Published online Apr 10, 2016. doi: 10.5306/wjco.v7.i2.270
Peer-review started: August 26, 2015
First decision: October 13, 2015
Revised: December 6, 2015
Accepted: December 18, 2015
Article in press: December 21, 2015
Published online: April 10, 2016
Processing time: 229 Days and 8.2 Hours
Laparoscopy-related tumor implantations of gynecological malignancies into the subcutaneous tissue are rarely diagnosed. We report an interesting case of a 46-year-old female who presented with an abdominal subcutaneous metastasis of a borderline ovarian tumor. The patient received a laparoscopic unilateral adnexectomy for a solid-cystic tumor of the right ovary. Histopathological workup showed a papillary borderline tumor of mucinous type. Nine days later she underwent a hysterectomy, left adnexectomy, appendectomy and omentectomy. Exploration of the peritoneum revealed no intraperitoneal implants. Further exploration showed a non-invasive implant of a borderline tumor in the subcutaneous tissue above the fascia that had no contact to the peritoneum. It is hypothesized that tumor cells may have been implanted during a previous laparoscopy, the most recent of which had been fourteen years prior to her current presentation. Various risk factors for port-site malignancies have been identified. Tumor manipulation and extraction of tumor tissue without a protective bag may contribute to development of trocar-site metastasis.
Core tip: Trocar-site subcutaneous metastasis rarely develops after laparoscopic surgery. Avoidance of over-manipulation of the tumor and use of a protective bag for removal of tumor tissue may minimize the risk of this complication.
- Citation: Banys-Paluchowski M, Yeganeh B, Luettges J, Maibach A, Langenberg R, Krawczyk N, Paluchowski P, Maul H, Gebauer G. Isolated subcutaneous implantation of a borderline ovarian tumor: A case report and review of the literature. World J Clin Oncol 2016; 7(2): 270-274
- URL: https://www.wjgnet.com/2218-4333/full/v7/i2/270.htm
- DOI: https://dx.doi.org/10.5306/wjco.v7.i2.270
Minimally invasive surgery is a standard procedure to clarify undefined intraabdominal masses and its use is increasing in gastroenterologic surgery, gynecology and general surgery. However, tumor seeding into abdominal wall tissues may occur during laparoscopic removal of the mass[1]. We report an interesting case of subcutaneous implantation of a borderline ovarian tumor (BOT) and hypothesize that the implantation occurred during last laparoscopy conducted 14 years prior to presentation.
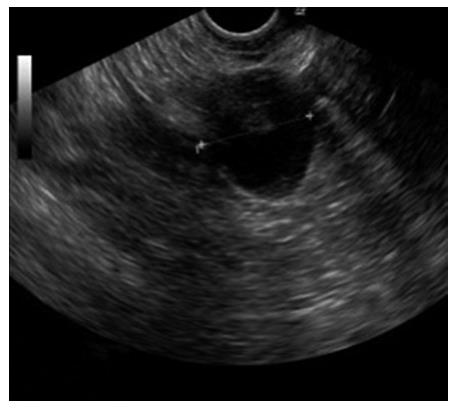
A 46-year-old female presented at our Gynecological Cancer Center (certified by the German Cancer Society) with a cyst with intracystic papillary projections in the right ovary, measuring 2.5 cm × 2.8 cm (Figure 1). Her left ovary and uterus were sonographically normal and she had no free intraabdominal fluid. The patient was asymptomatic. She reported no abdominal discomfort or changes in her bowel or bladder habit. CA125 was 38.7 U/mL. Her concurrent medical history included obesity, latent hypothyroidism and World Health Organization (WHO) grade I arterial hypertension. She had undergone two previous laparoscopies, one for an ectopic pregnancy in 1990, and another for a sterilization procedure six years later, and had had one vaginal birth. Her family history was unremarkable.
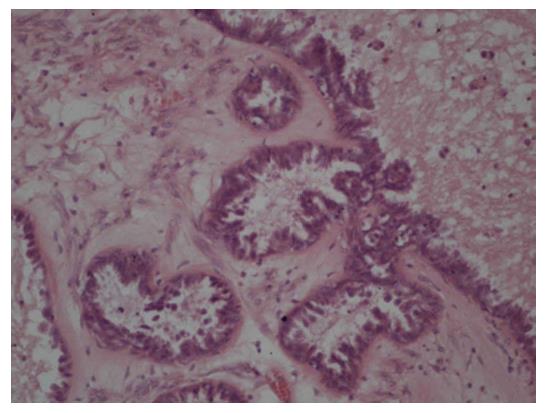
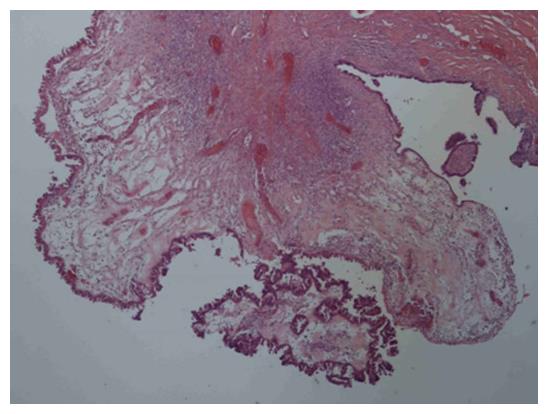
We recommended one-sided laparoscopic adnexectomy. Laparoscopy revealed an enlarged right ovary with smooth surface. The patient’s uterus and left ovary were macroscopically unremarkable and she had no ascites. A right adnexectomy was performed, with a protective bag used for retrieval of the intact tumor. Histopathological analysis revealed a mucinous papillary borderline tumor (Figure 2). After discussion in the interdisciplinary tumor board meeting, explorative laparotomy with adequate surgical staging was recommended according to national treatment guidelines[2]. Nine days after the laparoscopy, the patient received median exploratory laparotomy with hysterectomy, left adnexectomy, multiple peritoneal biopsies, appendectomy and omentectomy. Pelvic and para-aortic lymph nodes were clinically unsuspicious. Systematic staging revealed no intraperitoneal implants. During further exploration, a cystic-solid structure (5 cm × 5 cm) was found in the subcutaneous fat tissue above the fascia with no contact with the peritoneal cavity. This tumor was located far from the trocar sites used during laparoscopy nine days prior to laparotomy. The tumor was completely excised; histological analysis showed a non-invasive implant of a papillary borderline tumor (Figure 3). The patient had an uneventful recovery and received no further therapy. After five years of follow-up she is alive and disease-free.
Tumor implantation in the surgical wound is a rare complication of oncological surgery (Table 1). In the present report, the patient was diagnosed with a synchronous abdominal and subcutaneous manifestation of a BOT. This heterogeneous group of tumors with characteristic histological features comprises 15%-20% of all epithelial ovarian malignancies with an incidence of 1.8-4.8 per 100000 women per year[3]. In contrast to ovarian cancer, BOTs occur at a younger age and have excellent prognosis. However, recurrences are observed in one-tenth of patients; tumor stage, postoperative macroscopic disease and the presence of invasive and noninvasive implants are predictors of relapse risk and survival. In a large meta-analysis of more than 4000 patients, the most reliable prognostic indicator for advanced stage tumors was the type of peritoneal implant; the survival of patients with noninvasive implants was 95%, compared to only 66% for invasive implants[4]. With regard to histological subtype, the majority of borderline tumors are serous, followed by mucinous subtype, whereas most ovarian cancers are serous but the mucinous type is rare. According to the WHO definition, the principal diagnostic feature of a borderline tumor is the absence of obvious stromal invasion; their mitotic activity and nuclear abnormalities are between clearly benign and unquestionably malignant tumours of a similar cell type. In many cases, extensive specimen sampling is necessary to establish the diagnosis; a histological review by a reference pathologist may be helpful to adequately classify the tumor.
| Ref. | No. of patients | Cohort | Type of surgery | Results |
| Chua et al[9], 2011 | 11027 from 17 studies | Various cancers (colon, rectal, gynecological, gastric, liver, urological) | Laparoscopic surgery or diagnostic surgery for malignant abdominal disease | The incidence of PSM is low (< 2%); in eight randomized trials comparing laparoscopy to open surgery for cancer, there was no statistical difference in the development of PSM or wound metastasis (none of these eight trials included gynaecological cancer patients) |
| Zivanovic et al[1], 2008 | 1694 (retrospective analysis of all laparoscopies in a 16-yr period) | Various gynecological cancers (ovarian, perotineal, uterine, cervical) | Laparoscopy in women with malignant disease | Laparoscopy-related subcutaneous tumor implantation occurred in 20 cases (1.18%); only one case of “isolated” PSM was reported; subcutaneous tumor implantations usually occurred with carcinomatosis, with synchronous metastases to other sites; patients whose preceding laparoscopy was performed for advanced or recurrent abdominopelvic disease were more at risk for developing PSM; patients who developed PSM within 7 mo after laparoscopy had a median survival of 12 mo compared to 37 mo in patients who developed PSM > 7 mo after laparoscopy |
| Martínez et al[10], 2010 | 1216 (retrospective analysis of all laparoscopies) | Uterine (921) and cervical (295) cancer | Laparoscopy in women with malignant disease | The overall incidence of PSM was very low (0.4% per procedure) after a median follow-up of 49 mo; the median time to the development of PSM was 8 mo; patients with peritoneal carcinomatosis were most at risk for developing PSM |
| Ndofor et al[8], 2011 | 181 | Various gynecological cancers (uterine, cervical, ovarian) | Robotic surgery (DaVinci Surgical system) in women with malignant disease | PSM occurred in two cases (1.1%); there were no cases of isolated PSM |
| Heitz et al[5], 2009 | 66 | Ovarian cancer | Laparoscopy followed by cytoreductive laparotomy with resection of port sites | Port site metastasis were present in 47% of patients; patients with ascites, higher tumor stage and peritoneal carcinomatosis were more at risk of developing PSM |
The use of laparoscopy in BOTs and ovarian cancer remains controversial. Heitz et al[5] retrospectively analyzed a cohort of ovarian cancer patients who had laparoscopy before definite cytoreductive surgery. In 66 patients, port sites were excised and histopathologically examined: Abdominal wall metastasis was revealed in 47% of cases. However, this finding did not influence clinical outcome. The authors concluded that patients with highly suspected ovarian malignancy and ascites were the most likely to benefit from a direct referral to a cancer center. In their review, Wang et al[6] reported that the risk of an early occurrence of port site metastases (PSM) was the highest in patients with ovarian cancer, ascites, and diagnostic or palliative procedures for malignancy. A more recent review by Ramirez et al[7] showed that port site recurrences may occur in patients with various gynecological malignancies, such as ovarian, cervical, uterine, vaginal and fallopian tube carcinoma and are isolated to the tissue-manipulating port in most patients. Ndofor et al[8] evaluated the rate of PSM after robotic endoscopic surgery for gynecological cancer. In a cohort of 181 patients undergoing robotic oncological operations, two (1.1%) developed trocar site metastases. Both patients were diagnosed with other metastases as well; there were no cases of isolated port site recurrences. In the present report, since the subcutaneous tumor was localized far from the trocar incisions used during the laparoscopy nine days before laparotomy and had no contact to the intraperitoneal cavity, it may be hypothesized that tumor cells may have been implanted during one of the previous laparoscopies, the most recent of which had been fourteen years prior to her current presentation.
It is not yet clear which mechanisms play a role during trocar site implantation of tumor cells. Direct implantation may occur through forced retrieval of the mass or by instruments that had contact with tumor cells during dissection. One possible way to minimize the risk of tumor seeding is the use of a protective bag for removal of all tissue. Tumor seeding may occur during excision of the diseased organ in case of over-manipulation leading to tumor spillage, and dissemination in the peritoneal cavity[8]. Since a higher rate of trocar site recurrences has been reported in patients with advanced ovarian cancer, ascites and omental cake, the risk may be reduced by avoidance of laparoscopic surgery in patients with advanced malignancy. In case of gynecological cancer suspicion, patients benefit from a referral to a gynecological oncologist[11]. In case of laparoscopy for advanced ovarian cancer, careful closure of the peritoneum, rectus sheath, and skin followed by chemotherapy or cytoreductive surgery with excision of the trocar sites within seven days has been recommended as well[12]. Among other factors, the effects of gas turbulence and high pressure peritoneum during laparoscopy have been discussed as a potential mechanism of the development of PSM. Since carbon dioxide exposure stimulates the growth of ovarian cancer cells in vitro, it has been speculated that CO2 used during laparoscopy may increase the rate of wound metastasis[13]. Probably, a multifactorial mechanism may be responsible, in which the main parameters are tumor manipulation, forced removal of the unprotected tissue and high pressure pneumoperitoneum.
The estimated incidence of port site metastasis following laparoscopic surgery is 1%-2%. Several risk factors, such as tumor manipulation and extraction of tumor tissue without a protective bag, have been identified. In patients with advanced malignancy and ascites, the possibility of this complication should be kept in mind when choosing laparoscopic surgery.
We thank Dr. Mustafa Anjari from the Department of Radiology, St Thomas’ Hospital, London, United Kingdom for revising the manuscript.
A 46-year-old female presented with an asymptomatic cyst.
A cyst with intracystic papillary projections in the right ovary, measuring 2.5 cm × 2.8 cm, no abdominal pain.
Cyst, benign ovarian tumor, borderline ovarian tumor, ovarian cancer.
CA125: 38.7 U/mL.
Ultrasound: An ovarian cyst with intracystic papillary projections, no ascites.
Mucinous papillary borderline tumor of the ovary with a non-invasive subcutaneous implant.
Laparoscopic right adnexectomy followed by median exploratory laparotomy with hysterectomy, left adnexectomy, multiple peritoneal biopsies, appendectomy and omentectomy.
Port site metastasis (or trocar site metastasis) is the development of a metastasis in a port site (usually following laparoscopy) or in a drain site.
The incidence of trocar site metastasis following laparoscopic surgery is low (1%-2%). Tumor manipulation and extraction of tumor tissue without a protective bag have been identified as risk factors.
This is an interesting case report and review of the literature of port site implants in ovarian malignancy.
P- Reviewer: Sodergren MH, Souza CA S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Zivanovic O, Sonoda Y, Diaz JP, Levine DA, Brown CL, Chi DS, Barakat RR, Abu-Rustum NR. The rate of port-site metastases after 2251 laparoscopic procedures in women with underlying malignant disease. Gynecol Oncol. 2008;111:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Wagner U, Harter P, Hilpert F, Mahner S, Reuß A, du Bois A, Petru E, Meier W, Ortner P, König K. S3-Guideline on Diagnostics, Therapy and Follow-up of Malignant Ovarian Tumours: Short version 1.0 - AWMF registration number: 032/035OL, June 2013. Geburtshilfe Frauenheilkd. 2013;73:874-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Fischerova D, Zikan M, Dundr P, Cibula D. Diagnosis, treatment, and follow-up of borderline ovarian tumors. Oncologist. 2012;17:1515-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 4. | Seidman JD, Kurman RJ. Ovarian serous borderline tumors: a critical review of the literature with emphasis on prognostic indicators. Hum Pathol. 2000;31:539-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 271] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Heitz F, Ognjenovic D, Harter P, Kommoss S, Ewald-Riegler N, Haberstroh M, Gomez R, Barinoff J, Traut A, du Bois A. Abdominal wall metastases in patients with ovarian cancer after laparoscopic surgery: incidence, risk factors, and complications. Int J Gynecol Cancer. 2010;20:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Wang PH, Yuan CC, Lin G, Ng HT, Chao HT. Risk factors contributing to early occurrence of port site metastases of laparoscopic surgery for malignancy. Gynecol Oncol. 1999;72:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Ramirez PT, Frumovitz M, Wolf JK, Levenback C. Laparoscopic port-site metastases in patients with gynecological malignancies. Int J Gynecol Cancer. 2004;14:1070-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Ndofor BT, Soliman PT, Schmeler KM, Nick AM, Frumovitz M, Ramirez PT. Rate of port-site metastasis is uncommon in patients undergoing robotic surgery for gynecological malignancies. Int J Gynecol Cancer. 2011;21:936-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Chua TC, Yan CD, Morris DL, Sugarbaker PH. Port-Site Metastasis Following Laparoscopic Surgery. In book: Advanced Laparoscopy, ed. Edited by Shamsa A. In: Tech; 2011. |
| 10. | Martínez A, Querleu D, Leblanc E, Narducci F, Ferron G. Low incidence of port-site metastases after laparoscopic staging of uterine cancer. Gynecol Oncol. 2010;118:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Chan JK, Kapp DS, Shin JY, Husain A, Teng NN, Berek JS, Osann K, Leiserowitz GS, Cress RD, O’Malley C. Influence of the gynecologic oncologist on the survival of ovarian cancer patients. Obstet Gynecol. 2007;109:1342-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | van Dam PA, DeCloedt J, Tjalma WA, Buytaert P, Becquart D, Vergote IB. Trocar implantation metastasis after laparoscopy in patients with advanced ovarian cancer: can the risk be reduced? Am J Obstet Gynecol. 1999;181:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 84] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Smidt VJ, Singh DM, Hurteau JA, Hurd WW. Effect of carbon dioxide on human ovarian carcinoma cell growth. Am J Obstet Gynecol. 2001;185:1314-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |