Saltz et al[46] | 2001 | 120 | EGFR + | Cetuximab plus Irinotecan | 22.5% major objective response rate 17% radiologic response rate | |
| Saltz et al[47] | 2004 | 57 | EGFR + | Cetuximab | 9% (CI: 3% to 19%) partial response Median survival of 6.4 mo. P value ?? | |
| Cunningham et al[48] | 2004 | 329 | EGFR + | Cetuximab plus irinotecan vs cetuximab alone | 22.9% partial response in combination arm, 10.8% partial response in the cetuximab monotherapy arm No difference in OS | |
| Jonker et al[49] | 2007 | 572 | EGFR + | Cetuximab compared to best supportive care | 6.1 mo OS in treatment arm vs 4.6 mo with supportive care. P = 0.005 Quality of life was better preserved in the cetuximab group (P < 0.05) | |
| Van Cutsem et al[56] | 2007 | 463 | EGFR+ | Panitumumab vs best supportive care | Median PFS 8 wk in treatment arm compared to 7.3 wk in patients receiving supportive care (HR 0.54; P < 0.0001) | |
| Amado et al[57] | 2008 | 427 | EGFR+ Wild type KRAS vs mutant KRAS | Panitumumab vs best supportive care | Reanalysis of Van Cutsem 2007 | In patients with wild type KRAS median PFS was 12.3 wk for panitumumab vs 7.3 wk for supportive care Response rates to panitumumab was 17% for patients with wild type KRAS compared to 0% in patients with mutant KRAS |
| Van Cutsem et al[51] CRYSTAL Trial | 2009 | 1198 | EGFR + | Cetuximab plus FOLFIRI vs FOLFIRI alone | HR for PFS in combination therapy 0.85 (P = 0.048) when compared to FOLFIRI alone There was no difference in OS (HR 0.93; P = 0.31) Although not significant PFS was improved with cetuximab in patients with wild-type-KRAS (HR 0.68; P = 0.07) | |
| Folprecht et al[55] CELIM trial | 2010 | 113 | Wild type KRAS vs mutant KRAS | Cetuximab plus FOLFOX vs Cetuximab plus FOLFIRI vs historical controls | Neoadjuvant setting | No survival difference between the two groups Higher response rates compared to historical controls (FOLFOX and FOLFIRI) Total 36 of 116 patients were able to receive R0 resection Improved outcomes limited to patients with wild type KRAS |
| Van Cutsem et al[52] | 2011 | 1198 | EGFR+ Wild type KRAS vs mutant KRAS | Cetuximab plus FOLFIRI vs FOLFIRI alone Wild type KRAS vs mutant KRAS | Reanalysis of data from CRYSTAL trial | Patients with wild type KRAS had improvements in OS from 20 to 23.5 mo PFS from 8.4 to 9.9 mo and response rates 39.7% to 57.3% with addition of Cetuximab to FOLFIRI |
| Bokemeyer et al[53] OPUS study | 2011 | 315 | Wild type KRAS/BRAF vs mutant KRAS/BRAF | Cetuximab plus FOLFOX | Improved PFS (HR 0.567) and response (OR 2.55) in patients with KRAS wild-type tumors | |
| Alberts et al[54] N0137 trial | 2012 | 2686 | Wild type KRAS vs mutant KRAS | Cetuximab plus FOLFOX vs FOLFOX alone | Locally advanced disease | No additional benefit of Cetuximab when added to FOLFOX-6 regimen in the setting of locally advance disease |
The observed improvement in response rate in the treatment of irinotecan-refractory cases led to further studies in treatment-naïve patients. The CRYSTAL study showed significantly improved PFS in metastatic CRC patients who received a cetuximab plus FOLFIRI (bolus 5-fluoruracil/leucovorin chemotherapy plus irinotecan) regimen compared to patients who underwent FOLFIRI treatment alone (Table 1). However, there was no significant difference in OS between the two groups[51]. Subsequent analysis of the study demonstrated improved OS (approximately 3.5 mo) in patients with wild-type KRAS who underwent cetuximab treatment. No significant response was observed in KRAS-mutant patients[52]. This study led to the FDA approval of cetuximab as a first line treatment in combination with FOLFIRI in patients with metastatic CRC. In the OPUS study, cetuximab was combined with FOLFOX-4 and an improved PFS [hazard ratio (HR) 0.567, P = 0.0064] was observed in KRAS wild-type metastatic CRC patients who received combination therapy[53]. In order to assess the role of cetuximab in earlier stages of the disease, N0137 trial was conducted: Stage III CRC patients were enrolled to receive cetuximab plus FOLFOX-6 combinations vs FOLFOX-6 in adjuvant settings after initial resection[54]. After a of median 28 mo follow-up in both wild-type and mutant KRAS groups, results of this trial showed no benefit of cetuximab when added to FOLFOX-6 regimen in the setting of locally advance disease. In order to compare the efficacy of cetuximab in combinations with FOLFOX-6 and FOLFIRI regimens as a neoadjuvant treatment in unresectable metastatic CRC patients, a phase II clinical trial (CELIM trial) was conducted[55]. Although there was no significant survival difference between the two groups, addition of cetuximab resulted in higher response rates compared to historical controls (FOLFOX and FOLFIRI). A total 36 of 116 patients were able to receive R0 resection. The result of this study was also consistent with previous studies demonstrating improved outcomes limited to patients with wild type KRAS.
Panitumumab, another monoclonal antibody for EGFR blockade, has also been investigated in CRC patients. This agent received FDA approval after prolonged PFS was demonstrated in an open-label phase III clinical trial in which patients were enrolled to receive single-agent panitumumab vs best supportive care (median PFS, 8 wk vs 7.3 wk; HR, 0.54; 95%CI: 0.44-0.66)[56]. Follow-up analysis of this study showed that the survival benefit was again, limited to wild-type KRAS CRC patients[57]. A retrospective study suggested potential resistance in patients with BRAF V600E mutation and requirement of wild-type BRAF for clinical benefit, along with wild-type KRAS[58]. Currently this drug is also approved as a first line agent in combination with FOLFOX4 in metastatic CRC patients with wild-type KRAS due to observed improvement in PFS in a randomized phase III clinical trial (PRIME trial; median PFS, 9.6 mo vs 8.0 mo; P = 0.02)[59]. A small phase II trial evaluated Panitumumab in 33 wild-type KRAS metastatic CRC patients whom were deemed ineligible for chemotherapy. The study demonstrated a PFS rate of 36.4%, objective response rate of 9.1% and stable disease in 54.5% of patients. The median PFS was 4.3 mo and OS was 7.1 mo, indicating a role for Panitumumab monotherapy in patients who are not eligible for chemotherapy[60].
The promising results observed with monoclonal antibodies either as monotherapy or in combination treatments in metastatic CRC patients were encouraging. However the relatively small magnitude of improvement in PFS was an early sign of emerging resistance to cetuximab therapy. Therefore, further clinical and translational studies were conducted to elucidate possible underlying mechanisms related to EGFR blockade resistance.
EGFR mutations were considered as a possible mechanism for EGFR blockade resistance in CRC patients. However, in a clinical study, only one out of the 293 CRC patients was found to be harboring an EGFR mutation[61]. Considering the rarity of EGFR mutations, they are unlikely to be a common cause of resistance to EGFR blockade in CRC patients.
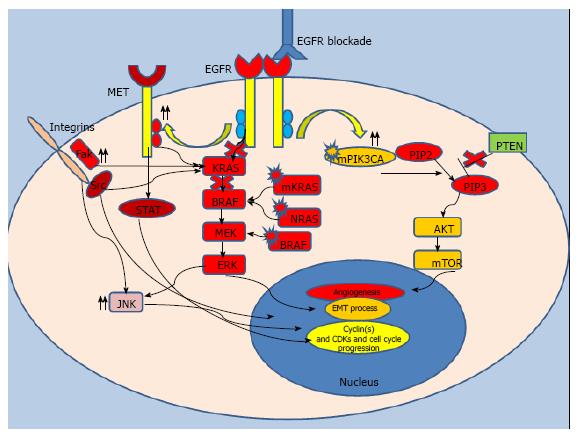
As mentioned above, a retrospective study first revealed that the KRAS-mutant CRC patients did not benefit from cetuximab therapy[52]. In a study by De Roock et al[62], KRAS 13D mutation was reported to be associated with cetuximab response unlike other KRAS mutations. However another retrospective study did not confirm this favorable outcome[63]. Whether KRAS 13D-mutant CRC tumors have a distinct clinical behavior from other KRAS mutations warrants further study. Similar to KRAS, a clinical study found NRAS to be a biomarker of anti-EGFR antibody resistance in CRC patients[64]. This association of NRAS mutations with EGFR blockade resistance needs to be validated in future studies.
Since disruptions downstream of EGFR cause autonomous activation of the signaling pathway and potential intrinsic resistance to EGFR blockade, clinical studies also investigated the utility of BRAF mutations as a biomarker in CRC patients (Figure 2). In two clinical trials, patients with CRC harboring BRAF mutations were reported to have a poor response to cetuximab; indicating BRAF mutations could also limit the efficacy of anti-EGFR treatment[58,65]. A large retrospective multicenter study looking at survival in patients with metastatic CRC and their mutation status; also reported similar findings[64]. Although current evidence strongly suggests that BRAF mutations might be a negative biomarker for monoclonal anti-EGFR treatment, guidelines do not currently preclude such patients from this targeted treatment approach.
A study of 23 PIK3CA-mutant metastatic CRC patients reported no resistance to anti-EGFR treatment[66]. However, in another cohort of 15 PIK3CA-mutant CRC patients, no objective response to panitumumab and cetuximab treatment was observed[67]. A recent study of patients with metastatic CRC harboring wild-type KRAS showed a trend towards improved PFS in patients with wild-type PIK3CA compared to PI3KCA-mutant patients who received cetuximab either as a monotherapy or in combination with chemotherapy (P = 0.06)[68]. Furthermore, a meta-analysis of 576 CRC patients reported a significantly lower PFS in patients harboring a PIK3CA mutation compared to patients with wild-type PIK3CA when treated with EGFR blockade[69]. Although a majority of the aforementioned studies suggest resistance to EGFR blockade in PIK3CA-mutant metastatic CRC patients with wild-type KRAS, the absence of a consensus precludes making a clear conclusion in this specific patient group. A recent study investigated the role of PTEN expression in anti-EGFR blockade therapy and reported potential resistance to cetuximab treatment in metastatic CRC patients with low PTEN expression[70]. Another study also demonstrated a significantly diminished response and worse PFS in patients with loss of PTEN function (32 wk vs 14 wk, P < 0.0001)[71]. Loss of PTEN expression was also studied in patients with KRAS wild-type CRC which again suggested a lack of benefit from EGFR blockade[68]. While accumulating evidence supports the hypothesis that PTEN loss may result in potential intrinsic resistance to EGFR blockade in CRC patients, further studies are needed to understand exact role of dysregulation of this gene.
The MET proto-oncogene, a receptor tyrosine kinase that belongs to the hepatocyte growth factor receptor, is also related to EGFR blockade resistance in CRC patients[72]. A mechanistic study suggested that, resistance essentially arises in cancer stem cells via increased rebound activity of the MET oncogene upon inhibition of EGFR signaling by monoclonal antibodies[73]. Another study identified increased Met activity driven by oncogenic Src and this upregulation was found to be related to cetuximab resistance[74]. Increased TGF-α expression mediated by the Met oncogene could be also another oncogenic pathway related to cetuximab resistance[75]. A mechanistic study reported that inhibition of both the Met and EGFR pathways by a bispecific monoclonal antibody may abrogate EGFR blockade resistance[76]. Although these studies suggest that upregulation of this tyrosine kinase pathway may be related to EGFR blockade resistance, the exact mechanism of resistance with Met activity is yet to be elucidated.
The response to EGFR blockade even in metastatic CRC patients with wild-type KRAS is unfortunately limited: Following an initial honeymoon period, a majority of patients develop disease progression within months of initiation[77]. Researchers investigated pathways possibly related to resistance in wild-type KRAS patients. An animal model study demonstrated that human epidermal growth factor receptor 2 (HER-2) amplification was associated with cetuximab resistance in a subset of metastatic CRC patients with wild-type KRAS, NRAS, BRAF and PIK3CA[78]. The authors also reported prolonged responses with concurrent inhibition of HER-2 and EGFR in their model. Another mechanistic cell line study corroborated the association of aberrant ERBB2 (HER-2) signaling with EGFR blockade resistance[79]. In a retrospective cohort study, HER-3 overexpression was also suggested to be a predictor of EGFR blockade resistance in metastatic CRC patients who underwent cetuximab and irinotecan treatment[80]. Similar to the aforementioned studies, all patients included in this study were also harboring wild-type KRAS. A recent translational study examined the emergence of resistance in CRC cells sensitive to EGFR blockade[81]. The authors examined the ultimate effect of continuous cetuximab treatment on the development of cetuximab-resistant clones. One of the cell lines developed a rescue mechanism by KRAS amplification and the other acquired a KRAS mutation. In the same study, the authors studied human samples and reported acquisition of KRAS mutation in 6 of 10 cases and KRAS amplification in another patient who developed resistance to cetuximab after an initial response. In another recent translational study, authors tested their theory with mathematical modeling; they hypothesized that KRAS-mutant CRC subclones might exist prior to initiation of anti-EGFR treatment[82]. According to this model, anti-EGFR monoclonal therapy results in the selective proliferation of resistant subclones. The time required for these subclones to become radiologically detectable corresponds to the time of disease progression and development of EGFR blockade resistance. This hypothesis is also supported by tumor heterogeneity, a consequence of variant clones arising from a single parental clone due to continuous diverse genetic alterations throughout carcinogenesis and metastasis. These diverse genetic signatures have also been demonstrated to cause mixed clinical responses to cytotoxic chemotherapy. Moreover, a recent whole-exome sequencing study of 129 wild-type KRAS CRC tumor samples identified potential resistance mechanisms resulting from mutations in other growth factor pathways such as FGFR, and PDGFRA[83]. This data suggests activation of potential bypass pathways related to other receptor tyrosine kinases abrogating the inhibitory effect of EGFR blockade.
Another possible resistance mechanism to anti-EGFR treatment could be attributed to ineffective structural interaction between the drug and its receptor[84]. Mutations in the extracellular domain of EGFR hindering the binding of cetuximab to EGFR were demonstrated in 2 of 10 study participants with resistance to cetuximab. Moreover, one of those two cases responded to panitumumab. Since the number of the patients in the study was limited, this specific mutation should be further studied in larger populations.
Some recent studies indicate that certain signaling mediators which inhibit apoptosis could potentially rescue malignant cells during initiation of anti-EGFR monoclonal treatments[85,86]. A recent study investigated acquired EGFR blockade resistance by analyzing circulating cancer cells and reported progressive genomic alterations throughout the acquisition of resistance suggesting that genomic instability in cancer cells may execute an important role in EGFR blockade resistance[87]. Activation of other oncogenic tyrosine kinases by the local microenvironment with closed loop feedback might be another key mechanism of resistance in wild type KRAS patients[73]. Further studies are required to elucidate the impact of tumor stroma on EGFR blockade resistance.
Given the limited duration of responses to EGFR blockade and the inevitable development of resistance in both KRAS mutant and KRAS wild-type patients, studies were enrolled to optimize treatment outcomes. Based on potential mechanisms of resistance to EGFR blockade identified in preclinical studies, clinical trials have been designed to combat resistance. Considering the role of PIK3CA mutations, a phase II trial investigated the combination of cetuximab with PX-866, an irreversiblepan-isoform inhibitor of PI3KCA, in 86 patients with KRAS wild type metastatic CRC. The authors reported no improvement in PFS, objective response rate, or OS with the addition PX-866[88]. Combinations of cetuximab with HER-2 and HER-3 targeting agents are currently being studied in clinical trials. The combination of cetuximab with pertuzumab (a monoclonal antibody that disrupts the dimerization of HER-2) showed clinical activity, but produced intolerable side effects and toxicity[89]. Studies combining cetuximab with HER-3 antagonists have demonstrated promising preliminary data, although final results are yet to be reported[90].
A xenograft study investigated a new agent to overcome EGFR mutation-related resistance. Sym004, a novel mixture of two nonoverlapping anti-EGFR monoclonal antibodies, has demonstrated binding and ligand-dependent activation in patients with EGFR mutations conferring resistance to conventional anti-EGFR antibodies in functional studies[91]. Phase I trials of Sym004 in metastatic CRC patients with wild type KRAS mutation and previous EGFR blockade response showed tumor radiologic regression in 17 of 39 patients (44%) and partial response in 5 patients (13%)[92]. Phase II clinical trials of Sym004 and early phase studies of other targeting agents of the EGFR pathways are currently being investigated which may enlighten the utility of concurrent/simultaneous inhibition of prosurvival pathways to abrogate EGFR blockade resistance.
Current state-of-the-science endorses clinical activity of the EGFR blockade in selected subsets of patients with treatment naïve and refractory metastatic CRC. However, growing evidence has advanced our understanding of the limitations of anti-EGFR treatment.
Although cetuximab and other monoclonal anti-EGFR antibodies effectively inhibit EGFR signaling; their clinical activity is limited to a short period of time. Disease heterogeneity, created by continuous and dynamic genetic alterations, distinct mutational signatures in the EGFR downstream signaling pathways, and rebound activation of other growth signals appears to be the primary driving force rescuing cancer cells from apoptosis. New subclones with new oncogenic fingerprints arising as a consequence of genomic instability, appear to be one of the most challenging factors in the era of targeted treatment. Frequently, this dynamic process and the associated genetic plasticity overcome the inhibitory effect of targeted agents and ultimately, disease progression occurs despite optimal treatment. Moreover, the addiction of cancer cells to a single oncogenic pathway appears to be limited, and the co-activation of rebound survival pathways further limits the impact of a single targeted agent. The lack of durability of the clinical responses to EGFR blockade observed in the aforementioned studies also represents the phenotypic characteristics of dynamic changes in genotype. Whether combinations of targeted agents along with alternate treatment cycles can overcome this acquired resistance requires further clinical studies. Studies are warranted to better understand the underpinnings of dynamic genomic alterations and its role in acquired resistance to targeting agents including EGFR blockades.
Manuscript source: Invited manuscript
Specialty Type: Oncology
Country of Origin: United States
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Milone M, Zoller M S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25542] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 2. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8406] [Cited by in RCA: 8971] [Article Influence: 690.1] [Reference Citation Analysis (0)] |
| 3. | Cohen S, Carpenter G, King L. Epidermal growth factor-receptor-protein kinase interactions. Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980;255:4834-4842. [PubMed] |
| 4. | Adamson ED, Rees AR. Epidermal growth factor receptors. Mol Cell Biochem. 1981;34:129-152. [RCA] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 163] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Roskoski R. The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem Biophys Res Commun. 2004;319:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 285] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 6. | Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1378] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 7. | Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682-4689. [PubMed] |
| 8. | Magudia K, Lahoz A, Hall A. K-Ras and B-Raf oncogenes inhibit colon epithelial polarity establishment through up-regulation of c-myc. J Cell Biol. 2012;198:185-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Torii S, Yamamoto T, Tsuchiya Y, Nishida E. ERK MAP kinase in G cell cycle progression and cancer. Cancer Sci. 2006;97:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 202] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 10. | Minden A, Lin A, McMahon M, Lange-Carter C, Dérijard B, Davis RJ, Johnson GL, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719-1723. [PubMed] |
| 11. | Haigis KM, Kendall KR, Wang Y, Cheung A, Haigis MC, Glickman JN, Niwa-Kawakita M, Sweet-Cordero A, Sebolt-Leopold J, Shannon KM. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet. 2008;40:600-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 497] [Cited by in RCA: 468] [Article Influence: 27.5] [Reference Citation Analysis (1)] |
| 12. | Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S, Mercer KL, Grochow R, Hock H, Crowley D. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell. 2004;5:375-387. [PubMed] |
| 13. | Parker PJ, Waterfield MD. Phosphatidylinositol 3-kinase: a novel effector. Cell Growth Differ. 1992;3:747-752. [PubMed] |
| 14. | Kennedy AL, Morton JP, Manoharan I, Nelson DM, Jamieson NB, Pawlikowski JS, McBryan T, Doyle B, McKay C, Oien KA. Activation of the PIK3CA/AKT pathway suppresses senescence induced by an activated RAS oncogene to promote tumorigenesis. Mol Cell. 2011;42:36-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 15. | Asnaghi L, Bruno P, Priulla M, Nicolin A. mTOR: a protein kinase switching between life and death. Pharmacol Res. 2004;50:545-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131-2142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 582] [Article Influence: 32.3] [Reference Citation Analysis (1)] |
| 17. | Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2599] [Cited by in RCA: 2761] [Article Influence: 131.5] [Reference Citation Analysis (0)] |
| 18. | Samuels Y, Diaz LA, Schmidt-Kittler O, Cummins JM, Delong L, Cheong I, Rago C, Huso DL, Lengauer C, Kinzler KW. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 743] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 19. | Roskoski R. RAF protein-serine/threonine kinases: structure and regulation. Biochem Biophys Res Commun. 2010;399:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 20. | Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7459] [Cited by in RCA: 7637] [Article Influence: 332.0] [Reference Citation Analysis (0)] |
| 21. | McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263-1284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1793] [Cited by in RCA: 1765] [Article Influence: 98.1] [Reference Citation Analysis (0)] |
| 22. | Ikehara N, Semba S, Sakashita M, Aoyama N, Kasuga M, Yokozaki H. BRAF mutation associated with dysregulation of apoptosis in human colorectal neoplasms. Int J Cancer. 2005;115:943-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Ikenoue T, Hikiba Y, Kanai F, Tanaka Y, Imamura J, Imamura T, Ohta M, Ijichi H, Tateishi K, Kawakami T. Functional analysis of mutations within the kinase activation segment of B-Raf in human colorectal tumors. Cancer Res. 2003;63:8132-8137. [PubMed] |
| 24. | Sakamoto K, Maeda S, Hikiba Y, Nakagawa H, Hayakawa Y, Shibata W, Yanai A, Ogura K, Omata M. Constitutive NF-kappaB activation in colorectal carcinoma plays a key role in angiogenesis, promoting tumor growth. Clin Cancer Res. 2009;15:2248-2258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 200] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 25. | Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284:31-53. [PubMed] |
| 26. | Osherov N, Levitzki A. Epidermal-growth-factor-dependent activation of the src-family kinases. Eur J Biochem. 1994;225:1047-1053. [PubMed] |
| 27. | Mao W, Irby R, Coppola D, Fu L, Wloch M, Turner J, Yu H, Garcia R, Jove R, Yeatman TJ. Activation of c-Src by receptor tyrosine kinases in human colon cancer cells with high metastatic potential. Oncogene. 1997;15:3083-3090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 149] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Biscardi JS, Tice DA, Parsons SJ. c-Src, receptor tyrosine kinases, and human cancer. Adv Cancer Res. 1999;76:61-119. [PubMed] |
| 29. | Aligayer H, Boyd DD, Heiss MM, Abdalla EK, Curley SA, Gallick GE. Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis. Cancer. 2002;94:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 194] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 30. | Irby RB, Mao W, Coppola D, Kang J, Loubeau JM, Trudeau W, Karl R, Fujita DJ, Jove R, Yeatman TJ. Activating SRC mutation in a subset of advanced human colon cancers. Nat Genet. 1999;21:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 359] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 31. | Kotenko SV, Pestka S. Jak-Stat signal transduction pathway through the eyes of cytokine class II receptor complexes. Oncogene. 2000;19:2557-2565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 157] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | David M, Wong L, Flavell R, Thompson SA, Wells A, Larner AC, Johnson GR. STAT activation by epidermal growth factor (EGF) and amphiregulin. Requirement for the EGF receptor kinase but not for tyrosine phosphorylation sites or JAK1. J Biol Chem. 1996;271:9185-9188. [PubMed] |
| 33. | Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 330] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 34. | Corvinus FM, Orth C, Moriggl R, Tsareva SA, Wagner S, Pfitzner EB, Baus D, Kaufmann R, Huber LA, Zatloukal K. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia. 2005;7:545-555. [PubMed] |
| 35. | Lockhart AC, Berlin JD. The epidermal growth factor receptor as a target for colorectal cancer therapy. Semin Oncol. 2005;32:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Mendelsohn J, Baselga J. Epidermal growth factor receptor targeting in cancer. Semin Oncol. 2006;33:369-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 509] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 37. | Sato JD, Kawamoto T, Le AD, Mendelsohn J, Polikoff J, Sato GH. Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol Biol Med. 1983;1:511-529. [PubMed] |
| 38. | Masui H, Kawamoto T, Sato JD, Wolf B, Sato G, Mendelsohn J. Growth inhibition of human tumor cells in athymic mice by anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res. 1984;44:1002-1007. [PubMed] |
| 39. | Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311-1318. [PubMed] |
| 40. | Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787-2799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 921] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 41. | Fracasso PM, Burris H, Arquette MA, Govindan R, Gao F, Wright LP, Goodner SA, Greco FA, Jones SF, Willcut N. A phase 1 escalating single-dose and weekly fixed-dose study of cetuximab: pharmacokinetic and pharmacodynamic rationale for dosing. Clin Cancer Res. 2007;13:986-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | Kawamoto T, Sato JD, Le A, Polikoff J, Sato GH, Mendelsohn J. Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc Natl Acad Sci USA. 1983;80:1337-1341. [PubMed] |
| 43. | Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550-6565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1009] [Cited by in RCA: 971] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 44. | Prewett MC, Hooper AT, Bassi R, Ellis LM, Waksal HW, Hicklin DJ. Enhanced antitumor activity of anti-epidermal growth factor receptor monoclonal antibody IMC-C225 in combination with irinotecan (CPT-11) against human colorectal tumor xenografts. Clin Cancer Res. 2002;8:994-1003. [PubMed] |
| 45. | Baselga J, Pfister D, Cooper MR, Cohen R, Burtness B, Bos M, D’Andrea G, Seidman A, Norton L, Gunnett K. Phase I studies of anti-epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin. J Clin Oncol. 2000;18:904-914. [PubMed] |
| 46. | Saltz L, Rubin M, Hochster H, Tchekmeydian N, Waksal H, Needle M, LoBuglio A. Cetuximab (IMC-C225) plus irinotecan (CPT-11) is active in CPT-11-refractory colorectal cancer (CRC) that expresses epidermal growth factor receptor (EGFR). Proc Am Soc Clin Oncol. 2001;20:3a. |
| 47. | Saltz LB, Meropol NJ, Loehrer PJ, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1306] [Cited by in RCA: 1283] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 48. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3767] [Cited by in RCA: 3708] [Article Influence: 176.6] [Reference Citation Analysis (1)] |
| 49. | Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1468] [Cited by in RCA: 1488] [Article Influence: 82.7] [Reference Citation Analysis (1)] |
| 50. | Pippas A, Lenz H, Mayer R, Mirtsching B, Cohn A, Windt P, Van Cutsem E. Analysis of EGFR status in metastatic colorectal cancer patients treated with cetuximab monotherapy. J Clin Oncol. 2005;23:3595. |
| 51. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2901] [Cited by in RCA: 3126] [Article Influence: 195.4] [Reference Citation Analysis (1)] |
| 52. | Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1452] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 53. | Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, Celik I, Schlichting M, Koralewski P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22:1535-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 586] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 54. | Alberts SR, Sargent DJ, Nair S, Mahoney MR, Mooney M, Thibodeau SN, Smyrk TC, Sinicrope FA, Chan E, Gill S. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 359] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 55. | Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher J, Weitz J. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 712] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 56. | Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1444] [Cited by in RCA: 1472] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 57. | Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2504] [Cited by in RCA: 2403] [Article Influence: 141.4] [Reference Citation Analysis (0)] |
| 58. | Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, De Dosso S, Mazzucchelli L, Frattini M, Siena S. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705-5712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1242] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 59. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-4705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1296] [Cited by in RCA: 1394] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 60. | Sastre J, Massuti B, Pulido G, Guillén-Ponce C, Benavides M, Manzano JL, Reboredo M, Rivera F, Grávalos C, Safont MJ. First-line single-agent panitumumab in frail elderly patients with wild-type KRAS metastatic colorectal cancer and poor prognostic factors: A phase II study of the Spanish Cooperative Group for the Treatment of Digestive Tumours. Eur J Cancer. 2015;51:1371-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Barber TD, Vogelstein B, Kinzler KW, Velculescu VE. Somatic mutations of EGFR in colorectal cancers and glioblastomas. N Engl J Med. 2004;351:2883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 250] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 62. | De Roock W, Jonker DJ, Di Nicolantonio F, Sartore-Bianchi A, Tu D, Siena S, Lamba S, Arena S, Frattini M, Piessevaux H. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304:1812-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 584] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 63. | Peeters M, Douillard JY, Van Cutsem E, Siena S, Zhang K, Williams R, Wiezorek J. Mutant KRAS codon 12 and 13 alleles in patients with metastatic colorectal cancer: assessment as prognostic and predictive biomarkers of response to panitumumab. J Clin Oncol. 2013;31:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 64. | De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V, Papamichael D, Laurent-Puig P. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1491] [Cited by in RCA: 1656] [Article Influence: 110.4] [Reference Citation Analysis (1)] |
| 65. | Laurent-Puig P, Cayre A, Manceau G, Buc E, Bachet JB, Lecomte T, Rougier P, Lievre A, Landi B, Boige V. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27:5924-5930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 525] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 66. | Prenen H, De Schutter J, Jacobs B, De Roock W, Biesmans B, Claes B, Lambrechts D, Van Cutsem E, Tejpar S. PIK3CA mutations are not a major determinant of resistance to the epidermal growth factor receptor inhibitor cetuximab in metastatic colorectal cancer. Clin Cancer Res. 2009;15:3184-3188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 67. | Sartore-Bianchi A, Martini M, Molinari F, Veronese S, Nichelatti M, Artale S, Di Nicolantonio F, Saletti P, De Dosso S, Mazzucchelli L. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69:1851-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 595] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 68. | Sood A, McClain D, Maitra R, Basu-Mallick A, Seetharam R, Kaubisch A, Rajdev L, Mariadason JM, Tanaka K, Goel S. PTEN gene expression and mutations in the PIK3CA gene as predictors of clinical benefit to anti-epidermal growth factor receptor antibody therapy in patients with KRAS wild-type metastatic colorectal cancer. Clin Colorectal Cancer. 2012;11:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 69. | Mao C, Yang ZY, Hu XF, Chen Q, Tang JL. PIK3CA exon 20 mutations as a potential biomarker for resistance to anti-EGFR monoclonal antibodies in KRAS wild-type metastatic colorectal cancer: a systematic review and meta-analysis. Ann Oncol. 2012;23:1518-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 70. | Loupakis F, Pollina L, Stasi I, Ruzzo A, Scartozzi M, Santini D, Masi G, Graziano F, Cremolini C, Rulli E. PTEN expression and KRAS mutations on primary tumors and metastases in the prediction of benefit from cetuximab plus irinotecan for patients with metastatic colorectal cancer. J Clin Oncol. 2009;27:2622-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 331] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 71. | Li FH, Shen L, Li ZH, Luo HY, Qiu MZ, Zhang HZ, Li YH, Xu RH. Impact of KRAS mutation and PTEN expression on cetuximab-treated colorectal cancer. World J Gastroenterol. 2010;16:5881-5888. [PubMed] |
| 72. | Krumbach R, Schüler J, Hofmann M, Giesemann T, Fiebig HH, Beckers T. Primary resistance to cetuximab in a panel of patient-derived tumour xenograft models: activation of MET as one mechanism for drug resistance. Eur J Cancer. 2011;47:1231-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 73. | Luraghi P, Reato G, Cipriano E, Sassi F, Orzan F, Bigatto V, De Bacco F, Menietti E, Han M, Rideout WM. MET signaling in colon cancer stem-like cells blunts the therapeutic response to EGFR inhibitors. Cancer Res. 2014;74:1857-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 74. | Song N, Liu S, Zhang J, Liu J, Xu L, Liu Y, Qu X. Cetuximab-induced MET activation acts as a novel resistance mechanism in colon cancer cells. Int J Mol Sci. 2014;15:5838-5851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Troiani T, Martinelli E, Napolitano S, Vitagliano D, Ciuffreda LP, Costantino S, Morgillo F, Capasso A, Sforza V, Nappi A. Increased TGF-α as a mechanism of acquired resistance to the anti-EGFR inhibitor cetuximab through EGFR-MET interaction and activation of MET signaling in colon cancer cells. Clin Cancer Res. 2013;19:6751-6765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 76. | Castoldi R, Ecker V, Wiehle L, Majety M, Busl-Schuller R, Asmussen M, Nopora A, Jucknischke U, Osl F, Kobold S. A novel bispecific EGFR/Met antibody blocks tumor-promoting phenotypic effects induced by resistance to EGFR inhibition and has potent antitumor activity. Oncogene. 2013;32:5593-5601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 77. | Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2724] [Cited by in RCA: 2763] [Article Influence: 162.5] [Reference Citation Analysis (0)] |
| 78. | Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, Corà D, Di Nicolantonio F, Buscarino M, Petti C. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011;1:508-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 738] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 79. | Yonesaka K, Zejnullahu K, Okamoto I, Satoh T, Cappuzzo F, Souglakos J, Ercan D, Rogers A, Roncalli M, Takeda M. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med. 2011;3:99ra86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 523] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 80. | Scartozzi M, Mandolesi A, Giampieri R, Bittoni A, Pierantoni C, Zaniboni A, Galizia E, Giustini L, Silva RR, Bisonni R. The role of HER-3 expression in the prediction of clinical outcome for advanced colorectal cancer patients receiving irinotecan and cetuximab. Oncologist. 2011;16:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 81. | Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, Valtorta E, Schiavo R, Buscarino M, Siravegna G. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1313] [Cited by in RCA: 1475] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 82. | Diaz LA, Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, Allen B, Bozic I, Reiter JG, Nowak MA. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486:537-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1259] [Cited by in RCA: 1354] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 83. | Bertotti A, Papp E, Jones S, Adleff V, Anagnostou V, Lupo B, Sausen M, Phallen J, Hruban CA, Tokheim C. The genomic landscape of response to EGFR blockade in colorectal cancer. Nature. 2015;526:263-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 373] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 84. | Montagut C, Dalmases A, Bellosillo B, Crespo M, Pairet S, Iglesias M, Salido M, Gallen M, Marsters S, Tsai SP. Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat Med. 2012;18:221-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 392] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 85. | Rosa R, Marciano R, Malapelle U, Formisano L, Nappi L, D’Amato C, D’Amato V, Damiano V, Marfè G, Del Vecchio S. Sphingosine kinase 1 overexpression contributes to cetuximab resistance in human colorectal cancer models. Clin Cancer Res. 2013;19:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 86. | Scartozzi M, Giampieri R, Maccaroni E, Mandolesi A, Biagetti S, Alfonsi S, Giustini L, Loretelli C, Faloppi L, Bittoni A. Phosphorylated AKT and MAPK expression in primary tumours and in corresponding metastases and clinical outcome in colorectal cancer patients receiving irinotecan-cetuximab. J Transl Med. 2012;10:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 87. | Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, Ponzetti A, Cremolini C, Amatu A, Lauricella C. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21:795-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 675] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 88. | Bowles DW, Kochenderfer M, Cohn A, Sideris L, Nguyen N, Cline-Burkhardt V, Schnadig I, Choi M, Nabell L, Chaudhry A. A Randomized, Phase II Trial of Cetuximab With or Without PX-866, an Irreversible Oral Phosphatidylinositol 3-Kinase Inhibitor, in Patients With Metastatic Colorectal Carcinoma. Clin Colorectal Cancer. 2016;pii:S1533-0028(16)30029-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 89. | Rubinson DA, Hochster HS, Ryan DP, Wolpin BM, McCleary NJ, Abrams TA, Chan JA, Iqbal S, Lenz HJ, Lim D. Multi-drug inhibition of the HER pathway in metastatic colorectal cancer: results of a phase I study of pertuzumab plus cetuximab in cetuximab-refractory patients. Invest New Drugs. 2014;32:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 90. | Temraz S, Mukherji D, Shamseddine A. Dual targeting of HER3 and EGFR in colorectal tumors might overcome anti-EGFR resistance. Crit Rev Oncol Hematol. 2016;101:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 91. | Sánchez-Martín FJ, Bellosillo B, Gelabert-Baldrich M, Dalmases A, Cañadas I, Vidal J, Martinez A, Argilés G, Siravegna G, Arena S. The First-in-class Anti-EGFR Antibody Mixture Sym004 Overcomes Cetuximab Resistance Mediated by EGFR Extracellular Domain Mutations in Colorectal Cancer. Clin Cancer Res. 2016;22:3260-3267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 92. | Dienstmann R, Patnaik A, Garcia-Carbonero R, Cervantes A, Benavent M, Roselló S, Tops BB, van der Post RS, Argilés G, Skartved NJ. Safety and Activity of the First-in-Class Sym004 Anti-EGFR Antibody Mixture in Patients with Refractory Colorectal Cancer. Cancer Discov. 2015;5:598-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |