Published online Dec 10, 2015. doi: 10.5306/wjco.v6.i6.189
Peer-review started: May 31, 2015
First decision: August 7, 2015
Revised: August 12, 2015
Accepted: August 30, 2015
Article in press: August 31, 2015
Published online: December 10, 2015
Processing time: 195 Days and 3.1 Hours
Radiotherapy is an established curative treatment method for prostate cancer. Optimal tumor control rates can only be achieved with high local doses, associated with a considerable risk of rectal toxicity. Apart from already widely adapted technical advances, as intensity-modulated radiation therapy, the application of spacers placed between the prostate and rectum has been increasingly used in the last years. Biodegradable spacers, including hydrogel, hyaluronic acid, collagen or an implantable balloon, can be injected or inserted in a short procedure under transrectal ultrasound guidance via a transperineal approach. A distance of about 1.0-1.5 cm is usually achieved between the rectum and prostate, excluding the rectal wall from the high isodoses. Several studies have shown well tolerated injection procedures and treatments. Apart from considerable reduction of rectal irradiation, a prospective randomized trial demonstrated a reduction of rectal toxicity after hydrogel injection in men undergoing prostate image-guided intensity-modulated radiation therapy. The results are encouraging for continuing evaluation in dose escalation, hypofractionation, stereotactic radiotherapy or re-irradiation trials in the future.
Core tip: Radiotherapy is widely used for the treatment of prostate cancer. Technical advances allow improved tumor control with increasing prescription doses, but rectal wall is known to be a dose-limiting organ. A new method that has been increasingly used in the last years is the application of a biodegradable spacer to increase the distance between the prostate and rectal wall. Clinical studies, including a prospective randomized trial, have reported considerable dosimetric advantages for the rectum, well tolerated insertion procedures and radiotherapy treatments.
- Citation: Pinkawa M. Current role of spacers for prostate cancer radiotherapy. World J Clin Oncol 2015; 6(6): 189-193
- URL: https://www.wjgnet.com/2218-4333/full/v6/i6/189.htm
- DOI: https://dx.doi.org/10.5306/wjco.v6.i6.189
Radiotherapy is an established curative treatment method for prostate cancer. Prospective randomized trials evaluating dose escalation have consistently shown significantly higher biochemical control rates for higher doses. However, significantly higher rectal toxicity rates resulted[1]. Rectal toxicity is regarded as the dose-limiting toxicity[2]. Rectal toxicity has been evaluated in a large number of studies and dose-volume correlations have been clearly established[3-5]. Apart from higher dose and larger volumes within specific isodoses, risk factors for toxicity after radiotherapy include history of prior abdominal surgery, advanced age, diabetes mellitus, concomitant use of androgen deprivation, hemorrhoids, and inflammatory bowel disease[6].
Most of the randomized dose escalation studies applied three-dimensional conformal techniques[1]. Several further technical advances have been introduced in the last years. Intensity-modulated radiotherapy (IMRT) techniques, currently regarded as a standard for prostate cancer treatment in an increasing number radiation oncology departments, result in improved dose conformality[7]. Image-guided radiotherapy (IGRT) is applied to show the prostate position before or even during each fraction, so that treatment margins and volumes can be reduced[8]. Using these techniques, new concepts as hypofractionated treatments or even stereotactic radiotherapy treatments have been introduced in the past, resulting in a considerable shortening of the external beam radiotherapy (EBRT) treatment period[9].
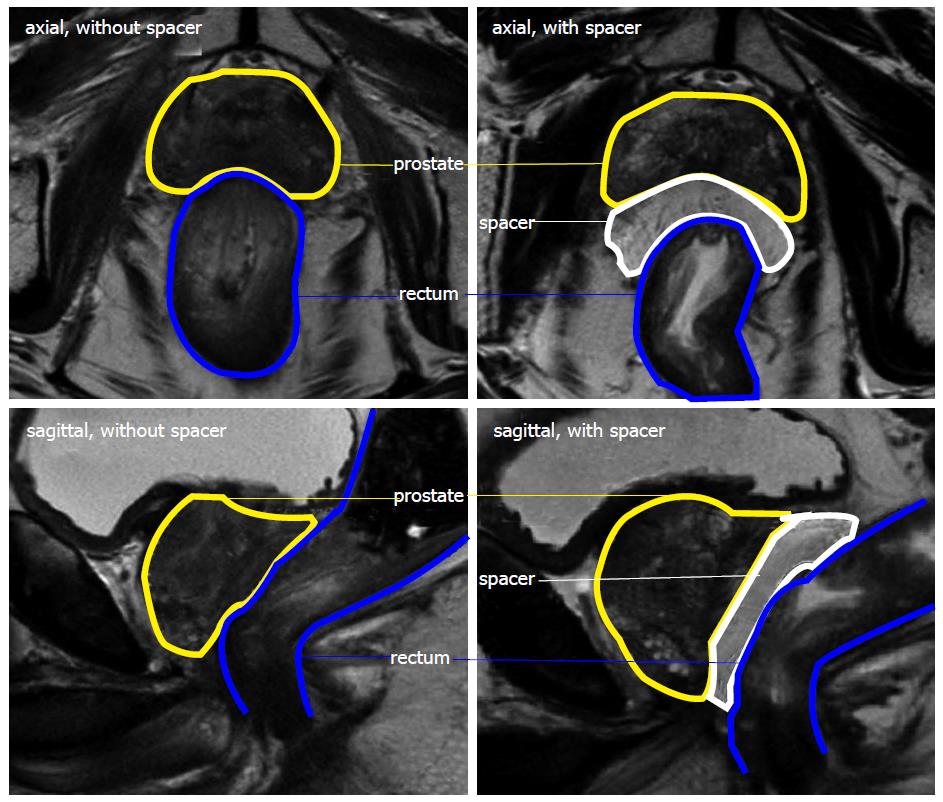
As the prostate is usually situated without a relevant distance to the rectal wall and EBRT requires safety margins around the prostate of about 4-10 mm (depending on several factors as patient positioning and IGRT method), the anterior rectal wall is always included in the planning target volume and thus the prescription isodose. The insertion of a spacer between the prostate and rectum is an increasingly used method to create a considerable distance between the prostate and rectum and thus exclude the rectum from the high dose volume. A high dose can be delivered safely with adequate margins[10].
Requirements for a spacer are a well tolerated insertion, a stable position during up to two months of radiotherapy and biodegradation. A spacer should not be allergenic or toxic. Studies in prostate cancer patients evaluated the effects of hyaluronic acid, human collagen, inflatable balloon or hydrogel as different spacer materials[11-14]. Hyaluronic acid is a natural polysaccharide component in connective tissue and extracellular matrix[15]. Human collagen is known from injections into the perineum to treat urinary incontinence[16]. An inflatable biodegradable balloon (PLCL, polylactide-co-ε-caprolactone) has been specifically introduced to be used as a spacer[17]. In the past absorbable polyethylene glycol (PEG) hydrogels have been applied in surgical procedures as lung, dural and vascular sealants[14]. Hydrogels are injected as liquids and polymerize in situ within < 10 s following the mixture of two precursor solutions.
A transperineal approach with transrectal ultrasound (TRUS) guidance is used for spacer implantation/ injection under local, spinal or light general anaesthesia. The actually selected anaesthesia will be chosen depending on the procedures planned (length and depth needed for incision, gold marker implantation, brachytherapy) and the respective local protocol. The approach is well known from prostate brachytherapy or gold marker implantation for IGRT. A needle is placed about 1-2cm anteriorly to the TRUS probe and forwarded to the prostatic apex. Prior hydrodissection facilitates spacer insertion. The spacer must be positioned between the rectal wall and the Denonvilliers’ fascia[18]. In a series including 243 prostatectomy specimens, Villers et al[19] reported that prostate cancer invaded Denonvilliers’ fascia in 19% of cases, but no patients presented a tumour progression through the full thickness of the fascia. Thus, the risk of tumour cell displacement can be regarded to be minimal.
Prada et al[13] performed hyaluronic acid injections without hydrodissection. Hydrogel or human collagen are injected following prior hydrodissection - the same-18 gauge spinal needle is used for hydrodissection and spacer injection[12,18]. Injection of fluid spacers is less invasive in comparison to the balloon implantation. However, a balloon can be deflated and repositioned if required.
An incision of 3-5 mm is required before implantation of a biodegradable balloon. The incision allows the dilatator and the introducer sheath to be inserted into the perineum. The dilatator is advanced towards the prostate base over the needle and the needle removed subsequently. The introducer sheath acts as a working channel for the introduction of the balloon. The balloon is filled with warm saline and sealed with a biodegradable plug following a full inflation[11,20].
As demonstrated in several studies, the injection or insertion of a spacer results in a distance of about 1.0-1.5 cm between the prostate and rectum, so that the rectal wall and planning target volume do not overlap[11-13,21]. The largest study included 100 patients after hydrogel injection[22]. A higher injected volume can result in a larger separation. Hydrogel is usually inserted in standardized 10-15 mL systems[14,22]. Comparably to the hydrogel studies, a mean separation of 12.7 mm was achieved with 20 mL human collagen injections in a pilot study[12]. The inflation of a balloon with nearly 20 mL of saline can result in mean prostate-rectum separation > 1.5 cm[11]. Though different injection volumes have not been compared in studies, an increasing volume can be potentially associated with toxicity related to the pressure on the rectal wall or even the prostate. A volume of 10-15 mL and a resulting distance of 1.0-1.5 cm appear to be very effective and well tolerable for the patients[11-14,22].
This separation results in a considerable dosimetric advantage for the rectum. In EBRT studies, relative rectal wall volume reductions of > 70% within the 90% isodose levels have been shown comparing treatment plans prior and following spacer insertion, i.e., only < 5% of rectal volume is included in the 70Gy isodose when a prescription dose of 78-79Gy is used[11,15,21]. Guidelines recommend to limit this volume to 20%[23], so that these recommendations can be met without problems. Thus, to reach an optimal dose distribution, treatment planning after spacer insertion must include much lower objectives for the rectum. The information from the dose-volume histogram indicates a low risk of rectal toxicity. On the other hand, it implicates the potential for safe delivery of new hypofractionated and stereotactic treatments or re-irradiation concepts without a relevant risk of higher grade rectal toxicity.
Spacer studies have been reported after several different treatment concepts, as low-dose rate[24] and high-dose rate (HDR) brachytherapy[22,25] with or without additional EBRT, hypofractionated EBRT concepts[26] or proton and heavy ion concepts[27,28]. Rare spacer-related complications have been reported in the literature, as focal rectal necrosis or ulceration as a result of unintentional injection of hydrogel into the rectal wall or urinary retention, usually resolving within a short time[14].
Vanneste et al[29] calculated the cost-effectiveness of treating prostate cancer patients with and without a spacer, using a decision-analytic Markov model. According to the Dutch health costs, the spacer was found to be cost-effective for prostate cancer patients due to less severe toxicity and a reduction in treatment costs associated with side effects.
Taking into account a lack of long-term clinical experience with spacers, radiobiological models can be used to estimate long-term toxicity. They correlate prior data from the treatment plan and long-term toxicity[30]. Mean normal tissue complication probability (NTCP) for severe proctitis, necrosis, fistula or ≥ grade 2 rectal bleeding was found to be reduced by ≥ 50% comparing data before vs after hydrogel spacer injection. A clear advantage was shown for conventional and IMRT techniques[31].
The vast majority of published clinical studies have used hyaluronic acid or hydrogel. Studies with hyaluronic acid included smaller patient groups. Prada et al[25] did not observe grade 2 or higher toxicity after HDR brachytherapy as monotherapy (single 19Gy fraction) in an analysis of 40 patients and a median follow-up of 18 mo. Chapet et al[26] reported the acute toxicity of a hypofractionated IMRT with 3.1Gy fractions up to 62Gy total dose in 36 patients, without any grade 2 or higher toxicities.
PEG hydrogel stability during treatment has been shown in studies, so that a constant prostate-rectum separation can be expected[32]. Hydrogel starts to liquify about 3 mo after injection, is absorbed within about 6 mo and cleared via renal filtration. Prostate position variability is similar with or without hydrogel, so that IGRT is still required with a spacer to keep safety margins small. However, in contrast to patients without a spacer, larger posterior displacements were not found with a spacer[32].
A learning curve has been reported in a study including 64 patients, showing an increasingly symmetrical hydrogel distribution and significantly larger prostate-rectum distances with the same hydrogel volume. As a consequence, an improved dosimetric rectum protection and smaller acute bowel quality of life changes resulted[10].
Gastrointestinal toxicity (GI) was analyzed in a study including 48 patients in a multi-institutional prospective study. Grade 2 acute GI toxicity was reported in only 12% of patients (no grade 3-4 toxicity). Grade 1 late GI toxicity was found in 7% of patients within 12 mo after treatment (corresponding to two patients, one of them with grade 1 at baseline; no patients with grade 2-4 toxicity)[14].
In a prospective randomized multicenter study 222 patients were randomized between a treatment with and without hydrogel (149 patients with and 73 without spacer). Patients were treated after fiducial marker placement (IGRT) with 1.8Gy fractions up to a total dose of 79.2Gy, using an IMRT technique. There were no device-related adverse events, rectal perforations, serious bleedings or infections within either groups[21]. Mean rectal volume within the 70Gy isodose was reduced from 12% to 3%. As also reported in a prior case control study[33], similar acute rectal toxicity was observed in both patient groups. However, a significant reduction in late (3-15 mo) rectal toxicity in the spacer group was observed (2% vs 7%). There was no late rectal toxicity greater than grade 1 in the spacer group. At 15 mo, 12% and 21% of spacer and control patients experienced 10-point declines in bowel quality of life (EPIC questionnaire, Expanded Prostate Cancer Index Composite)[21].
The number of published studies reporting clinical data with spacer materials for prostate cancer radiotherapy is increasing. Hydrogel, hyaluronic acid, collagen or an implantable balloon, can be injected or inserted under transrectal ultrasound guidance. Most studies, including several studies with more than 50 patients treated with a spacer and a recently published prospective randomized study, evaluate the effects of a hydrogel spacer (Figure 1). A distance of about 1.0-1.5 cm is usually achieved between the prostate and rectum, excluding the rectal wall from high isodoses. Procedure or spacer related complications are rare and treatments well tolerated. Reduced late toxicity rates have been shown in a prospective randomized study. Long-term results with a follow-up > 2 years are not available yet. Presently available results are encouraging for the design of further clinical studies.
P- Reviewer: Brajuskovic GN, Ilie CP, Johnny K
S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Viani GA, Stefano EJ, Afonso SL. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys. 2009;74:1405-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 342] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 2. | Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60:1013-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Akimoto T, Muramatsu H, Takahashi M, Saito J, Kitamoto Y, Harashima K, Miyazawa Y, Yamada M, Ito K, Kurokawa K. Rectal bleeding after hypofractionated radiotherapy for prostate cancer: correlation between clinical and dosimetric parameters and the incidence of grade 2 or worse rectal bleeding. Int J Radiat Oncol Biol Phys. 2004;60:1033-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Fiorino C, Sanguineti G, Cozzarini C, Fellin G, Foppiano F, Menegotti L, Piazzolla A, Vavassori V, Valdagni R. Rectal dose-volume constraints in high-dose radiotherapy of localized prostate cancer. Int J Radiat Oncol Biol Phys. 2003;57:953-962. [PubMed] |
| 5. | Huang EH, Pollack A, Levy L, Starkschall G, Dong L, Rosen I, Kuban DA. Late rectal toxicity: dose-volume effects of conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2002;54:1314-1321. [PubMed] |
| 6. | Budäus L, Bolla M, Bossi A, Cozzarini C, Crook J, Widmark A, Wiegel T. Functional outcomes and complications following radiation therapy for prostate cancer: a critical analysis of the literature. Eur Urol. 2012;61:112-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 7. | Pinkawa M, Piroth MD, Holy R, Djukic V, Klotz J, Krenkel B, Eble MJ. Combination of dose escalation with technological advances (intensity-modulated and image-guided radiotherapy) is not associated with increased morbidity for patients with prostate cancer. Strahlenther Onkol. 2011;187:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Pinkawa M, Pursch-Lee M, Asadpour B, Gagel B, Piroth MD, Klotz J, Nussen S, Eble MJ. Image-guided radiotherapy for prostate cancer. Implementation of ultrasound-based prostate localization for the analysis of inter- and intrafraction organ motion. Strahlenther Onkol. 2008;184:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Pinkawa M, Schoth F, Böhmer D, Hatiboglu G, Sharabi A, Song D, Eble MJ. Current standards and future directions for prostate cancer radiation therapy. Expert Rev Anticancer Ther. 2013;13:75-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Pinkawa M, Klotz J, Djukic V, Schubert C, Escobar-Corral N, Caffaro M, Piroth MD, Holy R, Eble MJ. Learning curve in the application of a hydrogel spacer to protect the rectal wall during radiotherapy of localized prostate cancer. Urology. 2013;82:963-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Melchert C, Gez E, Bohlen G, Scarzello G, Koziol I, Anscher M, Cytron S, Paz A, Torre T, Bassignani M. Interstitial biodegradable balloon for reduced rectal dose during prostate radiotherapy: results of a virtual planning investigation based on the pre- and post-implant imaging data of an international multicenter study. Radiother Oncol. 2013;106:210-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Noyes WR, Hosford CC, Schultz SE. Human collagen injections to reduce rectal dose during radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:1918-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Prada PJ, Fernández J, Martinez AA, de la Rúa A, Gonzalez JM, Fernandez JM, Juan G. Transperineal injection of hyaluronic acid in anterior perirectal fat to decrease rectal toxicity from radiation delivered with intensity modulated brachytherapy or EBRT for prostate cancer patients. Int J Radiat Oncol Biol Phys. 2007;69:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Uhl M, Herfarth K, Eble MJ, Pinkawa M, van Triest B, Kalisvaart R, Weber DC, Miralbell R, Song DY, DeWeese TL. Absorbable hydrogel spacer use in men undergoing prostate cancer radiotherapy: 12 month toxicity and proctoscopy results of a prospective multicenter phase II trial. Radiat Oncol. 2014;9:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Wilder RB, Barme GA, Gilbert RF, Holevas RE, Kobashi LI, Reed RR, Solomon RS, Walter NL, Chittenden L, Mesa AV. Cross-linked hyaluronan gel reduces the acute rectal toxicity of radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;77:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Faerber GJ, Richardson TD. Long-term results of transurethral collagen injection in men with intrinsic sphincter deficiency. J Endourol. 1997;11:273-277. [PubMed] |
| 17. | Levy Y, Paz A, Yosef RB, Corn BW, Vaisman B, Shuhat S, Domb AJ. Biodegradable inflatable balloon for reducing radiation adverse effects in prostate cancer. J Biomed Mater Res B Appl Biomater. 2009;91:855-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Hatiboglu G, Pinkawa M, Vallée JP, Hadaschik B, Hohenfellner M. Application technique: placement of a prostate-rectum spacer in men undergoing prostate radiation therapy. BJU Int. 2012;110:E647-E652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Villers A, McNeal JE, Freiha FS, Boccon-Gibod L, Stamey TA. Invasion of Denonvilliers’ fascia in radical prostatectomy specimens. J Urol. 1993;149:793-798. [PubMed] |
| 20. | Kouloulias V, Kalogeropoulos T, Platoni K, Georgakopoulos J, Matsopoulos G, Chaldeopoulos D, Beli I, Pantelakos P, Asimakopoulos C, Kouvaris J. Feasibility and radiation induced toxicity regarding the first application of transperineal implementation of biocompatible balloon for high dose radiotherapy in patients with prostate carcinoma. Radiat Oncol. 2013;8:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Mariados N, Sylvester J, Shah D, Karsh L, Hudes R, Beyer D, Kurtzman S, Bogart J, Hsi A, Kos M. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: dosimetric and clinical effects of perirectal spacer application in men undergoing IG-IMRT. Int J Radiat Oncol Biol Phys. 2015;92:971-977. [RCA] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 305] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 22. | Strom TJ, Wilder RB, Fernandez DC, Mellon EA, Saini AS, Hunt DC, Pow-Sang JM, Spiess PE, Sexton WJ, Poch MA. A dosimetric study of polyethylene glycol hydrogel in 200 prostate cancer patients treated with high-dose rate brachytherapy±intensity modulated radiation therapy. Radiother Oncol. 2014;111:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Lawton CA, Michalski J, El-Naqa I, Buyyounouski MK, Lee WR, Menard C, O’Meara E, Rosenthal SA, Ritter M, Seider M. RTOG GU Radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:383-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 319] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 24. | Beydoun N, Bucci JA, Chin YS, Malouf D, Enari E, Painter SD. First report of transperineal polyethylene glycol hydrogel spacer use to curtail rectal radiation dose after permanent iodine-125 prostate brachytherapy. Brachytherapy. 2013;12:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Prada PJ, Jimenez I, González-Suárez H, Fernández J, Cuervo-Arango C, Mendez L. High-dose-rate interstitial brachytherapy as monotherapy in one fraction and transperineal hyaluronic acid injection into the perirectal fat for the treatment of favorable stage prostate cancer: treatment description and preliminary results. Brachytherapy. 2012;11:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Chapet O, Decullier E, Bin S, Faix A, Ruffion A, Jalade P, Fenoglietto P, Udrescu C, Enachescu C, Azria D. Prostate hypofractionated radiation therapy with injection of hyaluronic acid: acute toxicities in a phase 2 study. Int J Radiat Oncol Biol Phys. 2015;91:730-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Rucinski A, Brons S, Richter D, Habl G, Debus J, Bert C, Haberer T, Jäkel O. Ion therapy of prostate cancer: daily rectal dose reduction by application of spacer gel. Radiat Oncol. 2015;10:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Weber DC, Zilli T, Vallee JP, Rouzaud M, Miralbell R, Cozzi L. Intensity modulated proton and photon therapy for early prostate cancer with or without transperineal injection of a polyethylen glycol spacer: a treatment planning comparison study. Int J Radiat Oncol Biol Phys. 2012;84:e311-e318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Vanneste BG, Pijls-Johannesma M, Van De Voorde L, van Lin EN, van de Beek K, van Loon J, Ramaekers BL, Lambin P. Spacers in radiotherapy treatment of prostate cancer: is reduction of toxicity cost-effective? Radiother Oncol. 2015;114:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Luxton G, Keall PJ, King CR. A new formula for normal tissue complication probability (NTCP) as a function of equivalent uniform dose (EUD). Phys Med Biol. 2008;53:23-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Pinkawa M, Corral NE, Caffaro M, Piroth MD, Holy R, Djukic V, Otto G, Schoth F, Eble MJ. Application of a spacer gel to optimize three-dimensional conformal and intensity modulated radiotherapy for prostate cancer. Radiother Oncol. 2011;100:436-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Pinkawa M, Piroth MD, Holy R, Escobar-Corral N, Caffaro M, Djukic V, Klotz J, Eble MJ. Spacer stability and prostate position variability during radiotherapy for prostate cancer applying a hydrogel to protect the rectal wall. Radiother Oncol. 2013;106:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Pinkawa M, Piroth MD, Holy R, Escobar-Corral N, Caffaro M, Djukic V, Klotz J, Eble MJ. Quality of life after intensity-modulated radiotherapy for prostate cancer with a hydrogel spacer. Matched-pair analysis. Strahlenther Onkol. 2012;188:917-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |