Published online Apr 10, 2016. doi: 10.5306/wjco.v7.i2.160
Peer-review started: July 7, 2015
First decision: September 18, 2015
Revised: January 5, 2016
Accepted: February 14, 2016
Article in press: February 16, 2016
Published online: April 10, 2016
Processing time: 280 Days and 7.8 Hours
Twenty-five years ago, Nembrot and colleagues reported amplification of the estrogen receptor alpha gene (ESR1) in breast cancer, initiating a broad and still ongoing scientific debate on the prevalence and clinical significance of this genetic aberration, which affects one of the most important genes in breast cancer. Since then, a multitude of studies on this topic has been published, covering a wide range of divergent results and arguments. The reported prevalence of this alteration in breast cancer ranges from 0% to 75%, suggesting that ESR1 copy number analysis is hampered by technical and interpreter issues. To date, two major issues related to ESR1 amplification remain to be conclusively addressed: (1) The extent to which abundant amounts of messenger RNA can mimic amplification in standard fluorescence in situ hybridization assays in the analysis of strongly expressed genes like ESR1, and (2) the clinical relevance of ESR1 amplification: Such relevance is strongly disputed, with data showing predictive value for response as well as for resistance of the cancer to anti-estrogen therapies, or for subsequent development of cancers in the case of precursor lesions that display amplification of ESR1. This review provides a comprehensive summary of the various views on ESR1 amplification, and highlights explanations for the contradictions and conflicting data that could inform future ESR1 research.
Core tip: The estrogen receptor alpha gene gene (ESR1) is one of the most important genes in breast cancer, but the prevalence of ESR1 amplification is matter of ongoing debate. A number of studies suggest that technical issues and lack of standards contribute to the discrepant findings. Future studies should focus on the potential clinical relevance of this phenomenon.
- Citation: Holst F. Estrogen receptor alpha gene amplification in breast cancer: 25 years of debate. World J Clin Oncol 2016; 7(2): 160-173
- URL: https://www.wjgnet.com/2218-4333/full/v7/i2/160.htm
- DOI: https://dx.doi.org/10.5306/wjco.v7.i2.160
In 1990, when Nembrot et al[1] reported on amplification of the estrogen receptor alpha encoding gene ESR1 in breast cancer, it was not possible to foresee that, two and a half decades later, conflicting data on the prevalence and possible clinical significance of this alteration would lead to an ongoing debate[2,3]. The relevance of the controversy results from the importance of the gene for the treatment of breast cancer. ESR1 encodes the estrogen receptor alpha (ERα), which is a cellular receptor for the steroid hormone estrogen, a key molecule that regulates the growth and differentiation of the mammary gland[4-8]. ERα is activated by estrogen and drives cell proliferation in breast cancer[9-11]. About two thirds of breast cancers express ERα at the time of diagnosis, making the ERα-protein the most frequently applied clinical biomarker and molecular therapy target for this tumor type[9,12-15].
Gene amplification is a critical mechanism for oncogenic activation of a gene[16,17], and is believed to be a marker for oncogene addiction[18,19]. The success of Herceptin® in treating breast and gastric cancers in which the ERBB2 gene [that encodes the human epidermal growth factor 2 (HER2)] is amplified has impressively demonstrated the clinical value of gene amplification[20,21]. The report of frequent ESR1 amplification as a candidate marker for optimal response of proliferating breast disease to anti-estrogenic Tamoxifen monotherapy, thus attracted considerable attention in the scientific community[22-29].
However, the accounts of ESR1 amplification were challenged from the outset. Watts et al[30] used the same method as Nembrot et al[1], but reported an unexpected lower incidence of copy number increase in 1991. This already suggested that differences in laboratory protocols, interpretation of results, and tissue sampling may represent major challenges in the analysis of ESR1 amplification. To date, articles that present a wide range of diverging data and arguments[22,31], continue to be published, and debate or address the topic of ESR1 amplification. An intense dialog flared up following the report that frequent ESR1 amplification was detected in a large series of breast cancers and that regarding clinical data suggested a particular benefit of Tamoxifen treatment for these patients[24-26,28,29,32-38]. This controversy was especially evident in response to the suggestion that pre-mRNA artifacts could explain the conflicting results reported in a 2012 fluorescence in situ hybridization (FISH) study on ESR1 amplification[3,22,23,39,40], which appeared to be a self-fulfilling prophecy concerning mRNA artifacts that were discussed as far back as 2008[36]. Conclusions ranged from no ESR1 amplification in breast cancer[38] and reports of ESR1 amplification being “fictional”[39], to reports of frequent prevalence, with predictive significance for response or resistance to anti-estrogen therapy[24-26,40,41]. In addition, whether amplification of ESR1 is an early or late event, and whether it can be implemented as a potential marker for prophylactic anti-estrogen treatment[3,42], is also unresolved.
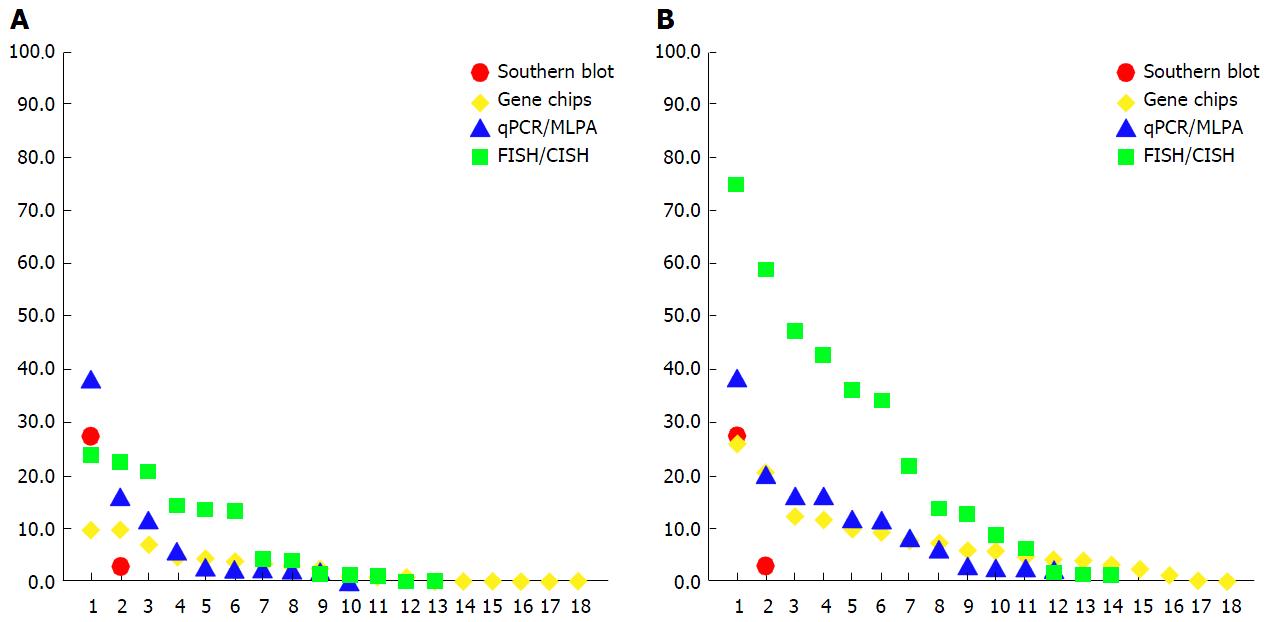
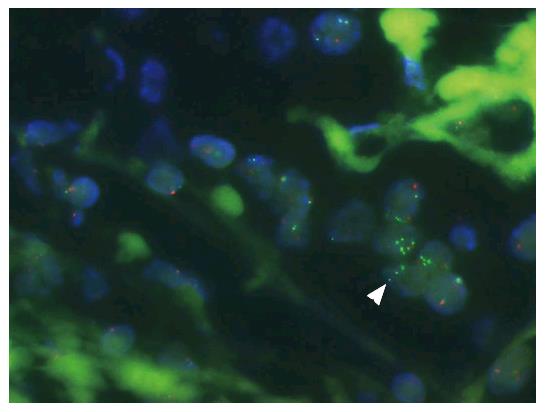
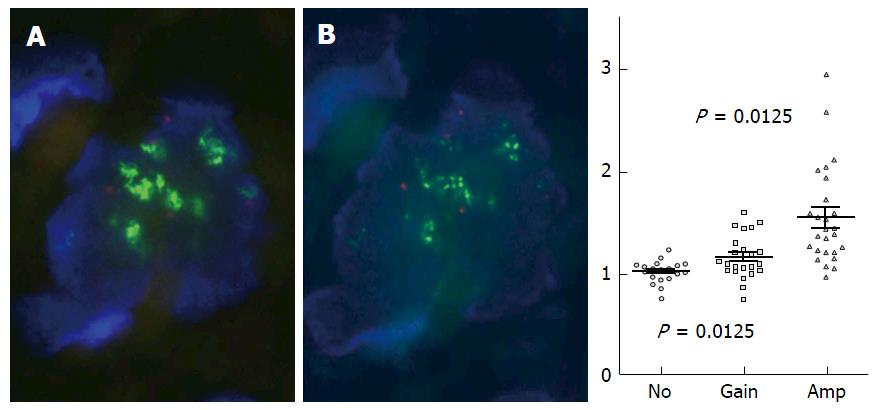
Various studies have published ESR1 amplification frequencies that range from 0% to 75% (Figure 1, Appendices A-D), depending on methods used, sample cohorts, and threshold definitions[31]. The amplification is typically described as occurring in a mosaic pattern, indicating heterogeneous and low level increases in copy number. Nuclei often show only few additional gene copies in tight clusters of the homogeneously staining region (HSR) type[40] (Figure 2).
This pattern of amplification is of particular relevance for understanding the scientific debate on ESR1 amplification in breast cancer. To gain clarity on the prevalence of gene amplification requires first that the term be defined. In general, “gene amplification” is defined as an increase in the gene copy number in a cell, independent of the ratio of gene copy number to centromere copy number[43,44]. However, as testing for human epidermal growth factor receptor 2 (HER2, ERBB2) became more frequent, the term “gene amplification” was reserved for amplifications with an average gene to centromere ratio of ≥ 2.0 or ≥ 2.2 (or > 6 copies per nucleus), simply because the threshold for predicting the response to therapy was determined at this level[45-47]. As a consequence, low level gene amplification - with a ratio less than 2.0 but greater than 1.0 - was neglected. However, for studies in which low copy number increases associated with gene amplification are investigated, the exclusion of amplifications with these low ratios[48] can have major consequences with respect to the prevalence of the genetic alteration, and can decrease the frequencies determined to considerably lower numbers in study cohorts, as shown in Figure 1 and Appendices A-D.
Low-level gene copy number alterations such as ESR1 amplifications often present as a continuum of one-to-several additional ESR1 copies, and minor changes of the threshold cut off value can have a major impact on study outcome. For example, using a cut off of > 2.0 instead of ≥ 2.0 for amplification calling, or a ratio 2.2 instead of 1.8, can change the amplification frequency by almost 50%[22,49,50]. In a recent study done with use of next-generation sequencing (NGS), the threshold of ≥ 6 average copies [as recommended for ERBB2 (HER2) testing[46]] in tissue samples with tumor purity of > 20% resulted in only 0.8% ESR1 amplification across samples[51].
The low level and heterogeneous character of ESR1 amplification suggests that “classical ERBB2 (HER2)” thresholds may not be optimal for ESR1 analysis[26,40]: This is even more true when non-morphologic methods are applied for analyzing isolated DNA, wherein the choice of normalization references has a critical impact on analysis outcome. Indeed, several investigators employing quantitative polymerase chain reaction (qPCR) have demonstrated that the prevalence of ESR1 amplification depends massively on the choice of the reference genes or sequences[32,34,40], and have suggested that variable deletion frequencies of reference genes are responsible for this phenomenon[32,34].
In fact, use of some assays with reference genes that have a lower frequency of deletion in breast cancer (approximately 18% vs approximately 30%) - according to The Cancer Genome Atlas (TCGA)[52] - also led to the detection of lower frequencies of ESR1 amplification (ASXL2, EIF5B and PVR vs ESR2). Note, however, that when reference genes such as PIEZO2 (FAM38B) are used, which have higher deletion frequencies (approximately 30%), the frequency of ESR1 amplification remains low[34], suggesting that factors other than reference gene alterations may also contribute to the outcome of qPCR studies. This is also exemplified in cases when two qPCR assays lead to different ESR1 amplification results, even though the different reference genes (ESR2 and SOD2) used had similar deletion frequencies (approximately 30%) according to TCGA.
These assays highlight a huge difference (approximately half a dimension) in the dynamic range for the same samples, pointing to the impact of technical issues, in addition to the status of the reference gene, on study outcome[53] (Supplementary Tables S1-S3 and Supplementary Graphs S1-S4).
Tumor heterogeneity and plasticity have been increasingly recognized over the last few years as common properties of cancer[54-64] that make molecular diagnosis difficult[22,29,40,57,58,64-66]. Heterogeneity is an obvious issue in the analyses of isolated DNA, where mixtures of cells with normal copy numbers and low level amplification may easily result in an “average” copy number that ranges below the definition threshold for the amplification status or even within the background noise of measurement[40]. In the case of heterogeneous cancers analyzed with use of in situ methods (such as FISH), the choice of the tumor area(s) for analysis is of the utmost importance[40]. Accordingly, approaches that classify the tumor gene status based on a minimum of successful hybridized tumor cells[67] might fail to detect aberrations that are only present only in a minor portion of the cancer bulk.
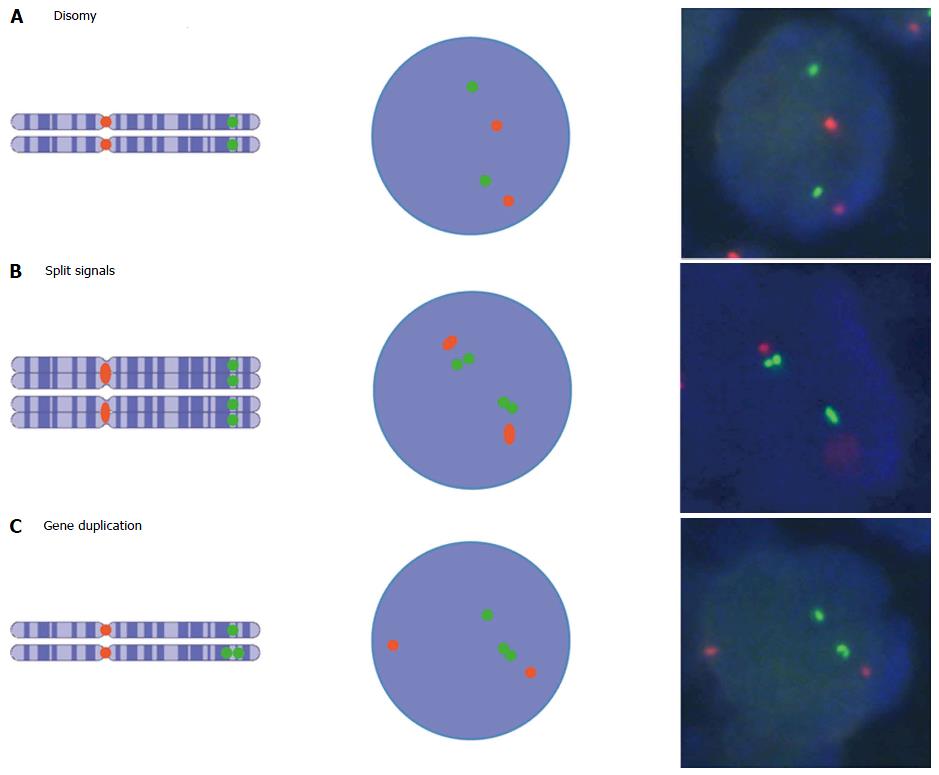
Besides the purity and analyzability of tumor cells, the interpretation of FISH signals also contributes to analysis outcome. For example, automated FISH scoring systems are limited in their ability to count individual signals in tight clusters, and instead measure the ratio of fluorescence intensities[68]. But because fluorescence intensities and signal numbers can vary due to various technical issues that are independent of copy number, automated analysis may be considerably less sensitive than the human eye. This may be particularly true in the case of the low-level clusters that are typical of ESR1[31,32], because identification of individual gene copies is highly dependent on the interpretation of signal patterns. A major challenge in determining low level gene copy numbers that are also heterogeneously distributed is the identification of FISH signals that represent a single gene copy. For example, distinguishing dense clusters of multiple gene copies (which often appear as one large signal) from true single gene copies is difficult; and additionally it is virtually impossible to detect tandem gene duplications (which occur commonly in breast cancer genomes[69]) with use of established FISH scoring criteria that recommend that “doublets” (i.e., two tightly adjacent signals) be interpreted as a single gene copy.
Such a FISH signal-counting recommendation is often applied, based on the interpretation guidelines for ERBB2 (HER2) FISH testing[70]. However, the recommendation to count two adjacent signals as one is based on studies that determine numerical chromosome aberrations, not focal changes in gene copy number[71,72]. This approach is warranted for chromosome enumeration that uses probes that do not hybridize within the centromere region: Following the s-phase of the cell cycle, chromosomes consist of two chromatids, each of which contains one gene copy. Accordingly, FISH analysis displays two signals nearby for these two gene copies[73] (Figure 3), even though chromosome number is not pathologically increased in these cases and such signals should be counted as one.
In contrast to chromosome enumeration, the increased number of gene copies on one chromatid or chromosome is relevant for determining gene copy number: In this case, signal doublets should be considered to represent two gene copies. Studies that combine FISH and gene chip or Southern blot technologies show that FISH signal doublets represent two gene copies on one chromosome, and as such constitute gene amplification[74-77] (Figure 3 and Supplementary Figures S1-S7).
Gene chip technology and NGS are powerful techniques for detecting genomic alterations with high accuracy and objectivity, compared to morphologic methods that evaluate results based on the interpretation of individual observers. Nevertheless, use of these methods is associated with serious pitfalls. In 2008, a published summary of gene chip analyses listed a huge range of amplification frequencies reported in different studies and different tumor populations, ranging from 7% to 35%, even for ERBB2 (HER2)[33]. Over the years, various limitations have led to a reconsideration of these methods. Most importantly, it has to be taken into account that the isolated DNA was analyzed as an average of many different cells. But also methodological limitations due to technology-related background of measurement and the quality of the gene chip hybridization (call rates) have to be considered[22,29,36,40,78]. The large amount of data involved in these studies also makes these methods strongly dependent on the specific computational approaches and algorithms that are used[78-80].
The general impact of the normalization reference and the computational method used to analyze raw data played a key role in the rediscovery of ESR1 amplification, based on the analysis of 22 breast cancers with use of Affymetrix 10K SNP gene chips[24,81]. This is illustrated in Supplementary Figures S1-S7 and the Supplementary Optical Dataset S1, as well as in a video documentation (Supplementary Video Clips S1 + S2).
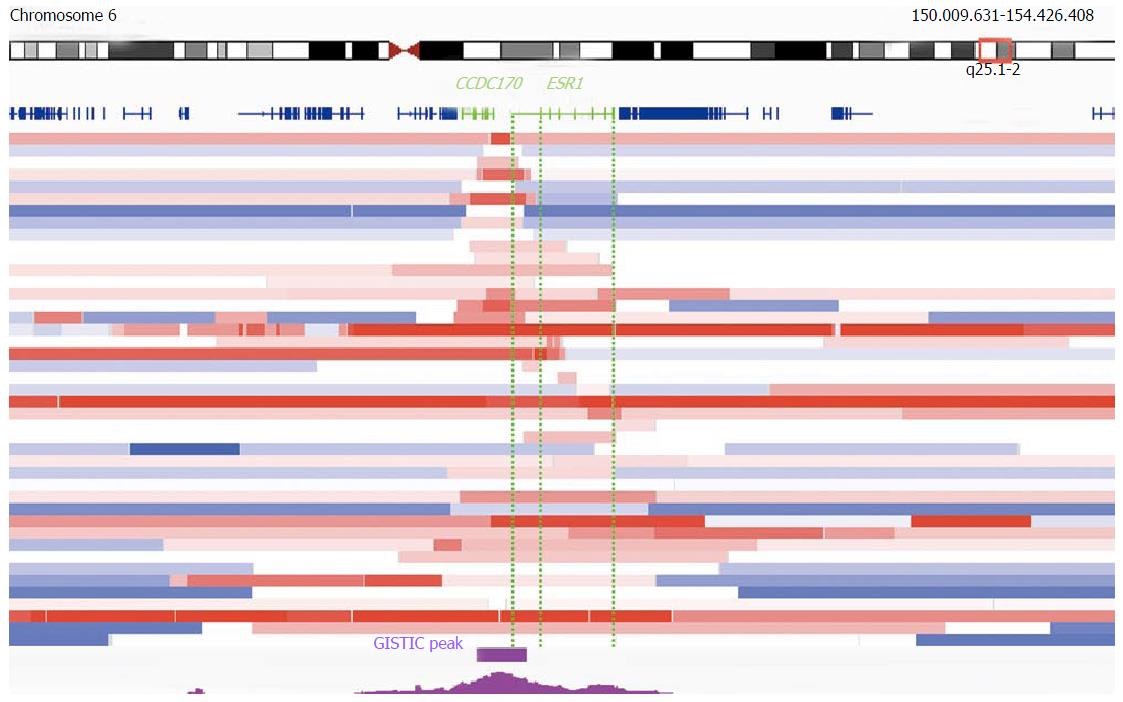
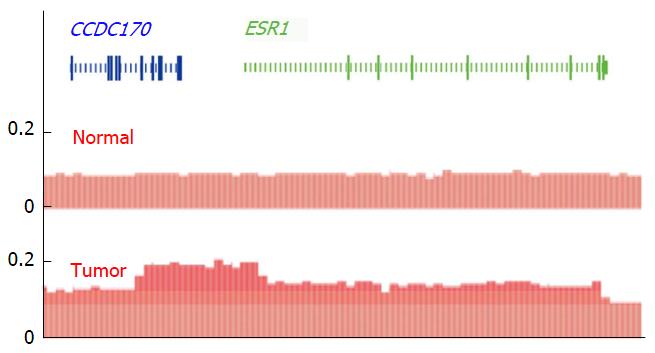
TCGA provides the largest and most advanced gene chip copy number database for isolated DNA from tumor samples[82,83]. A meta-analysis of TCGA dataset offers new insights into ESR1 copy number alterations beyond the published FISH studies. For example, two tumor entities with the most frequently reported ESR1 amplification (by FISH analysis), i.e., breast and endometrial cancers[24,84], are top ranked also by Genomic Identification of Significant Targets in Cancer (GISTIC) analysis (2016-06-01 stddata_2015-04-02 regular peel off)[52]. For GISTIC, TCGA defines gene amplification as a linear copy number increase that exceeds the genome-wide median (adjusted to diploid) by more than 0.1 copies[83,85]. Overall, focal (smaller than half a chromosome arm) and high level (increase of more than one copy) ESR1 amplifications are determined by GISTIC in 16.2%, 6.8% and 2.2% of breast cancers (n = 1080), respectively. Furthermore GISTIC analysis demonstrates that ESR1 undergoes focal amplifications significantly (threshold q = 0.25) above the genome-wide average rate in breast cancer (residual q after GISTIC peel-off = 0.096)[52,86,87]. TCGA also confirms the existence of very small amplifications, with ESR1 and CCDC170 being the only genes in the GISTIC peak[52] (Figure 4): Although some amplifications are due to structural alterations that involve only untranslated part of ESR1, others are limited specifically to the entire gene and its flanking regions (Figure 4)[52,88], providing additional strong support for a clonal selection process that targets the ESR1 locus[86]. Amplifications that were limited to ESR1 - but included parts of CCDC170 - were also found in another SNP gene chip study[81] (supporting Figures 1 and 5) and by NGS, in a breast tumor that was sensitive to estradiol treatment[89] (Figure 5). Obviously, novel and publicly available databases such as TCGA collection[52] were not available at the time when the debate on ESR1 amplification started, but the latest upgrade is still not considered in all publications to date[90].
FISH allows the analysis of gene copy number variations at a single cell level, and its morphological localization. This makes FISH especially well-suited for taking tissue heterogeneity into account. But signal interpretation with use of FISH is dependent on observer subjectivity and is prone to influence by signal artifacts; FISH probes may also detect pre-mRNA transcripts of ESR1, and such hybridization could mimic DNA hybridization signals[39,40]. Such mRNA artifacts may lead to ESR1 amplification calling by FISH that is false positive in cases with amplification signals that are exclusively limited to the nucleotide sequence of ESR1[24]. These artifacts may also explain the lower rates of amplification found by other methods[39]. The significance of this phenomenon has, however, not yet been fully evaluated, and some reports indicate that it does not have a major impact: These include cases with ESR1 amplifications whose extensions have been mapped (by FISH) to the gene locus only[24]. For example, FISH amplification signals are detectable even after RNase treatment of the tissue section prior to the FISH analysis, indicating that DNA is targeted[40] (Figure 6).
Also, in at least 50% of tested cancers, ESR1 amplification identified by FISH can be confirmed by multiplex ligation-dependent probe amplification PCR[40] (Figure 6), and additionally, a qPCR could show that tumors with ESR1 amplification (determined by FISH) average significantly higher ESR1 copy numbers than do tumors without ESR1 amplification (also detected by FISH) (Supplementary Tables S1-S3, Supplementary Graphs S1-S4)[53].
It is important to understand that failed validation of FISH-determined ESR1 amplifications by qPCR or gene chip analysis may be due to tumor purity and heterogeneity. Quantitative analysis of DNA isolated from tissue samples is always prone to underestimation of gene copy numbers - especially when tumor samples are not microdissected. This is because, due to the presence of non-cancerous cells present in a tumor sample, cancer cell purity typically ranges between 20%-80% in breast cancer, and is often overestimated in histological analyses[91]. The “contaminating” non-cancerous tissue inevitably “dilutes” the tumor DNA, and leads to underestimation of changes in gene copy number[22,29,40,52,91]. Clearly this dilution effect is even greater when low-level and heterogeneous alterations (e.g., the ESR1 amplification) are analyzed[52,62,88].
A causal relationship between ESR1 copy numbers and increased expression of ERα protein, which drives cell proliferation[9-11], provides the molecular underpinning of the potential clinical relevance of ESR1 amplification. Expression of the ERα-protein itself has long been used for decades as a biomarker for initiating anti-estrogen treatment in breast cancer[9]. However, regardless of the prevalence of gene amplification across a tumor type, the increase in copy number for a given gene is a well-known mechanism for increasing its expression[16,17,44,92,93]; accordingly, gene amplification is assumed to be a marker for a tumor’s addiction to the expression of the amplified gene[18,19].
Several reports, including those that use DNA-specific methods for ESR1 copy number determination, have documented a significant correlation between ESR1 gene amplification and ERα protein expression[24,25,28,50,67,83-97] (Table 1).
| Ref. | Patients (n) | ESR1 CNI (%) | ERα-negative CNI (%) | Method for CNI detection | |
| Correlation or association found | |||||
| 1 | Nembrot et al[1] | 22 | 27.3 | 0 | Western blot |
| 2 | Holst et al[24] | 1652 | 36.1 | 1.3 | FISH |
| 3 | Tomita et al[25] | 133 | 33.8 | 0 | FISH |
| 4 | Moelans et al[28] | 135 | 8.1 | 27.3 | MLPA |
| 5 | Tsiambas et al[50] | 60 | 21.6 | - | FISH |
| 6 | Dunbier et al[95] | 44 | 20.5 | 0 | Gene chip |
| 7 | Laenkholm et al[96] | 220 | 42.4 | 8.8 | FISH |
| 8 | Singer et al[41] | 394 | 47.5 | 1 | FISH |
| 9 | Lin et al[67] | 150 | 12.7 | 5.9 | FISH |
| 10 | Pentheroudakis et al[97] | 1010 | 58.8 | 12.5 | FISH |
| 11 | Li et al[93] | 219 | - | - | Gene chip |
| 12 | Soysal et al[3] | 58 | 15.5 | 0 | FISH |
| No correlation or association not found | |||||
| 1 | Watts et al[30] | 37 | 2.7 | 0 | Western blot |
| 2 | Reis-Filho et al[34] | 70 | 11.4 | 25 | Gene chip |
| 3 | Vincent-Salomon et al[35] | 341 | 0.9 | 66.70% | Gene chip |
| 4 | Moelans et al[125] | 39 | Approximately 20 | - | MLPA |
| 5 | Ooi et al[39] | 51 | 5.9 | 0 | FISH/MLPA |
| 6 | Markiewicz et al[99] | 281 | 11.7 | 66.7 | qPCR |
| 7 | Chen et al[2] | 301 | 8.6 | 46.2 | FISH |
Nevertheless, it is important to keep in mind that other mechanisms in addition to amplification also regulate a gene’s transcription, and its translation into protein. In fact, some studies[1,34,39,98,99] challenge the correlation between ESR1 copy number and expression levels (Table 1). Nonetheless, it is clear that here, too, the majority of discrepant findings are likely due to technical and methodological reasons. These could include a low number of cases analyzed, false negative ERα expression test results, or lack of statistical power due to low frequencies of tumors with ESR1 copy number alterations detected. However, ESR1 amplification has been described in ERα-protein negative breast cancers with poor survival[99] and it is conceivable that general genetic instability drives ERα-independent 6q amplification in these tumors. Accordingly, such cases are unlikely to be associated with ERα-protein expression and could contribute to the findings that challenge the correlation between ESR1 copy number and ERα expression levels.
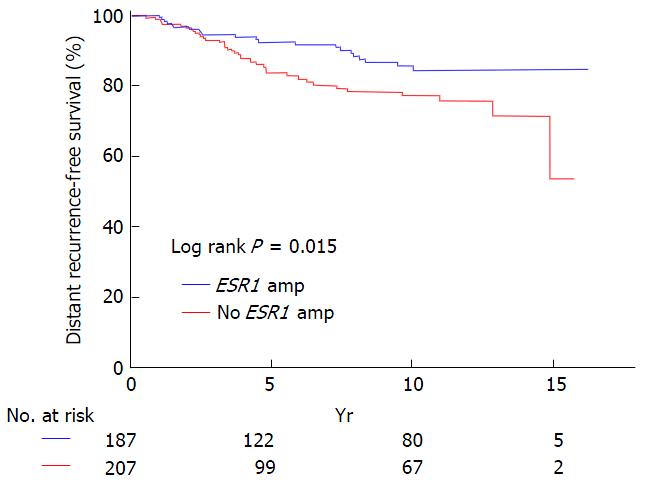
The controversy about the relevance of ESR1 amplification in breast cancer derives from claims that this genetic aberration is a potential predictive marker for optimal response to endocrine therapy. Three studies used FISH for gene copy number determination, and reported that breast cancer patients who were treated with Tamoxifen, and who showed ESR1 amplification in their tumors, had a better disease-specific survival than did patients without this alteration. These studies also included a retrospective analysis of the Tamoxifen-only arm of the prospective randomized ABCSG-06 trial (Figure 7)[24,25,41]. Additionally, a qPCR study found a worse outcome for patients whose tumors were ERα-negative and had ESR1 amplification, while there was no association with survival in patients with ERα-positive cancers that received Tamoxifen treatment[99]. In contrast, another study suggested that ESR1 amplification predicted resistance to Tamoxifen therapy[26], although the results were not reproduced in a follow-up study of the randomized Danish cohort of the BIG-98 trial[27].
These discrepant results can be explained by differences in the kinds and mechanisms of amplification. For example, gene amplification driven by general genetic instability may be a marker for aggressive tumors[100] with unfavorable prognosis, that are less likely to respond to any therapy. Such amplifications, with lack of ERα protein expression[99], would dominate the results of survival analysis in aggressive tumor subsets[26]. Other tumors might amplify a gene specifically driven by the tumors’ addiction to the respective pathway[19]. Indeed, this mechanism has been suggested in two independent studies that observed focal ESR1 amplifications of low-level copy number change in long-term estrogen-deprived (LTED) MCF7 breast cancer cell lines, with use of DNA-specific gene chips and qPCR for ESR1 copy number determination. And yet another experimental study showed that breast-cancer-derived xenografts respond to estrogen treatment of tumor cells that harbor ESR1 amplification, as determined by NGS[89,101].
Furthermore, in one clinical phase II study for evaluating anti-estrogen treatment, a focal ESR1 amplification appeared after therapy in one out of 49 tumors analyzed by NGS[102]. These functional studies provide strong evidence for the potential clinical relevance of ESR1 amplification as a mechanism of ERα pathway regulation. One additional study used LTED MCF7 cells to show a change of ESR1 gene status detectable by FISH; however, the FISH signals were RNase-sensitive and no ESR1 copy number increase was detectable by ESR1 qPCR, suggesting that the FISH results may have been due to probe hybridization to abundant RNA[103].
Gene amplifications in human cancers are markers of the tumor’s dependence on the encoded protein, and point to a potential target of therapy[18,20,21,45-47,104,105]. However, the effects of therapy depend on effective target neutralization, and indicate that target levels must be relevant for effective inactivation by antagonistic drugs. In other words, the success of therapy might depend on the fold change in the amplified gene’s copy number.
Accordingly, gene amplification is a well-known mechanism that underlies drug resistance[106-113]. Even in the case of HER2 (ERBB2), the mechanism of “receptor overcrowding” (and thus the level of receptor gene expression) was believed to be responsible for turning a marker for response into one for resistance depending on the level of gene expression[113].
And while the threshold for therapy response was determined at a doubled gene dose in the case of ERBB2 (HER2), amplifications of other genes [e.g., EGFR, ERBB3 (HER3), and PIK3CA in lung cancer] might be relevant at lower levels[40,73,114-123]. This, as well as a tumor’s heterogeneity regarding the amplification status of a gene, should be taken into account when considering gene amplification as a maker for therapeutic response or resistance.
There is growing evidence that low-level gene copy number amplifications represent an adaptation by tissues to selective pressures[89,101], even in normal (non-transformed) cells[62]. It is obvious that such alterations in growth-regulating pathways can increase the risk for cancerous outgrowth[10,93]. The appearance of ESR1 amplification in precancerous lesions, and their increased frequency during neoplastic transformation, suggest that such amplification is an early event that is potentially cancer-initiating and that drives cell proliferation[3,24,42,124,125]; what is not as clear is whether ESR1 amplification alone is sufficient to transform cells. Nevertheless, detection of ESR1 amplification in breast cancer precursor lesions might help to identify patients at high cancer risk, and it has thus been suggested that such patients could benefit from prophylactic anti-estrogen treatment[3].
The debate on ESR1 amplification in breast cancer is mainly based on methodological issues, including technical limitations, quality of application, and interpretation of results using the standard methods that are available today. The controversy about the frequency of low level ESR1 amplifications in particular, highlights the need for methodically advanced and sensitive approaches that will allow consistent findings.
The power of high throughput screening methods has enabled us to draw integrated maps of malignancy schematic landscapes from a bird's eye perspective. But cancer is not yet vanquished, and zooming in to details of these genetic landscapes could open new dimensions of insights into hidden and undiscovered molecular pathways of malignancy (rat runs) that might be missed from a bird’s eye viewpoint.
A future perspective could comprise a combination of the existing cancer landscapes and detailed information derived from sensitive targeted approaches that will enable us to develop eagles eyes. Use of established morphological imaging methods such as FISH, as well as newly developed NGS-based approaches, could combine the objectivity of computational analysis algorithms with the resolution of single-cell analyses. These methods could integrate spatial and morphological, objective and high resolution measurement within tissues[126], and are in the process of being developed. Initial results have been published with use of single-nucleus sequencing[56,61,127], but challenges of using NGS data processing and whole genome amplification still remain to be tackled[79,80,128].
The reproducibility of research on potential drug targets is low - successful only in about a fifth of studies published[129-131]. While the established FISH method seems to be a valuable approach for studying the clinical relevance of ESR1 amplification or gene status in breast cancer[3,24-26,40,41,50,96] (Figure 7), there is no established consensus on how the interpretation of signal patterns or of gene status classification thresholds and definitions. As such, the nature of the ESR1 gene status on the level of nucleic acids (DNA or RNA) might appear to be of secondary importance when considering a reproducible phenomenon that has an established standard diagnostic method and that is potentially applicable as a clinical marker[3]. In contrast, studies on the potential clinical significance and status definitions of detectable phenomena seem to be rather reasonable. In this context, the robustness and predictive power of a clinically applicable marker may be more important than its molecular properties.
Richard Horton recently commented that “much of the scientific literature, perhaps half, may simply be untrue”, pointing to the recent P-values of “5 sigma” set in particle physics. And the idea, that, regarding scientific publications, “something has gone fundamentally wrong with one of our greatest human creations”, highlights the need for open debates and paper critiques in science[132]. However, scientific debates will be rewarded when, besides the P-values and technical methodology, we do not lose sight of the goals of medical research[133].
P- Reviewer: Nayak BS, Shao R, Sheu JJC, Wang L, Vaclav V S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Nembrot M, Quintana B, Mordoh J. Estrogen receptor gene amplification is found in some estrogen receptor-positive human breast tumors. Biochem Biophys Res Commun. 1990;166:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Chen JR, Hsieh TY, Chen HY, Yeh KY, Chen KS, ChangChien YC, Pintye M, Chang LC, Hwang CC, Chien HP. Absence of estrogen receptor alpha gene (ESR1) gene amplification in a series of breast cancers in Taiwan. Virchows Arch. 2014;464:689-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Soysal SD, Kilic IB, Regenbrecht CR, Schneider S, Muenst S, Kilic N, Güth U, Dietel M, Terracciano LM, Kilic E. Status of estrogen receptor 1 (ESR1) gene in mastopathy predicts subsequent development of breast cancer. Breast Cancer Res Treat. 2015;151:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Russo J, Russo IH. Differentiation and breast cancer. Medicina (B Aires). 1997;57 Suppl 2:81-91. [PubMed] |
| 5. | Prall OW, Rogan EM, Sutherland RL. Estrogen regulation of cell cycle progression in breast cancer cells. J Steroid Biochem Mol Biol. 1998;65:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Russo J, Hu YF, Yang X, Russo IH. Developmental, cellular, and molecular basis of human breast cancer. J Natl Cancer Inst Monogr. 2000;17-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 151] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Russo J, Hu YF, Silva ID, Russo IH. Cancer risk related to mammary gland structure and development. Microsc Res Tech. 2001;52:204-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Brisken C, O’Malley B. Hormone action in the mammary gland. Cold Spring Harb Perspect Biol. 2010;2:a003178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 300] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 9. | Allred DC. Issues and updates: evaluating estrogen receptor-alpha, progesterone receptor, and HER2 in breast cancer. Mod Pathol. 2010;23 Suppl 2:S52-S59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 10. | Yue W, Yager JD, Wang JP, Jupe ER, Santen RJ. Estrogen receptor-dependent and independent mechanisms of breast cancer carcinogenesis. Steroids. 2013;78:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 11. | Zabransky DJ, Park BH. Estrogen receptor and receptor tyrosine kinase signaling: use of combinatorial hormone and epidermal growth factor receptor/human epidermal growth factor receptor 2-targeted therapies for breast cancer. J Clin Oncol. 2014;32:1084-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Sunderland MC, Osborne CK. Tamoxifen in premenopausal patients with metastatic breast cancer: a review. J Clin Oncol. 1991;9:1283-1297. [PubMed] |
| 13. | Stierer M, Rosen H, Weber R, Hanak H, Spona J, Tüchler H. Immunohistochemical and biochemical measurement of estrogen and progesterone receptors in primary breast cancer. Correlation of histopathology and prognostic factors. Ann Surg. 1993;218:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 87] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Silverberg SG, Kurman RJ, Nogales F, Mutter GL, Kubik-Huch RA, Tavassoli FA. Tumours of the uterine corpus: Epithelial tumors and related lesions. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon: IARC Press 2003; 221-249. |
| 15. | Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Rowden D, Solky AJ. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 562] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 16. | Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009;458:719-724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2798] [Cited by in RCA: 2419] [Article Influence: 151.2] [Reference Citation Analysis (0)] |
| 17. | Santarius T, Shipley J, Brewer D, Stratton MR, Cooper CS. A census of amplified and overexpressed human cancer genes. Nat Rev Cancer. 2010;10:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 426] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 18. | Sharma SV, Settleman J. Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev. 2007;21:3214-3231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 314] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 19. | Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008;7:504-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 660] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 20. | Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177-182. [PubMed] |
| 21. | Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8204] [Cited by in RCA: 8131] [Article Influence: 338.8] [Reference Citation Analysis (0)] |
| 22. | Holst F, Moelans CB, Filipits M, Singer CF, Simon R, van Diest PJ. On the evidence for ESR1 amplification in breast cancer. Nat Rev Cancer. 2012;12:149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Albertson DG. ESR1 amplification in breast cancer: controversy resolved? J Pathol. 2012;227:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Holst F, Stahl PR, Ruiz C, Hellwinkel O, Jehan Z, Wendland M, Lebeau A, Terracciano L, Al-Kuraya K, Jänicke F. Estrogen receptor alpha gene (ESR1) gene amplification is frequent in breast cancer. Nat Genet. 2007;39:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 274] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 25. | Tomita S, Zhang Z, Nakano M, Ibusuki M, Kawazoe T, Yamamoto Y, Iwase H. Estrogen receptor alpha gene gene ESR1 amplification may predict endocrine therapy responsiveness in breast cancer patients. Cancer Sci. 2009;100:1012-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Nielsen KV, Ejlertsen B, Müller S, Møller S, Rasmussen BB, Balslev E, Lænkholm AV, Christiansen P, Mouridsen HT. Amplification of ESR1 may predict resistance to adjuvant tamoxifen in postmenopausal patients with hormone receptor positive breast cancer. Breast Cancer Res Treat. 2011;127:345-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Ejlertsen B, Aldridge J, Nielsen KV, Regan MM, Henriksen KL, Lykkesfeldt AE, Müller S, Gelber RD, Price KN, Rasmussen BB. Prognostic and predictive role of ESR1 status for postmenopausal patients with endocrine-responsive early breast cancer in the Danish cohort of the BIG 1-98 trial. Ann Oncol. 2012;23:1138-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Moelans CB, Monsuur HN, de Pinth JH, Radersma RD, de Weger RA, van Diest PJ. ESR1 amplification is rare in breast cancer and is associated with high grade and high proliferation: A multiplex ligation-dependent probe amplification study. Anal Cell Pathol (Amst). 2010;33:13-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 29. | Holst F, Simon R, Tennstedt P. Estrogen Receptor (ESR1) Amplification in Breast Cancer: A Current Review. Connection. 2009;13:44-49. |
| 30. | Watts CK, Handel ML, King RJ, Sutherland RL. Oestrogen receptor gene structure and function in breast cancer. J Steroid Biochem Mol Biol. 1992;41:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Literature on ESR1 Copy Number Alterations in Gynecological Tissue Lesions. Available from: http: //dx.doi.org/10.6084/m6089.figshare.1015605. |
| 32. | Brown LA, Hoog J, Chin SF, Tao Y, Zayed AA, Chin K, Teschendorff AE, Quackenbush JF, Marioni JC, Leung S. ESR1 gene amplification in breast cancer: a common phenomenon? Nat Genet. 2008;40:806-807; author reply 810-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Horlings HM, Bergamaschi A, Nordgard SH, Kim YH, Han W, Noh DY, Salari K, Joosse SA, Reyal F, Lingjaerde OC. ESR1 gene amplification in breast cancer: a common phenomenon? Nat Genet. 2008;40:807-808; author reply 810-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Reis-Filho JS, Drury S, Lambros MB, Marchio C, Johnson N, Natrajan R, Salter J, Levey P, Fletcher O, Peto J. ESR1 gene amplification in breast cancer: a common phenomenon? Nat Genet. 2008;40:809-810; author reply 810-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Vincent-Salomon A, Raynal V, Lucchesi C, Gruel N, Delattre O. ESR1 gene amplification in breast cancer: a common phenomenon? Nat Genet. 2008;40:809; author reply 810-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Holst F, Stahl P, Hellwinkel O, Dancau A, Krohn A, Wuth L, Heupel C, Lebeau A, Terracciano L, Al-Kuraya K. ESR1 gene amplification in breast cancer: a common phenomenon? Nature genetics. 2008;40:810-812. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Albertson DG. Conflicting evidence on the frequency of ESR1 amplification in breast cancer. Nat Genet. 2008;40:821-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Adélaïde J, Finetti P, Charafe-Jauffret E, Wicinski J, Jacquemier J, Sotiriou C, Bertucci F, Birnbaum D, Chaffanet M. Absence of ESR1 amplification in a series of breast cancers. Int J Cancer. 2008;123:2970-2972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Ooi A, Inokuchi M, Harada S, Inazawa J, Tajiri R, Kitamura SS, Ikeda H, Kawashima H, Dobashi Y. Gene amplification of ESR1 in breast cancers--fact or fiction? A fluorescence in situ hybridization and multiplex ligation-dependent probe amplification study. J Pathol. 2012;227:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Moelans CB, Holst F, Hellwinkel O, Simon R, van Diest PJ. ESR1 amplification in breast cancer by optimized RNase FISH: frequent but low-level and heterogeneous. PLoS One. 2013;8:e84189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Singer CF, Holst F, Steurer S, Burandt E, Samonigg H, Lax S, Jakesz R, Rudas M, Stöger H, Greil R. Filipits M, Simon R, Gnant M. Estrogen receptor alpha gene (ESR1) gene amplification status and clinical outcome in tamoxifen-treated postmenopausal patients with endocrine-responsive early breast cancer: An analysis of the prospective ABCSG-6 trial. Proceedings of the ASCO Annual Meeting; 2012. J Clin Oncol. 2012;30 suppl:abstr 10501. |
| 42. | Verschuur-Maes AH, Moelans CB, de Bruin PC, van Diest PJ. Analysis of gene copy number alterations by multiplex ligation-dependent probe amplification in columnar cell lesions of the breast. Cell Oncol (Dordr). 2014;37:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | The American Heritage Science Dictionary. Boston: Houghton Mifflin Harcourt, 2005. . |
| 44. | Bizari L, Silva AE, Tajara EH. Gene amplification in carcinogenesis. Genet Mol Biol. 2006;29:1-7. |
| 45. | Kallioniemi OP, Kallioniemi A, Kurisu W, Thor A, Chen LC, Smith HS, Waldman FM, Pinkel D, Gray JW. ERBB2 amplification in breast cancer analyzed by fluorescence in situ hybridization. Proc Natl Acad Sci USA. 1992;89:5321-5325. [PubMed] |
| 46. | Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2665] [Cited by in RCA: 2596] [Article Influence: 144.2] [Reference Citation Analysis (0)] |
| 47. | Sauter G, Lee J, Bartlett JM, Slamon DJ, Press MF. Guidelines for human epidermal growth factor receptor 2 testing: biologic and methodologic considerations. J Clin Oncol. 2009;27:1323-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 386] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 48. | Tabarestani S, Ghaderian SM, Rezvani H. Detection of Gene Amplification by Multiplex Ligation-Dependent Probe Amplification in Comparison with In Situ Hybridization and Immunohistochemistry. Asian Pac J Cancer Prev. 2015;16:7997-8002. [PubMed] |
| 49. | Nessling M, Richter K, Schwaenen C, Roerig P, Wrobel G, Wessendorf S, Fritz B, Bentz M, Sinn HP, Radlwimmer B. Candidate genes in breast cancer revealed by microarray-based comparative genomic hybridization of archived tissue. Cancer Res. 2005;65:439-447. [PubMed] |
| 50. | Tsiambas E, Georgiannos SN, Salemis N, Alexopoulou D, Lambropoulou S, Dimo B, Ioannidis I, Kravvaritis C, Karameris A, Patsouris E. Significance of estrogen receptor 1 (ESR-1) gene imbalances in colon and hepatocellular carcinomas based on tissue microarrays analysis. Med Oncol. 2011;28:934-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Jeselsohn R, Yelensky R, Buchwalter G, Frampton G, Meric-Bernstam F, Gonzalez-Angulo AM, Ferrer-Lozano J, Perez-Fidalgo JA, Cristofanilli M, Gómez H. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res. 2014;20:1757-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 519] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 52. | TCGA Research Network. TCGA Copy Number Portal. Lyon: IARC Press 2015; Available from: http://www.broadinstitute.org/tcga/home. |
| 53. | Holst F. ESR1-Amplifikationen in humanen gynäkologischen Tumoren. Department of Biology. Hamburg: University of Hamburg, 2012. . |
| 54. | Liu W, Laitinen S, Khan S, Vihinen M, Kowalski J, Yu G, Chen L, Ewing CM, Eisenberger MA, Carducci MA. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med. 2009;15:559-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 544] [Cited by in RCA: 505] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 55. | Navin N, Krasnitz A, Rodgers L, Cook K, Meth J, Kendall J, Riggs M, Eberling Y, Troge J, Grubor V. Inferring tumor progression from genomic heterogeneity. Genome Res. 2010;20:68-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 381] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 56. | Navin N, Kendall J, Troge J, Andrews P, Rodgers L, McIndoo J, Cook K, Stepansky A, Levy D, Esposito D. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472:90-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1880] [Cited by in RCA: 1930] [Article Influence: 137.9] [Reference Citation Analysis (0)] |
| 57. | Yap TA, Gerlinger M, Futreal PA, Pusztai L, Swanton C. Intratumor heterogeneity: seeing the wood for the trees. Sci Transl Med. 2012;4:127ps10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 395] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 58. | Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12:323-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1383] [Cited by in RCA: 1433] [Article Influence: 110.2] [Reference Citation Analysis (0)] |
| 59. | Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6102] [Cited by in RCA: 5953] [Article Influence: 457.9] [Reference Citation Analysis (0)] |
| 60. | Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1549] [Cited by in RCA: 1801] [Article Influence: 150.1] [Reference Citation Analysis (0)] |
| 61. | Francis JM, Zhang CZ, Maire CL, Jung J, Manzo VE, Adalsteinsson VA, Homer H, Haidar S, Blumenstiel B, Pedamallu CS. EGFR variant heterogeneity in glioblastoma resolved through single-nucleus sequencing. Cancer Discov. 2014;4:956-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 230] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 62. | Black JC, Atabakhsh E, Kim J, Biette KM, Van Rechem C, Ladd B, Burrowes PD, Donado C, Mattoo H, Kleinstiver BP. Hypoxia drives transient site-specific copy gain and drug-resistant gene expression. Genes Dev. 2015;29:1018-1031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 63. | Nawaz S, Yuan Y. Computational pathology: Exploring the spatial dimension of tumor ecology. Cancer Lett. 2015;pii:S0304-3835(15)00694-1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Alizadeh AA, Aranda V, Bardelli A, Blanpain C, Bock C, Borowski C, Caldas C, Califano A, Doherty M, Elsner M. Toward understanding and exploiting tumor heterogeneity. Nat Med. 2015;21:846-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 544] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 65. | Tan DS, Lambros MB, Marchiò C, Reis-Filho JS. ESR1 amplification in endometrial carcinomas: hope or hyperbole? J Pathol. 2008;216:271-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 66. | Janiszewska M, Liu L, Almendro V, Kuang Y, Paweletz C, Sakr RA, Weigelt B, Hanker AB, Chandarlapaty S, King TA. In situ single-cell analysis identifies heterogeneity for PIK3CA mutation and HER2 amplification in HER2-positive breast cancer. Nat Genet. 2015;47:1212-1219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 67. | Lin CH, Liu JM, Lu YS, Lan C, Lee WC, Kuo KT, Wang CC, Chang DY, Huang CS, Cheng AL. Clinical significance of ESR1 gene copy number changes in breast cancer as measured by fluorescence in situ hybridisation. J Clin Pathol. 2013;66:140-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Furrer D, Jacob S, Caron C, Sanschagrin F, Provencher L, Diorio C. Validation of a new classifier for the automated analysis of the human epidermal growth factor receptor 2 (HER2) gene amplification in breast cancer specimens. Diagn Pathol. 2013;8:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 69. | McBride DJ, Etemadmoghadam D, Cooke SL, Alsop K, George J, Butler A, Cho J, Galappaththige D, Greenman C, Howarth KD, Lau KW, Ng CK, Raine K, Teague J, Wedge DC, Cancer Study Group AO, Caubit X, Stratton MR, Brenton JD, Campbell PJ, Futreal PA, Bowtell DD. Tandem duplication of chromosomal segments is common in ovarian and breast cancer genomes. J Pathol. 2012;227:446-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 70. | Dako Denmark A/S™. HER2 FISH pharmDx™ Interpretation Guide - Breast Cancer, 2010. . |
| 71. | Hopman AH, Ramaekers FC, Raap AK, Beck JL, Devilee P, van der Ploeg M, Vooijs GP. In situ hybridization as a tool to study numerical chromosome aberrations in solid bladder tumors. Histochemistry. 1988;89:307-316. [PubMed] |
| 72. | Hopman AH, Poddighe PJ, Smeets AW, Moesker O, Beck JL, Vooijs GP, Ramaekers FC. Detection of numerical chromosome aberrations in bladder cancer by in situ hybridization. Am J Pathol. 1989;135:1105-1117. [PubMed] |
| 73. | Martin V, Mazzucchelli L, Frattini M. An overview of the epidermal growth factor receptor fluorescence in situ hybridisation challenge in tumour pathology. J Clin Pathol. 2009;62:314-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 74. | Lupski JR, de Oca-Luna RM, Slaugenhaupt S, Pentao L, Guzzetta V, Trask BJ, Saucedo-Cardenas O, Barker DF, Killian JM, Garcia CA. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell. 1991;66:219-232. [PubMed] |
| 75. | Fortna A, Kim Y, MacLaren E, Marshall K, Hahn G, Meltesen L, Brenton M, Hink R, Burgers S, Hernandez-Boussard T. Lineage-specific gene duplication and loss in human and great ape evolution. PLoS Biol. 2004;2:E207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 221] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 76. | Braude I, Vukovic B, Prasad M, Marrano P, Turley S, Barber D, Zielenska M, Squire JA. Large scale copy number variation (CNV) at 14q12 is associated with the presence of genomic abnormalities in neoplasia. BMC Genomics. 2006;7:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 77. | Moles KJ, Gowans GC, Gedela S, Beversdorf D, Yu A, Seaver LH, Schultz RA, Rosenfeld JA, Torchia BS, Shaffer LG. NF1 microduplications: identification of seven nonrelated individuals provides further characterization of the phenotype. Genet Med. 2012;14:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 78. | Koike A, Nishida N, Yamashita D, Tokunaga K. Comparative analysis of copy number variation detection methods and database construction. BMC Genet. 2011;12:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 79. | Teo SM, Pawitan Y, Ku CS, Chia KS, Salim A. Statistical challenges associated with detecting copy number variations with next-generation sequencing. Bioinformatics. 2012;28:2711-2718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 80. | Treangen TJ, Salzberg SL. Repetitive DNA and next-generation sequencing: computational challenges and solutions. Nat Rev Genet. 2012;13:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1085] [Cited by in RCA: 1129] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 81. | Ruiz C. Identification and validation of amplification target genes in breast cancer. Philosophisch-Naturwissenschaftliche Fakultät. Basel: Universität Basel; 2006. |
| 82. | TCGA Research Network. The Cancer Genome Atlas. 2015. Available from: http://cancergenome.nih.gov/. |
| 83. | Zack TI, Schumacher SE, Carter SL, Cherniack AD, Saksena G, Tabak B, Lawrence MS, Zhsng CZ, Wala J, Mermel CH. Pan-cancer patterns of somatic copy number alteration. Nat Genet. 2013;45:1134-1140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1483] [Cited by in RCA: 1407] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 84. | Lebeau A, Grob T, Holst F, Seyedi-Fazlollahi N, Moch H, Terracciano L, Turzynski A, Choschzick M, Sauter G, Simon R. Oestrogen receptor gene (ESR1) amplification is frequent in endometrial carcinoma and its precursor lesions. J Pathol. 2008;216:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 85. | Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899-905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3271] [Cited by in RCA: 3053] [Article Influence: 203.5] [Reference Citation Analysis (0)] |
| 86. | Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, Getz G. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 2011;12:R41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1825] [Cited by in RCA: 2502] [Article Influence: 178.7] [Reference Citation Analysis (0)] |
| 87. | Beroukhim R, Getz G, Nghiemphu L, Barretina J, Hsueh T, Linhart D, Vivanco I, Lee JC, Huang JH, Alexander S. Assessing the significance of chromosomal aberrations in cancer: methodology and application to glioma. Proc Natl Acad Sci USA. 2007;104:20007-20012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 869] [Cited by in RCA: 828] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 88. | Veeraraghavan J, Tan Y, Cao XX, Kim JA, Wang X, Chamness GC, Maiti SN, Cooper LJ, Edwards DP, Contreras A. Recurrent ESR1-CCDC170 rearrangements in an aggressive subset of oestrogen receptor-positive breast cancers. Nat Commun. 2014;5:4577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 89. | Li S, Shen D, Shao J, Crowder R, Liu W, Prat A, He X, Liu S, Hoog J, Lu C. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Rep. 2013;4:1116-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 506] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 90. | Thomas C, Gustafsson JÅ. Estrogen receptor mutations and functional consequences for breast cancer. Trends Endocrinol Metab. 2015;26:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 91. | Carter SL, Cibulskis K, Helman E, McKenna A, Shen H, Zack T, Laird PW, Onofrio RC, Winckler W, Weir BA. Absolute quantification of somatic DNA alterations in human cancer. Nat Biotechnol. 2012;30:413-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1637] [Cited by in RCA: 1527] [Article Influence: 117.5] [Reference Citation Analysis (0)] |
| 92. | Claycomb JM, Orr-Weaver TL. Developmental gene amplification: insights into DNA replication and gene expression. Trends Genet. 2005;21:149-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 93. | Li Q, Seo JH, Stranger B, McKenna A, Pe’er I, Laframboise T, Brown M, Tyekucheva S, Freedman ML. Integrative eQTL-based analyses reveal the biology of breast cancer risk loci. Cell. 2013;152:633-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 258] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 94. | Schuur ER, Weigel RJ. Monoallelic amplification of estrogen receptor-alpha expression in breast cancer. Cancer Res. 2000;60:2598-2601. [PubMed] |
| 95. | Dunbier AK, Anderson H, Ghazoui Z, Lopez-Knowles E, Pancholi S, Ribas R, Drury S, Sidhu K, Leary A, Martin LA. ESR1 is co-expressed with closely adjacent uncharacterised genes spanning a breast cancer susceptibility locus at 6q25.1. PLoS Genet. 2011;7:e1001382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 96. | Laenkholm AV, Knoop A, Ejlertsen B, Rudbeck T, Jensen MB, Müller S, Lykkesfeldt AE, Rasmussen BB, Nielsen KV. ESR1 gene status correlates with estrogen receptor protein levels measured by ligand binding assay and immunohistochemistry. Mol Oncol. 2012;6:428-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 97. | Pentheroudakis G, Kotoula V, Eleftheraki AG, Tsolaki E, Wirtz RM, Kalogeras KT, Batistatou A, Bobos M, Dimopoulos MA, Timotheadou E. Prognostic significance of ESR1 gene amplification, mRNA/protein expression and functional profiles in high-risk early breast cancer: a translational study of the Hellenic Cooperative Oncology Group (HeCOG). PLoS One. 2013;8:e70634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 98. | Thomas C, Gustafsson JA. Not enough evidence to include ESR1 amplification. Nat Rev Cancer. 2011;11:823. [DOI] [Full Text] |
| 99. | Markiewicz A, Wełnicka-Jaśkiewicz M, Skokowski J, Jaśkiewicz J, Szade J, Jassem J, Zaczek AJ. Prognostic significance of ESR1 amplification and ESR1 PvuII, CYP2C19*2, UGT2B15*2 polymorphisms in breast cancer patients. PLoS One. 2013;8:e72219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 100. | Cancer Genome Atlas Research N, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2944] [Cited by in RCA: 4064] [Article Influence: 338.7] [Reference Citation Analysis (0)] |
| 101. | Aguilar H, Solé X, Bonifaci N, Serra-Musach J, Islam A, López-Bigas N, Méndez-Pertuz M, Beijersbergen RL, Lázaro C, Urruticoechea A. Biological reprogramming in acquired resistance to endocrine therapy of breast cancer. Oncogene. 2010;29:6071-6083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 102. | Quenel-Tueux N, Debled M, Rudewicz J, MacGrogan G, Pulido M, Mauriac L, Dalenc F, Bachelot T, Lortal B, Breton-Callu C. Clinical and genomic analysis of a randomised phase II study evaluating anastrozole and fulvestrant in postmenopausal patients treated for large operable or locally advanced hormone-receptor-positive breast cancer. Br J Cancer. 2015;113:585-594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 103. | Tomita S, Abdalla MO, Fujiwara S, Matsumori H, Maehara K, Ohkawa Y, Iwase H, Saitoh N, Nakao M. A cluster of noncoding RNAs activates the ESR1 locus during breast cancer adaptation. Nat Commun. 2015;6:6966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 104. | Albanell J, Baselga J. Trastuzumab, a humanized anti-HER2 monoclonal antibody, for the treatment of breast cancer. Drugs Today (Barc). 1999;35:931-946. [PubMed] |
| 105. | Pegram M, Slamon D. Biological rationale for HER2/neu (c-erbB2) as a target for monoclonal antibody therapy. Semin Oncol. 2000;27:13-19. [PubMed] |
| 106. | Stark GR, Wahl GM. Gene amplification. Annu Rev Biochem. 1984;53:447-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 527] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 107. | Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinänen R, Palmberg C, Palotie A, Tammela T, Isola J, Kallioniemi OP. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1061] [Cited by in RCA: 1033] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 108. | Koivisto P, Visakorpi T, Kallioniemi OP. Androgen receptor gene amplification: a novel molecular mechanism for endocrine therapy resistance in human prostate cancer. Scand J Clin Lab Invest Suppl. 1996;226:57-63. [PubMed] |
| 109. | Smith KA, Chernova OB, Groves RP, Stark MB, Martínez JL, Davidson JN, Trent JM, Patterson TE, Agarwal A, Duncan P. Multiple mechanisms of N-phosphonacetyl-L-aspartate resistance in human cell lines: carbamyl-P synthetase/aspartate transcarbamylase/dihydro-orotase gene amplification is frequent only when chromosome 2 is rearranged. Proc Natl Acad Sci USA. 1997;94:1816-1821. [PubMed] |
| 110. | Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, Trapman J, Cleutjens K, Noordzij A, Visakorpi T. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314-319. [PubMed] |
| 111. | Koivisto P, Kolmer M, Visakorpi T, Kallioniemi OP. Androgen receptor gene and hormonal therapy failure of prostate cancer. Am J Pathol. 1998;152:1-9. [PubMed] |
| 112. | Bubendorf L, Kolmer M, Kononen J, Koivisto P, Mousses S, Chen Y, Mahlamäki E, Schraml P, Moch H, Willi N. Hormone therapy failure in human prostate cancer: analysis by complementary DNA and tissue microarrays. J Natl Cancer Inst. 1999;91:1758-1764. [PubMed] |
| 113. | Bates M, Sperinde J, Köstler WJ, Ali SM, Leitzel K, Fuchs EM, Paquet A, Lie Y, Sherwood T, Horvat R. Identification of a subpopulation of metastatic breast cancer patients with very high HER2 expression levels and possible resistance to trastuzumab. Ann Oncol. 2011;22:2014-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 114. | Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, Haney J, Witta S, Danenberg K, Domenichini I. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;97:643-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1267] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 115. | Cappuzzo F, Toschi L, Domenichini I, Bartolini S, Ceresoli GL, Rossi E, Ludovini V, Cancellieri A, Magrini E, Bemis L. HER3 genomic gain and sensitivity to gefitinib in advanced non-small-cell lung cancer patients. Br J Cancer. 2005;93:1334-1340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 116. | Cappuzzo F, Varella-Garcia M, Shigematsu H, Domenichini I, Bartolini S, Ceresoli GL, Rossi E, Ludovini V, Gregorc V, Toschi L. Increased HER2 gene copy number is associated with response to gefitinib therapy in epidermal growth factor receptor-positive non-small-cell lung cancer patients. J Clin Oncol. 2005;23:5007-5018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 283] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 117. | Ribeiro FR, Henrique R, Martins AT, Jerónimo C, Teixeira MR. Relative copy number gain of MYC in diagnostic needle biopsies is an independent prognostic factor for prostate cancer patients. Eur Urol. 2007;52:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 118. | Jensen KC, Turbin DA, Leung S, Miller MA, Johnson K, Norris B, Hastie T, McKinney S, Nielsen TO, Huntsman DG. New cutpoints to identify increased HER2 copy number: analysis of a large, population-based cohort with long-term follow-up. Breast Cancer Res Treat. 2008;112:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 119. | Dahabreh IJ, Linardou H, Siannis F, Kosmidis P, Bafaloukos D, Murray S. Somatic EGFR mutation and gene copy gain as predictive biomarkers for response to tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2010;16:291-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 120. | Ålgars A, Lintunen M, Carpén O, Ristamäki R, Sundström J. EGFR gene copy number assessment from areas with highest EGFR expression predicts response to anti-EGFR therapy in colorectal cancer. Br J Cancer. 2011;105:255-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 121. | Dahabreh IJ, Linardou H, Kosmidis P, Bafaloukos D, Murray S. EGFR gene copy number as a predictive biomarker for patients receiving tyrosine kinase inhibitor treatment: a systematic review and meta-analysis in non-small-cell lung cancer. Ann Oncol. 2011;22:545-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 122. | Fidler MJ, Morrison LE, Basu S, Buckingham L, Walters K, Batus M, Jacobson KK, Jewell SS, Coon J, Bonomi PD. PTEN and PIK3CA gene copy numbers and poor outcomes in non-small cell lung cancer patients with gefitinib therapy. Br J Cancer. 2011;105:1920-1926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 123. | Woelber L, Hess S, Bohlken H, Tennstedt P, Eulenburg C, Simon R, Gieseking F, Jaenicke F, Mahner S, Choschzick M. EGFR gene copy number increase in vulvar carcinomas is linked with poor clinical outcome. J Clin Pathol. 2012;65:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 124. | Burkhardt L, Grob TJ, Hermann I, Burandt E, Choschzick M, Jänicke F, Müller V, Bokemeyer C, Simon R, Sauter G. Gene amplification in ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2010;123:757-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 125. | Moelans CB, de Weger RA, Monsuur HN, Maes AH, van Diest PJ. Molecular differences between ductal carcinoma in situ and adjacent invasive breast carcinoma: a multiplex ligation-dependent probe amplification study. Anal Cell Pathol (Amst). 2010;33:165-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 126. | Kalisky T, Blainey P, Quake SR. Genomic analysis at the single-cell level. Annu Rev Genet. 2011;45:431-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 127. | Shapiro E, Biezuner T, Linnarsson S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat Rev Genet. 2013;14:618-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 803] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 128. | Ning L, Liu G, Li G, Hou Y, Tong Y, He J. Current Challenges in Bioinformatics of Single Cell Genomics. Front Oncol. 2014;4:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 129. | Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov. 2011;10:712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1131] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 130. | Arrowsmith J. Trial watch: Phase II failures: 2008-2010. Nat Rev Drug Discov. 2011;10:328-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 422] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 131. | Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:e124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6269] [Cited by in RCA: 4986] [Article Influence: 249.3] [Reference Citation Analysis (0)] |
| 132. | Horton R. Offline: What is medicine’s 5 sigma? The Lancet. 2015;385:1380. |
| 133. | Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature. 2012;483:531-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1889] [Cited by in RCA: 1719] [Article Influence: 132.2] [Reference Citation Analysis (0)] |