Published online Oct 10, 2015. doi: 10.5306/wjco.v6.i5.166
Peer-review started: March 24, 2015
First decision: April 27, 2015
Revised: May 9, 2015
Accepted: June 1, 2015
Article in press: June 2, 2015
Published online: October 10, 2015
Processing time: 210 Days and 12.4 Hours
AIM: To investigate whether selenomethionine (SLM) reduces mucositis incidence in patients with head and neck squamous cell cancer (HNSCC) undergoing concurrent chemoradiation (CRT).
METHODS: In this multi-institutional, randomized, double-blind phase II trial, patients with Stage III or IV HNSCC received SLM 3600 μg/m2 or placebo twice daily for 7 d prior to CRT, once daily during CRT, and daily for 3 wk following CRT. CRT consisted of 70 Gy at 2 Gy per fraction with cisplatin 100 mg/m2 IV on days 1, 22, and 43.
RESULTS: Eighteen patients were randomized, 10 received SLM, and there were no differences in baseline factors. There was no difference in mucositis or patient-reported side effects between groups. There was no difference in overall or relapse-free survival at 12 mo.
CONCLUSION: Addition of SLM to CRT for HNSCC was well-tolerated but did not lower the incidence of severe mucositis or improve quality of life or survival outcomes.
Core tip: This is an international, randomized, double-blind, placebo-controlled phase II trial evaluating the addition of selenomethionine (SLM) to concurrent chemoradiation for locally advanced squamous cell carcinoma of the head and neck. The addition of SLM was well tolerated, but did not lead to a difference in the rates of mucositis, or quality of life outcomes vs placebo.
- Citation: Mix M, Singh AK, Tills M, Dibaj S, Groman A, Jaggernauth W, Rustum Y, Jameson MB. Randomized phase II trial of selenomethionine as a modulator of efficacy and toxicity of chemoradiation in squamous cell carcinoma of the head and neck. World J Clin Oncol 2015; 6(5): 166-173
- URL: https://www.wjgnet.com/2218-4333/full/v6/i5/166.htm
- DOI: https://dx.doi.org/10.5306/wjco.v6.i5.166
Head and neck squamous cell cancers (HNSCC) are occurring with increasing incidence[1]. Worldwide, approximately 350000 diagnoses are expected annually[2]. HNSCC is often related to tobacco and alcohol exposure[3], human papilloma virus exposure[4], or some combination of these factors.
Over the past 2 decades, concurrent chemoradiation therapy (CRT) without surgery has demonstrated the ability to cure many HNSCC patients and preserve important functions such as speech and swallowing. Nevertheless, even with the improvements of modern therapy, 5 year overall survival (OS) can be as low at 30%-40%[5,6]. Moreover, both the acute and late side effects with concurrent CRT (e.g., mucositis, xerostomia, etc.) can be severe. Acute effects can be sufficiently severe to necessitate a treatment “break” during therapy. Each day of treatment prolongation can reduce local control and survival by 2%-5%[7-9].
Pre-clinical literature suggested that organic selenium (Se) compounds including L-selenomethionine (SLM) might have both anti-tumor[10-15] and anti-toxicity[12,14,16-19] effects when combined with CRT, potentially widening the very narrow therapeutic window in HNSCC. This promising dual anti-tumor and anti-toxicity effect lead to human studies combining chemotherapy and Se supplementation[20-22].
This double blind, randomized, multi-institutional trial was performed to assess whether SLM supplementation can reduce the incidence of grades 3 or 4 mucositis in HNSCC patients treated with concurrent CRT over 7 wk.
Patients with stage III-IV HNSCC who were planned for definitive treatment with 7 wk of concurrent cisplatin and radiation were offered the opportunity to participate on this phase II trial. All patients had biopsy-proven locally-advanced HNSCC of oral cavity, oropharynx, hypopharynx, larynx, nasopharynx or paranasal sinuses, and had an eastern cooperative oncology group (ECOG) performance status of 0-2. Excluded were those who underwent definitive surgery (anything beyond excisional biopsy) or those with Stage IVc disease (non-regional metastatic disease), as well as those with malignancy within the previous five years. Prior radiotherapy was not permitted. HIV or hepatitis C positivity, platinum hypersensitivity, inability to tolerate oral medications (in absence of feeding tube), symptomatic peripheral neuropathy, planned use of amifostine, and significant comorbidity were all excluding factors.
This double blind, placebo-controlled, randomized, multi-institutional trial was designed to assess whether SLM supplementation can reduce the incidence of grades 3 or 4 mucositis in HNSCC patients treated with concurrent CRT over 7 wk. The trial was planned to recruit 80 patients but, due to funding constraints, recruitment was suspended after 18 patients and an interim analysis was performed to see if a sufficiently promising effect could be discerned to warrant further funding.
The primary objective of this trial was to assess whether SLM reduces the incidence of grades 3 or 4 mucositis in HNSCC patients treated with concurrent CRT over 7 wk. Secondary objectives included assessment of the effect of SLM on tumor complete response (CR) rate, progression-free survival (PFS), OS and quality of life (QOL). In addition, an assessment of whether SLM reduces incidence and severity of other treatment-related toxicity including xerostomia, renal impairment, hearing loss, and myelosuppression was performed. In New Zealand patients only, an exploratory objective was to assess the impact of SLM on plasma free cisplatin and plasma Se pharmacokinetics and on pharmacodynamics markers of biological activity of Se.
Written informed consent was obtained from all patients. Following registration and fulfillment of all eligibility criteria, patients were allocated to either the control or treatment arm in a 1:1 fashion using a permuted block randomization scheme based on blocks of size 4, stratified by site. The randomization list was generated by the study biostatistician. The trial was approved by the Roswell Park Cancer Institute Institutional Review Board and the Northern Y Regional Ethics Committee in New Zealand. The ClinicalTrials.gov identifier is NCT01682031.
Radiation therapy structures and doses were consistent with the radiation therapy oncology group 0522 trial that was current at the time of this protocol. Briefly, the primary tumor, gross adenopathy and margin were treated to 70 Gy at 2 Gy per fraction in 35 daily treatments, 5 d a week over 7 wk. The at-risk but clinically-negative nodal regions were treated to 56 Gy in 35 daily treatments, 5 d a week over 7 wk.
Simulation was performed with appropriate immobilization in the treatment position. CT-based planning was required, and dose was specified at the ICRU-50 reference point. Volumes were created according to the 1993 ICRU Report #50[23]. 3D conformal planning was used, and IMRT was acceptable where feasible. Heterogeneity corrections were not utilized. The planning target volume was encompassed by the 90% isodose line. Beam energies of ≥ 6 MeV were utilized.
Cisplatin was dosed at 100 mg/m2 intravenously over 3 h in 1000 mL of normal saline on days 1, 22, and 43 of radiation therapy. Institution-specific standard pre-medication protocols for hydration and anti-emetics were used.
SLM was supplied as 800 μg capsules or matching placebo capsules (Sabinsa Corp., NJ). The number of capsules taken was the closest equivalent to a dose of 3600 μg/m2. This dose was taken twice daily orally for 7 d prior to initiation of CRT, based on pharmacokinetic modeling aiming to achieve a serum level prior to commencing CRT that approximated the steady-state concentration expected with prolonged once-daily dosing of 3600 µg/m2. Once CRT commenced, SLM/placebo dosing was once daily and continued until 3 wk after completion of CRT. Only for patients who were unable to tolerate capsules was dosing allowed division to 2-3 doses/d. Patients who were unable to swallow capsules or required tube feeding during or after CRT were asked to open the capsules and add the contents to their liquid feed. All patients were provided a diary to record capsule usage.
QOL assessments were carried out with the EORTC quality of life questionnaire (QLQ) C-30 version 3, and the EORTC QLQ - H and N35 module. Patients completed QOL assessments at baseline visit, weeks 4 and 7 during treatment, 6-8 wk post-treatment, and at 3 mo intervals following completion.
After completion of therapy, patients were seen in follow-up every 3 mo for 2 years, then every 6 mo to 5 years. This included physical examination and speech/swallow evaluation, assessment for adverse events and QOL, as well as documentation of weight, ECOG performance status, and adverse events. Relapse was defined as local, regional, or distant. Disease was measured where appropriate using the RECIST 1.0 Criteria[24]. Completion surgery to sites of remaining disease after CRT was performed if clinically appropriate.
Sample size calculations were based on a ≥ grade 3 mucositis rate of 50% in published randomized studies of similar schedules of concurrent cisplatin and radiation for HNSCC. This study used the Phase IIb 3-region design concept allowing decisions of: (1) clearly improved proportion with endpoint of interest; (2) promising benefits in the proportion with endpoint of interest; or (3) not worth pursuing[25]. With this design the chance of concluding there is an improvement in the proportion with ≥ grade 3 mucositis remains the same as the standard 0.025 (one-sided) cut-off for evidence of benefit. The lower cut-off fixes a 12.5% chance of concluding SLM is not worth pursuing if the true benefit is a reduction from 50% to 30% in rates of ≥ grade 3 mucositis.
The primary analysis was by intention-to-treat. Grade 3-4 mucositis, overall grades 3 and 4 toxicity, and tumor response were to be compared as difference in proportions with 95%CIs. Kaplan-Meier PFS curves and the proportion with an event at 1 year for PFS were to be compared simultaneously to obtain more global sensitivity to differences in time-to-event. The means between study groups and the proportion of patients completing CRT as initially planned were to be compared between groups using the student’s t test. Comparisons will be adjusted for baseline differences in prognostic factors using logistic, Cox or linear regression as appropriate. Distributions of time to event variables will be estimated using the Kaplan-Meier method. Log-rank tests were used for the comparison of survival distributions among study groups. Continuous endpoints will be summarized using means, standard deviations and percentiles. Statistical analysis was done using SAS, version 9.1, statistical software (SAS Institute Inc., Cary, NC).
Three interim analyses were planned: the first after 20 patients have completed CRT to ensure toxicity in the SLM arm was not unacceptably high and the second and third after one third and two thirds of the patients had been followed for at least 18 mo.
Ten patients received SLM and 8 received placebo capsules. Median age was 57, 17 patients were male. There was no significant difference in race between the two groups. Stage was evenly matched, all patients having either stage IVA or IVB disease. See Table 1 for patient and disease characteristics.
| Characteristic | Placebo(n = 8) | Selenium(n = 10) | P value | |
| Median age | 55.5 | 59.5 | 0.700 | |
| Male sex | 7 | 10 | 0.165 | |
| Race | White | 4 | 8 | 0.180 |
| Other | 4 | 2 | ||
| Best response | CR | 7 | 6 | 0.196 |
| Not evaluable | 1 | 4 | ||
| T stage | 1 | 3 | 0 | 0.063 |
| 2 | 2 | 5 | ||
| 3 | 0 | 3 | ||
| 4 | 3 | 1 | ||
| X | 0 | 1 | ||
| N stage | 1 | 0 | 1 | 0.103 |
| 2 | 6 | 8 | ||
| 3 | 1 | 1 | ||
| X | 1 | 0 | ||
| M stage | 0 | 5 | 10 | 0.105 |
| X | 3 | 0 | ||
| Stage group | IVA | 7 | 8 | 0.108 |
| IVB | 1 | 1 | ||
| Unkn | 0 | 1 | ||
One patient randomized to SLM took one dose, complained of a “bad taste” and withdrew from the trial. All patients except one received the protocol-prescribed dose of radiation. This patient experienced a cerebrovascular event due to tumor involvement of the carotid artery, leading to abandonment of treatment. Eight patients received all three cycles of cisplatin as planned, 6 patients received two cycles, two received one cycle, and one patient had chemotherapy held altogether.
There was no grade 4 mucosal toxicity. Grade 3 mucositis was seen in 3 of 8 patients in the placebo group, and 2 of 10 patients in the SLM group. These results are summarized in Table 2. Hearing dysfunction was reported in 1 patient from each group. Elevated creatinine was noted in 1 patient in the placebo group, and was not seen within the SLM group. Regarding myelosuppression of placebo and SLM groups; anemia occurred in 1 and 0, leukopenia in 2 and 3, respectively. Non-mucosal adverse events are summarized by treatment group in Table 3.
| Mucositis grade | Placebo(n = 8) | SLM(n = 10) |
| 0 | 1 | 2 |
| 1 | 1 | 3 |
| 2 | 3 | 3 |
| 3 | 3 | 2 |
| Toxicity ≥ grade 2 | Placebo | SLM |
| Dermatitis | 0 | 2 |
| Dry mouth | 2 | 0 |
| Dysgeusia | 1 | 2 |
| Anemia | 1 | 0 |
| Leukopenia | 2 | 3 |
| Thrombocytopenia | 0 | 0 |
| Odyno-/dysphagia | 2 | 1 |
| Oral/throat pain | 2 | 0 |
| Phlegm | 1 | 3 |
| Elevated creatinine | 1 | 0 |
| Hearing dysfunction | 1 | 1 |
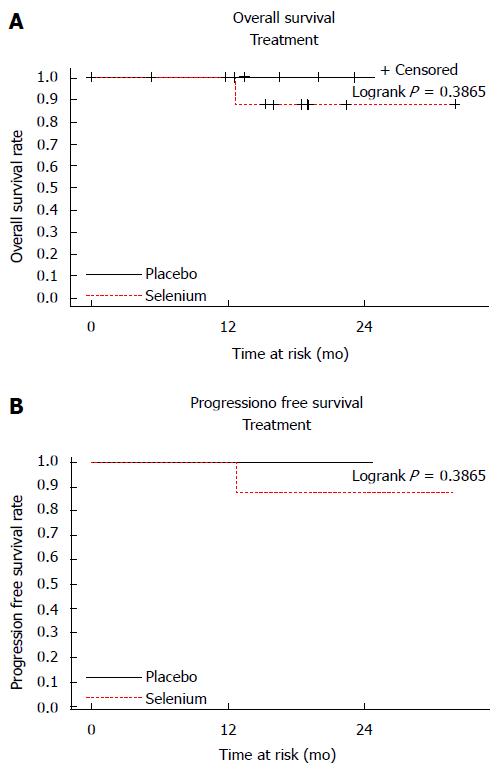
Only one patient (in the SLM group) failed to achieve a CR and died of locally persistent and widely metastatic disease. There was no discernible difference in OS or PFS. Kaplan-Meier survival curves are shown in Figure 1.
EORTC QOL questionnaire scores at baseline, weeks 4 and 7 of CRT, and during the 1 year follow-up period showed no significant differences between treatment groups (data not shown).
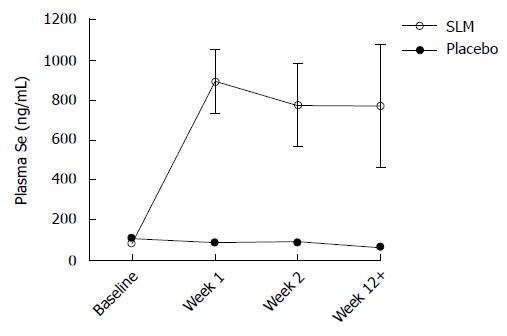
Blood draws to evaluate changes in plasma Se concentrations were undertaken in 8 patients from the NZ site. Baseline mean Se was similar in the SLM and placebo groups: 80.2 ng/mL and 105.1 ng/mL, respectively. Plasma concentrations tended to fall in the placebo group during and after CRT (Figure 2). In contrast, after taking SLM twice daily for 1 wk mean plasma Se rose to 890.4 ng/mL (range 475.0-1104.7) and similar levels were maintained with SLM once daily thereafter. About 1-2 wk after finishing SLM, plasma Se remained similar to on-treatment levels.
This small trial underwent an interim analysis after 18 of a planned 80 patients were accrued, to see if there was a sufficiently strong indication of efficacy to warrant further funding. No such signal of efficacy in either reduction of toxicity or improved therapeutic benefit was found, though given the single failure to achieve CR, no conclusion regarding the effect of SLM on CRT efficacy can be drawn from this trial. The reduction in incidence of grades 3-4 mucositis from 37.5% to 20% in the experimental group was consistent with the projected effect size of 20%, however patient numbers were too small for this difference to be significant.
Our findings agree with 2 other small studies of Se in HNSCC patients. Eroglu et al[26], in an observational study (without Se supplementation) of 47 consecutive patients receiving radiotherapy for HNSCC, found no correlation between serum Se levels and radiation toxicity[26]. Büntzel et al[27] performed a randomized phase II trial of 39 patients with advanced head and neck cancer. Patients either received no Se substitution or 500 μg sodium selenite orally on the days of radiotherapy and 300 μg on days without radiotherapy. There was no statistically significant difference in the incidence of severe toxicity overall; however the weekly patient analysis showed a significant reduction of dysphagia in the Se group at the last week of irradiation[27].
Our trial results stand in contrast to the findings of 3 other studies in patients with cancers other than HNSCC, which did show benefit to the addition of Se. Muecke et al[28], in a multi-center phase III trial with the primary endpoint of improving baseline serum Se levels in Se-deficient patients, found in post-operative patients with cervical cancer (n = 11) and uterine cancer (n = 70) a significant reduction in grade 2 or worse diarrhea (20.5% compared with 44.5%; P = 0.04) in the group supplemented with sodium selenite using the schedule by Buntzel above[28].
Jahangard-Rafsanjani et al[29] found that oral Se 200 μg twice daily significantly reduced oral mucositis in the setting of allogeneic stem cell transplantation for leukemia. In this 77 patient double-blind, randomized, placebo-controlled trial, the incidence of severe oral mucositis (grades 3-4) was significantly lower in the Se group (10.8% vs 35.1%, P < 0.05). Also, the duration of grades 2-4 mucositis was significantly shorter in the Se group (3.6 ± 1.84 d vs 5.3 ± 2.2 d, P = 0.014)[29]. A series of randomized trials reported by Asfour et al[30,31] using sodium selenite in conjunction with chemotherapy for patients with non-Hodgkin lymphoma revealed a small but significant survival advantage in those who achieved a CR to therapy.
Our own trial in stage III non-small cell lung cancer patients showed that SLM 4800 μg daily was well-tolerated in patients undergoing concurrent chemoradiation. The addition of SLM significantly reduced the incidence of myelosuppression and displayed a trend towards decreased rates of esophagitis and pneumonitis[32].
In contrast, a prior phase I trial from our group has shown that SLM did not limit irinotecan toxicity[21]. Furthermore, a phase 2, randomized, placebo-controlled trial of 140 localized prostate cancer patients undergoing active surveillance showed no difference in prostate specific antigen (PSA) velocity with 200 μg/d or 800 μg/d Se supplementation (as selenized yeast). In fact, in patients in the highest quartile of baseline Se, supplementation with high dose Se showed statistically significantly higher PSA velocity as compared with placebo (P = 0.018)[33].
There are a multitude of studies that have used Se supplementation to try to prevent the development of cancer in healthy patients, with mixed results[34-37]. While these studies are not directly relevant for comparison to our trial, some have argued that perhaps the discrepant results of prevention studies stem from the particular Se compound and dose selected for supplementation[38]. Similarly, it is possible that the discrepant results on toxicity and efficacy trials as described may stem from the use of different Se compounds and doses, in the setting of different tumor types.
With a mixed picture in human trials, the optimum form and dosing of Se is not yet known. The pre-clinical literature on the dual anti-tumor[10,11,14,15] and anti-toxicity[14,16-19] effects of organic Se compounds’ ability to widen narrow therapeutic windows in patients remains compelling. The organic Se compounds, such as Se-methyl-L-selenocysteine and selenite, are currently being evaluated for safety, pharmacokinetics and dose-dependency of pharmacodynamic mechanisms in phase I trials at our institutions.
Though the addition of SLM to concurrent chemoradiation for HNSCC was well-tolerated in this small trial, it did not significantly lower the incidence of severe mucositis or improve QOL outcomes. This is consistent with reports from 2 other studies of Se in HNSCC patients. Given that only a single failure to achieve CR was seen in this trial, no conclusion regarding effect of Se on treatment efficacy can be drawn from this trial.
Squamous cell carcinoma of the head and neck represents a significant worldwide health burden, and composes a substantial proportion of all cancer diagnoses. Concurrent radiotherapy and chemotherapy (CRT) has demonstrated the ability to cure a substantial number of patients, while maintaining important functions such as speech and swallowing. CRT, however, has significant acute side effects. Mucositis is one CRT side effect which can lead to interruptions of treatment. These interruptions are known to be associated with inferior outcomes. Because selenium (Se) -containing compounds have been suggested to effective protectors from radiation toxicity, the current trial was designed to evaluate the potential benefit of selenomethionine (SLM) in reducing rates and severity of mucositis during CRT. Patients received either SLM 3600 μg/m2 twice daily for one week prior to CRT, and once daily during CRT, or placebo, through a multicenter, randomized clinical trial.
As outcomes in cancers treated with radiotherapy continue to improve, there is increasing emphasis on the importance of toxicity mitigation. In this study, SLM failed to reduce the incidence and severity of mucositis during treatment with CRT.
The literature suggests a benefit for Se in protection from radiotherapy and chemotherapy induced toxicity. The current trial, however, failed to show benefit from the addition of Se to CRT treatment for head and neck cancer.
This study serves as additional evidence contributing to the current knowledge regarding Se as a potential radioprotcetor.
SLM: A naturally occurring amino acid containing Se, found in certain nuts, beans, and legumes. Mucositis: Painful inflammation of mucous membranes. This is a common side effect of cytotoxic therapies, such as chemotherapy and radiotherapy.
This is a good study to evaluate Se supplementation in CRT.
P- Reviewer: Dirier A, Lim SM, Sanabria A S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 1169] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25543] [Article Influence: 1824.5] [Reference Citation Analysis (7)] |
| 3. | Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, Bernstein L, Schoenberg JB, Stemhagen A, Fraumeni JF. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282-3287. [PubMed] |
| 4. | Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709-720. [PubMed] |
| 5. | Adelstein DJ, Li Y, Adams GL, Wagner H, Kish JA, Ensley JF, Schuller DE, Forastiere AA. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92-98. [PubMed] |
| 6. | Bourhis J, Lapeyre M, Tortochaux J, Rives M, Aghili M, Bourdin S, Lesaunier F, Benassi T, Lemanski C, Geoffrois L. Phase III randomized trial of very accelerated radiation therapy compared with conventional radiation therapy in squamous cell head and neck cancer: a GORTEC trial. J Clin Oncol. 2006;24:2873-2878. [PubMed] |
| 7. | Platek ME, McCloskey SA, Cruz M, Burke MS, Reid ME, Wilding GE, Rigual NR, Popat SR, Loree TR, Gupta V. Quantification of the effect of treatment duration on local-regional failure after definitive concurrent chemotherapy and intensity-modulated radiation therapy for squamous cell carcinoma of the head and neck. Head Neck. 2013;35:684-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Rades D, Stoehr M, Kazic N, Hakim SG, Walz A, Schild SE, Dunst J. Locally advanced stage IV squamous cell carcinoma of the head and neck: impact of pre-radiotherapy hemoglobin level and interruptions during radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:1108-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Nangia S, Chufal KS, Tyagi A, Bhatnagar A, Mishra M, Ghosh D. Selective nodal irradiation for head and neck cancer using intensity-modulated radiotherapy: application of RTOG consensus guidelines in routine clinical practice. Int J Radiat Oncol Biol Phys. 2010;76:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Altundag K, Silay YS, Altundag O, Yigitbasi OG, Gundeslioglu O, Gunduz M. Selenium supplementation may increase the efficacy of cetuximab in metastatic colorectal cancer patients. Med Hypotheses. 2005;64:1162-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Cao S, Durrani FA, Rustum YM. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin Cancer Res. 2004;10:2561-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Cao S, Durrani FA, Tóth K, Rustum YM. Se-methylselenocysteine offers selective protection against toxicity and potentiates the antitumour activity of anticancer drugs in preclinical animal models. Br J Cancer. 2014;110:1733-1743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Chintala S, Najrana T, Toth K, Cao S, Durrani FA, Pili R, Rustum YM. Prolyl hydroxylase 2 dependent and Von-Hippel-Lindau independent degradation of Hypoxia-inducible factor 1 and 2 alpha by selenium in clear cell renal cell carcinoma leads to tumor growth inhibition. BMC Cancer. 2012;12:293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Fischer JL, Mihelc EM, Pollok KE, Smith ML. Chemotherapeutic selectivity conferred by selenium: a role for p53-dependent DNA repair. Mol Cancer Ther. 2007;6:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Yang Y, Huang F, Ren Y, Xing L, Wu Y, Li Z, Pan H, Xu C. The anticancer effects of sodium selenite and selenomethionine on human colorectal carcinoma cell lines in nude mice. Oncol Res. 2009;18:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Stewart J, Ware J, Fortina P, Breaux J, Gulati S, Kennedy A. L-selenomethionine modulates high LET radiation-induced alterations of gene expression in cultured human thyroid cells. Oncol Rep. 2006;16:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Jeong SW, Jung HJ, Rahman MM, Hwang JN, Seo YR. Protective effects of selenomethionine against ionizing radiation under the modulation of p53 tumor suppressor. J Med Food. 2009;12:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Fischer JL, Lancia JK, Mathur A, Smith ML. Selenium protection from DNA damage involves a Ref1/p53/Brca1 protein complex. Anticancer Res. 2006;26:899-904. [PubMed] |
| 19. | Li L, Xie Y, El-Sayed WM, Szakacs JG, Franklin MR, Roberts JC. Chemopreventive activity of selenocysteine prodrugs against tobacco-derived nitrosamine (NNK) induced lung tumors in the A/J mouse. J Biochem Mol Toxicol. 2005;19:396-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Fakih MG, Pendyala L, Smith PF, Creaven PJ, Reid ME, Badmaev V, Azrak RG, Prey JD, Lawrence D, Rustum YM. A phase I and pharmacokinetic study of fixed-dose selenomethionine and irinotecan in solid tumors. Clin Cancer Res. 2006;12:1237-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Fakih MG, Pendyala L, Brady W, Smith PF, Ross ME, Creaven PJ, Badmaev V, Prey JD, Rustum YM. A Phase I and pharmacokinetic study of selenomethionine in combination with a fixed dose of irinotecan in solid tumors. Cancer Chemother Pharmacol. 2008;62:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Fakih M, Cao S, Durrani FA, Rustum YM. Selenium protects against toxicity induced by anticancer drugs and augments antitumor activity: a highly selective, new, and novel approach for the treatment of solid tumors. Clin Colorectal Cancer. 2005;5:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | International Commissino on Radiation Units and Measurements. ICRU report 50: Prescribing, Recording, and Reporting Photon Beam Therapy. Bethesda, MD, 1993. . |
| 24. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12751] [Cited by in RCA: 13078] [Article Influence: 523.1] [Reference Citation Analysis (0)] |
| 25. | Fleming TR, Richardson BA. Some design issues in trials of microbicides for the prevention of HIV infection. J Infect Dis. 2004;190:666-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Eroglu C, Unal D, Cetin A, Orhan O, Sivgin S, Oztürk A. Effect of serum selenium levels on radiotherapy-related toxicity in patients undergoing radiotherapy for head and neck cancer. Anticancer Res. 2012;32:3587-3590. [PubMed] |
| 27. | Büntzel J, Riesenbeck D, Glatzel M, Berndt-Skorka R, Riedel T, Mücke R, Kisters K, Schönekaes KG, Schäfer U, Bruns F. Limited effects of selenium substitution in the prevention of radiation-associated toxicities. results of a randomized study in head and neck cancer patients. Anticancer Res. 2010;30:1829-1832. [PubMed] |
| 28. | Muecke R, Schomburg L, Glatzel M, Berndt-Skorka R, Baaske D, Reichl B, Buentzel J, Kundt G, Prott FJ, Devries A. Multicenter, phase 3 trial comparing selenium supplementation with observation in gynecologic radiation oncology. Int J Radiat Oncol Biol Phys. 2010;78:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Jahangard-Rafsanjani Z, Gholami K, Hadjibabaie M, Shamshiri AR, Alimoghadam K, Sarayani A, Mojtahedzadeh M, Ostadali-Dehaghi M, Ghavamzadeh A. The efficacy of selenium in prevention of oral mucositis in patients undergoing hematopoietic SCT: a randomized clinical trial. Bone Marrow Transplant. 2013;48:832-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Asfour IA, El-Tehewi MM, Ahmed MH, Abdel-Sattar MA, Moustafa NN, Hegab HM, Fathey OM. High-dose sodium selenite can induce apoptosis of lymphoma cells in adult patients with non-Hodgkin’s lymphoma. Biol Trace Elem Res. 2009;127:200-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Asfour IA, Fayek M, Raouf S, Soliman M, Hegab HM, El-Desoky H, Saleh R, Moussa MA. The impact of high-dose sodium selenite therapy on Bcl-2 expression in adult non-Hodgkin’s lym phoma patients: correlation with response and survival. Biol Trace Elem Res. 2007;120:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Rajan S, Jameson M, de Groot C, Gomez J, Tills M, Dibaj S, Tan W, Ramnath N, Rustum Y, Singh AK. Concurrent Carboplatin, Paclitaxel, and Selenomethionine in Combination With Radiation for Patients With Unresectable Stage III Non-Small Cell Lung Cancer: A Phase 2 Multicenter Trial. Int J Radiat Oncol. 2013;87:S76-S77. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Stratton MS, Algotar AM, Ranger-Moore J, Stratton SP, Slate EH, Hsu CH, Thompson PA, Clark LC, Ahmann FR. Oral selenium supplementation has no effect on prostate-specific antigen velocity in men undergoing active surveillance for localized prostate cancer. Cancer Prev Res (Phila). 2010;3:1035-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Algotar AM, Stratton MS, Ahmann FR, Ranger-Moore J, Nagle RB, Thompson PA, Slate E, Hsu CH, Dalkin BL, Sindhwani P. Phase 3 clinical trial investigating the effect of selenium supplementation in men at high-risk for prostate cancer. Prostate. 2013;73:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Marshall JR, Tangen CM, Sakr WA, Wood DP, Berry DL, Klein EA, Lippman SM, Parnes HL, Alberts DS, Jarrard DF. Phase III trial of selenium to prevent prostate cancer in men with high-grade prostatic intraepithelial neoplasia: SWOG S9917. Cancer Prev Res (Phila). 2011;4:1761-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Klein EA, Thompson IM, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1223] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 37. | Duffield-Lillico AJ, Slate EH, Reid ME, Turnbull BW, Wilkins PA, Combs GF, Park HK, Gross EG, Graham GF, Stratton MS. Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J Natl Cancer Inst. 2003;95:1477-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 181] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 38. | Marshall JR, Ip C, Romano K, Fetterly G, Fakih M, Jovanovic B, Perloff M, Crowell J, Davis W, French-Christy R. Methyl selenocysteine: single-dose pharmacokinetics in men. Cancer Prev Res (Phila). 2011;4:1938-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |