Published online Oct 10, 2015. doi: 10.5306/wjco.v6.i5.156
Peer-review started: March 12, 2015
First decision: May 13, 2015
Revised: June 6, 2015
Accepted: June 18, 2015
Article in press: June 19, 2015
Published online: October 10, 2015
Processing time: 215 Days and 7.6 Hours
AIM: To prospectively determine the safety and tolerability of oral L-selenomethionine (SLM) with concurrent chemoradiation (CCRT) for Stage III non-small cell lung cancer (NSCLC) and estimate if the incidence and/or severity of adverse events could be reduced by its use.
METHODS: Sixteen patients with stage III NSCLC were accrued to this single arm, phase II study. CCRT consisted of radiation given at 2 Gy per fraction for 30-33 fractions, 5 d per week with concurrent weekly IV paclitaxel 50 mg/m2 followed by carboplatin dosed at an area under the time-concentration curve of 2. SLM was dosed in a loading phase at 4800 μg twice daily for one week prior to CCRT followed by once daily dosing during treatment.
RESULTS: No selenium-related toxicity was observed. Analysis revealed grade 3 or higher esophagitis in 3 of 16 patients (19%), pneumonitis in 0, leukopenia in 2 (12.5%), and anemia in 1 (6%); the latter two were significantly reduced when compared to the protocol-stated expected rate of 35% (P = 0.045 for leukopenia, and P < 0.01 for anemia). Median overall survival was 14.9 mo and median failure-free survival was 9 mo (95%CI: 3.3-21.5).
CONCLUSION: There may be some protective benefit of selenium in the setting of CCRT for inoperable NSCLC. The data suggests decreased rates of myelosuppression when compared to similarly-treated historical and contemporary controls. Further evaluation of selenium in this setting may be warranted.
Core tip: This was a prospective international phase II trial with 16 patients seeking to evaluate the effect of selenomethionine on acute toxicity in the setting of concurrent chemoradiaiton for locally advanced, inoperable non-small cell lung cancer. Selenium proved to be well tolerated and led to significantly reduced rates of myelosuppression.
- Citation: Mix M, Ramnath N, Gomez J, Groot C, Rajan S, Dibaj S, Tan W, Rustum Y, Jameson MB, Singh AK. Effects of selenomethionine on acute toxicities from concurrent chemoradiation for inoperable stage III non-small cell lung cancer. World J Clin Oncol 2015; 6(5): 156-165
- URL: https://www.wjgnet.com/2218-4333/full/v6/i5/156.htm
- DOI: https://dx.doi.org/10.5306/wjco.v6.i5.156
Concurrent chemoradiation (CCRT) is the standard of care for inoperable, locally-advanced non-small cell lung cancer (NSCLC)[1]. Even though there have been improvements in radiation delivery and less utilization of elective nodal irradiation (ENI), a significant proportion of patients continue to experience severe acute toxicities including esophagitis, myelosuppression and pneumonitis. Grade 3-4 esophagitis rates as high as 28% were reported in one study utilizing weekly carboplatin and paclitaxel in CCRT for inoperable NSCLC[2]. A meta-analysis reports that the addition of chemotherapy to radiation in this setting increases severe esophagitis rates from 4% to 18%[3]. Significant rates of high grade leukopenia and neutropenia have also been seen in the literature, with upper limits approximating 50%[4,5].
Given their short- and long-term effects on quality of life and the potential to interrupt therapy, it is important to reduce the incidence and severity of acute toxicities caused by CCRT. Several pharmacological agents that may protect against normal tissue toxicity have been studied, including organic thiophosphates such as amifostine. Although some protection by this agent during CCRT in NSCLC was suggested in Radiation Therapy Oncology Group (RTOG) study 9801, amifostine was not able to significantly reduce esophagitis rates[6,7]. In addition, side effects including marked hypotension and the requisite IV route of delivery have precluded its widespread adoption in this setting.
Preclinical data from our institution and others suggest that the organic selenium (Se) compound L-selenomethionine (SLM) has properties that confer protection on normal tissues from toxicities of chemotherapy and radiation, while enhancing their anti-tumor effects[8-17]. The dual properties of SLM to reduce normal tissue toxicity while increasing antitumor efficacy led to consideration[18] and implementation of early human studies combining chemotherapy with Se in a variety of tumors[19,20]. On the basis of this early clinical work, we hypothesized that SLM might reduce the major toxic effects of CCRT in NSCLC patients including esophagitis, pneumonitis, and myelosuppression. This might, in turn, reduce treatment interruptions and lead to increased local tumor control and survival. We therefore conducted a phase II multi-institutional study to determine the effects of SLM on acute toxicities as well as efficacy of concurrently-administered carboplatin, paclitaxel, and radiation in patients with unresectable stage III NSCLC.
Patients with Stage III NSCLC from Roswell Park Cancer Institute (RPCI) and Waikato Hospital were eligible for recruitment. The study was approved by the RPCI institutional review board and the Northern Y Regional Ethics Committee in New Zealand. Patients were screened for eligibility during clinic visits. Eligible patients were given information describing the study in readily understandable language and detailing the investigational nature of the study. Patients were subsequently required to provide their written consent in order to participate in the study. ClinicalTrials.gov identifier: NCT00526890.
Patients were eligible if: they had histologically- or cytologically-confirmed stage IIIA-IIIB squamous cell carcinoma, adenocarcinoma, large cell carcinoma, or NSCLC not otherwise specified; age ≥ 18; ECOG PS 0-1; weight loss ≤ 5% in the 3 mo before study entry; no invasive malignancy in the prior 3 years; no prior radiotherapy to the thorax/neck or chemotherapy; no pleural effusion; serum creatinine ≤ 1.5 mg/dL; serum bilirubin and glutamic-oxaloacetic transaminase ≤ 1.5 times the upper limit of normal; hemoglobin ≥ 8.0 g/dL; absolute granulocyte count ≥ 2000/mm3; and platelet count ≥ 100000/mm3. Patients were ineligible if they: were pregnant or of childbearing potential and refusing appropriate contraception; had a prior myocardial infarct within the preceding 6 mo or had symptomatic heart disease (angina, congestive heart failure, uncontrolled arrhythmia); had a serious concomitant infection including post-obstructive pneumonia; or had undergone major surgery other than biopsy in the previous 2 wk.
The pre-treatment evaluation included a complete medical history and physical examination with determination of the Eastern Cooperative Oncology Group (ECOG) performance status (PS) and questions about recent weight loss and concurrent non-malignant diseases. A complete blood count with differential and platelet count was also required, along with a biochemical survey, measurement of electrolytes, magnesium and serum transaminase levels, all of which had to be performed within 14 d of enrolment. Imaging studies included computed tomography (CT) scans of the chest and upper abdomen and CT or magnetic resonance imaging of the brain. At least weekly, an interval history and physical examination was performed by a member of the study team to prospectively assess and collect data regarding PS, weight loss, and symptoms of esophagitis and other toxicities. The complete blood count with differential, absolute granulocyte count, platelet count and serum creatinine levels were determined weekly. Particular attention was paid to patients’ pain levels and the medications required for control of symptomatic esophagitis. Toxicity was scored using National Cancer Institute Common Toxicity Criteria (CTC), version 3.0. Patients were evaluated with the same assessments 1 and 3 mo after treatment completion, at 3-mo intervals for 2 years then every 6 mo. CT scanning of the thorax was performed 3 mo after treatment and at each follow-up visit thereafter. Blood selenium levels were drawn at baseline, then weekly for the duration of therapy in order to monitor response of serum levels to supplementation.
An exact two-stage design was used to evaluate excess toxicity early on, and cease treatment if appropriate. The goal was for 10 patients in stage 1, with plan to stop accrual if ≥ 4 patients experienced excessive toxicity. Stage 2 was planned to accrue an additional 20 patients, with the bar set at ≥ 7 patients with excessive toxicity for stopping early. Total accrual was therefore set at 30 patients, and was expected to take a maximum of 6 years. Excessive toxicity was defined as: Grades 3-4 esophagitis, pneumonitis, or myelosuppression which caused delay of CCRT > 2 wk despite corrective measures. The study closed due to poor accrual in 2010 after the recruiting 16 patients. Changing practice patterns including desires to use alternative systemic agents, and a shift away from ENI (see below) were the primary reasons for unacceptable accrual. The decision to terminate the trial was made by the investigators for the aforementioned reasons. As the accrual goal exceeded 50%, we elected to retrospectively evaluate the collected data according to protocol specifications.
CT simulation was performed for all patients. Intravenous contrast was recommended but not required for improved delineation of targets. Dose inhomogeneity corrections were not used. The radiation therapy (RT) delivered was determined according to optimal dose distribution. Dose was 2 Gy per fraction, 30-33 fractions, 5 d per week for 6-6½ wk. Patients received megavoltage portal imaging for verification prior to treatment initiation, and at least weekly thereafter. Patients were treated with megavoltage equipment with at least 6 MeV photons using 3D conformal radiotherapy techniques. The planning target volume included a minimum margin of 1.5 cm around the gross tumor volume (GTV). A clinical tumor volume (CTV) was treated to an intermediate dose ranging from 40-46 Gy. The CTV included the elective nodal volumes, consisting of ipsilateral hilar, upper and lower paratracheal (levels 2, 4), and subcarinal lymph nodes. Aortic nodes (levels 5-6 were also included for left sided tumors. Ipsilateral supraclavicular lymph nodes were included if the primary tumor was located in the upper lobe or mainstem bronchus. Electron beams were permitted for elective treatment of supraclavicular lymph nodes. Individual custom blocking was used to spare normal tissues. Each field was treated each day. Protocol-specified dose constraints were as follows; total lung V20 < 32%, esophagus V55 < 66%, mean esophageal dose < 45 Gy, and maximal spinal cord dose < 45 Gy.
Patients did not receive induction chemotherapy. Concurrent chemotherapy consisted of paclitaxel (50 mg/m2) infused over 1 h, followed by carboplatin dosed at an area under the plasma concentration-time curve of 2 mg/mL per minute, infused over 30 min. These were given intravenously once weekly, 30 min before thoracic RT, for 6 wk, beginning on day 1 of RT. Patients received pre-medications and antiemetics as per institutional standards. The use of erythropoietin was permitted. The use of granulocyte colony-stimulating factors was discouraged, and was not allowed as prophylaxis, or with intent to prevent delay of protocol-specified therapy. SLM 800 μg capsules (Sabinsa Corp., NJ) were dosed as follows for a total of 7 wk: patients received loading doses of SLM 4800 μg orally twice daily for one week prior to beginning CCRT followed by a maintenance dose of 4800 μg daily for six weeks, or until the completion of therapy. This loading dosing schedule was based on pharmacokinetic modeling aiming to achieve a serum level prior to commencing CCRT that approximated the steady-state concentration expected with prolonged daily dosing of 4800 μg[19].
Treatment response was determined as follows: Complete response (CR) required disappearance of all measurable disease, signs, symptoms, and biochemical changes related to the tumor. Partial response (PR) required a reduction of ≥ 50% of the sum of the products of the perpendicular diameters of all measurable lesions. Stable disease (SD) required < 50% reduction and ≤ 25% increase in the sum. An increase > 25% was registered as progressive disease (PD).
The primary endpoint examined was toxicity resulting from SLM/CCRT (in particular, the anticipated esophagitis, pneumonitis and myelosuppression). Secondary endpoints included effects of SLM on efficacy and survival. A protocol-dictated 35% rate of CTC grade ≥ 3 esophagitis, pneumonitis, and myelosuppression was utilized for comparative statistics. The lower bound of the statistical power for correctly concluding acceptable toxicity of SLM/CCRT is 0.81 if the true toxicity rate is reduced by 20% compared to historical controls. A 0.05 level was set for Type 1 error, and 95%CI were calculated using the Jennison and Turnbull method[21]. One-sided P-values were calculated. Median, overall, and failure-free survival rates were calculated using the Kaplan-Meier method, with 95%CI.
After the first 10 patients were enrolled, no excess toxicity was noted and the cohort was expanded. Patients were enrolled between January 2007 and December 2009. After enrollment of 16 patients, there was still no selenium-related excess toxicity but the study was closed due to poor accrual. Pre-treatment characteristics are shown in Table 1.
| Characteristic | n (%) | Characteristic | n (%) |
| Sex | Performance status | ||
| Male | 5 (31) | 0 | 7 (44) |
| Female | 11 (69) | 1 | 9 (57) |
| Age | Stage | ||
| Mean | 63.25 | IIIA | 7 (44) |
| Median | 61 | IIIB | 7 (44) |
| Range | 49-78 | III NOS | 2 (13) |
| Race | Smoking status | ||
| White | 11 (69) | Current | 3 (19) |
| Black | 2 (13) | Former | 13 (81) |
| Other | 3 (19) | ||
| Histology | |||
| Adenocarcinoma | 8 | ||
| Squamous Cell | 6 | ||
| NSCLC-NOS | 2 | ||
Treatment was completed as planned in 14/16 (87.5%) patients. Treatment was discontinued indefinitely in one patient due to severe esophagitis. In a second patient, the patient was given a treatment break, and was subsequently re-planned using an IMRT technique, thus was no longer receiving protocol-specified treatment. These discontinuances did not meet stopping rules per protocol, as they were not deemed to be selenium-related. From available dosimetric data (13/16), median radiation dose to the GTV and CTV was 66 Gy and 46 Gy respectively. Regarding mean esophageal dose in treated patients, mean and median values were 19 Gy and 21 Gy respectively. The median follow-up time was 14.9 mo (3.3-62). Adverse events are summarized in Table 2. Grade 3 esophagitis was seen in 3 patients, none of whom were current smokers [18.75% (95%CI 4.05-45.7)]. There were no instances of grade 3-4 pneumonitis, and rates of grade 3-4 anemia, leukopenia, and neutropenia were 6% (95%CI: 0.16%-30.2%), 12.5% (95%CI: 1.55-38.4), and 0% respectively. When compared to the protocol-specified expected toxicity rate of 35%, anemia was significantly reduced (P < 0.01) when compared to the protocol-specified expected toxicity rate of 35%, leukopenia was significantly reduced (P = 0.045). There were no adverse effects attributed to SLM alone.
| n = 16 | Grade 1-2 | Grade 3 | Grade 4 | Grade 3-4 (%) |
| Esophagitis | 6 | 3 | 0 | 19 |
| Pneumonitis | 4 | 0 | 0 | 0 |
| Anemia | 7 | 1 | 0 | 6 |
| Leukopenia | 8 | 2 | 0 | 13 |
| Neutropenia | 4 | 0 | 0 | 0 |
| Hypokalemia | 3 | 0 | 1 | 6 |
| Fatigue | 7 | 1 | 0 | 6 |
| Weight loss | 2 | 0 | 0 | 0 |
Median overall survival (OS) and failure-free survival (FFS) were 14.9 mo (95%CI: 7.5-43.8) and 9.1 mo (95%CI: 3.3-21.5) respectively. Eight patients (50%) had a PR, 4 patients (25%) had SD, and 3 patients (19%) exhibited PD as their best response. The overall response rate was 50% (95%CI: 24.7-75.4). One patient was not evaluable for response.
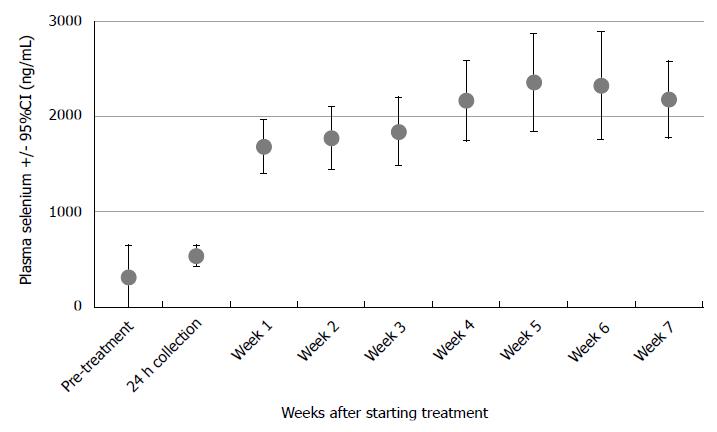
Baseline serum Se levels were available for 14 of 16 patients: the mean (standard deviation) value was 304 (604) ng/mL and the median value was 98 ng/mL. Trough Se levels rose for all patients during supplementation, shown in Figure 1. Levels were available for 14 of 16 patients at week 6, when mean and median values were 2324 and 2179 ng/mL respectively.
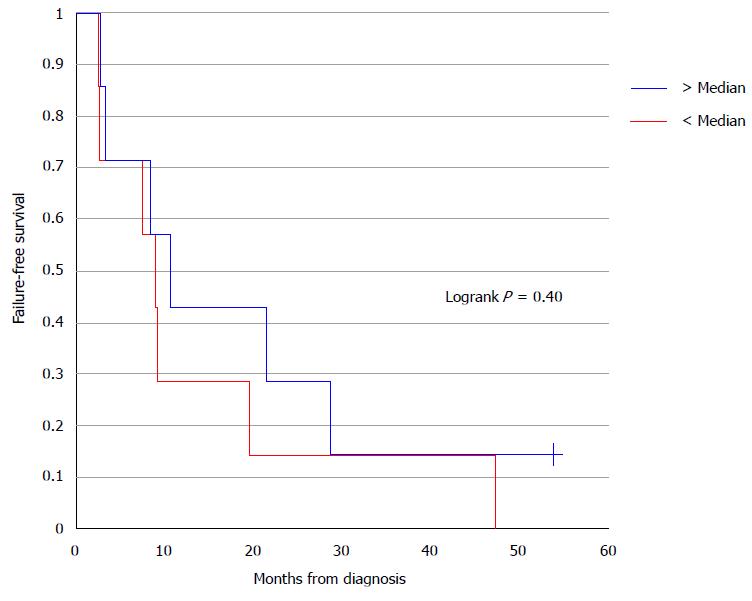
Baseline Se values and their relationship to FFS were analyzed. Baseline levels were dichotomized into two groups relative to the median value. No significant correlation was detected between baseline Se and FFS (P = 0.4016) (Figure 2). Similarly, baseline values were compared to severe esophagitis and/or myelosuppresion rates using Fisher’s exact test and there was no significant association with either toxicity (P = 1.00). Due to a paucity of data, an association between toxicity outcomes and week 7 serum Se levels could not be analyzed.
The addition of SLM 4800 μg daily to CCRT in inoperable stage III NSCLC was safe and well-tolerated. To our knowledge, this is the first study evaluating the use of SLM in this population. Leukopenia, anemia, neutropenia, and esophagitis rates appear to be improved compared to the protocol-specified incidence of 35%, however this figure was likely set too high in the context of more recent publications with regard to esophagitis. A more reasonable estimate for high grade esophagitis would be 18%[3]. Regarding the myelosuppresive endpoints, estimates based on similarly treated patients for leukopenia, anemia, and neutropenia, are 23%-51%, 3%-10%, and 15%-51%, respectively[2,4,5]. Given these estimates, the addition of selenium may have improved myelosuppresion.
At the time of this protocol’s inception, treatment of uninvolved regional nodal basins was standard of care, thus trials which utilized ENI are the best comparators for these data. Regarding esophagitis, our rate of 19% esophagitis compared favorably to the CCRT arm using both ENI and the same chemotherapeutic regimen in a phase III trial by Vokes et al[2] at 28%. Based on the observation that ENI doesn’t significantly reduce regional recurrence[22] while increasing toxicity, current paradigms have shifted towards involved field radiotherapy (IFRT) with consequent decreases in normal tissue irradiation and therefore toxicity. As expected, our results exceed esophagitis rates seen in similar patients treated using an IFRT technique, reported as low as 1%-8%[5,23,24]. One such trial, however, revealed numerically-increased rates of esophagitis compared to ours, with grade 3-4 toxicity of 28%[4]. Table 3 summarizes esophagitis rates for several studies evaluating CCRT in Stage III NSCLC, using a variety of CTV parameters and concurrent chemotherapeutic regimens.
| Ref. | Year | Design | No. of patients | Nodes | RT dose (Gy) | Chemo | Grade 3-4esophagitis | Grade 3-4pneumonitis |
| Furuse et al[27] | 1999 | Ind → RT | 314 | ENI | 56 | Cis/Vnd/Mit | 3% | - |
| CCRT | 561 | 2% | 1% | |||||
| Zatloukal et al[28] | 2004 | Ind → RT | 102 | ENI | 60 | Cis/Vno | 4% | |
| CCRT | 18% | 4% | ||||||
| Fournel et al[26] | 2005 | Ind → RT | 205 | ENI | 66 | Cis/Vno | 2% | |
| CCRT → Cons | Cis/Eto → Cis/Vno | 32% | 5% | |||||
| Belani et al[4] | 2005 | Ind → RT | 257 | IFRT | 63 | Cbp/Pac | - | - |
| Ind → CCRT | 19% | 4% | ||||||
| CCRT → Cons | 28% | 16% | ||||||
| Vokes et al[2] | 2007 | CCRT | 366 | ENI | 66 | Cbp/Pac | 28% | 4% |
| Ind → CCRT | 30% | 10% | ||||||
| Belderbos et al[25] | 2007 | Ind → RT | 158 | ENI | 662 | Cis/Gem | 5% | |
| CRT | Cis | 14% | 18% | |||||
| Socinski et al[41] | 2008 | Ind → CCRT | 69 | “ENI discouraged but allowed” | 74 | Cbp/Pac | 16% | 16% |
| Ind → CRT | Cbp/Gem | 39% | 37% | |||||
| Blumenschein et al[23] | 2011 | CCRT | 87 | “Selective nodal irradiation” | 63 | Cbp/Pac/ | 8% | 22% |
| Cet | ||||||||
| Curran et al[42] | 2011 | Ind → RT | 407 | ENI | 63 | Cis/Vnb | 4% | - |
| CCRT | 63 | Cis/Vnb | 22% | 13% | ||||
| CCRT | 69.63 | Cis/Eto | 45% | 15% | ||||
| Hoang et al[5] | 2012 | CCRT | 546 | IFRT | 60 | Cbp/Pac | < 1% | 1% |
| CCRT + Thl | Cbp/Pac/Thl | < 1% | 1% |
There were no instances of grade ≥ 3 pneumonitis in our study, which compares favorably with studies using a comparable CCRT regimen as well as other chemoradiation regimens (Table 3).
Regarding myelosuppression, we report rates of anemia, leukopenia, and neutropenia of 6%, 13%, and 0% respectively. The leukopenia rate is significantly decreased from the 35% benchmark dictated in protocol. The rates of both leukopenia and neutropenia are numerically decreased when compared to patients receiving CCRT with identical chemotherapeutic regimens (Table 4). The avoidance of severe neutropenia by adding SLM, if confirmed, would be clinically significant.
The current trial reports 50% PR as best response (95%CI: 24.7-75.4), and 19% PD. This figure is somewhat less than expected from historical controls. Vokes et al[2] reported 67% CR/PR and 9% PD, while Blumenschein et al[23] report 62% and 11%. Our results should be interpreted with caution given small patient numbers and wide confidence intervals, remembering that preclinical work with SLM strongly suggests a benefit in terms of tumor response with RT. However, it is important to be critically aware of the slightly lower response rate seen in this study when compared to similarly treated historical cohorts. It is critically important to be vigilant of tumor response rates when investigating agents purported to protect normal tissues.
The median OS in the current study is 14.9 mo. Similar survival rates were seen in larger groups of similarly-treated patients, ranging from 12-16.6 mo[2,4,25-28]. It should be noted that more recently-published series, using more contemporary radiation methods (i.e., IFRT as opposed to ENI) have demonstrated improved survival. For example, RTOG 0117 treated similar patients with similar chemotherapy, but used higher doses of radiation, and did not electively treat nodal volumes. This phase II study reported median survival of 25.9 mo[29]. It is not clear if the data presented here are directly comparable to this more modern cohort. Nevertheless, this represents a more current estimation of median survival in this patient population.
Broadly supportive of our findings, prior studies have found that Se compounds may limit chemotherapy toxicity. Jahangard-Rafsanjani et al[30] found that selenium significantly reduced oral mucositis in the setting of busulfan and cyclophosphamide-based high-dose chemotherapy followed by allogeneic stem cell transplantation for leukemia. In this 77-patient double-blind, randomized, placebo-controlled study, those receiving SLM (200 μg BID) experienced significantly less grades 3-4 oral mucositis (10.8% vs 35.1%, P < 0.05). The duration of grades 2-4 oral mucositis was also significantly shorter in the selenium group (3.6 ± 1.84 vs 5.3 ± 2.2 d, P = 0.014). Another trial evaluating Se in the form of selenokappacarrageenan given prior to cisplatin-based chemotherapy led to higher white blood cell counts on day 14 than in its absence; no comment on antitumor effect was made[31].
In a double-blind trial involving 62 women receiving cisplatin and cyclophosphamide for ovarian cancer, patients were randomized to antioxidant capsules with or without Se as selenized yeast[32]. Those receiving Se were found to have fewer toxicities including nausea, vomiting, stomatitis, alopecia, abdominal pain, weakness, and loss of appetite (all with P < 0.05). A formal assessment of antitumor activity wasn’t performed, however CA-125 levels were numerically lower in the Se group. Another trial randomized 50 patients receiving cisplatin-based chemotherapy to concurrent supplementation with sodium selenite, vitamin C and vitamin E vs placebo. There was no observed difference in toxicity, although 64% of patients within the experimental arm were noncompliant with therapy due to GI side effects and serum Se levels did not differ between the two groups, suggesting that Se intake was not significant[33]. A series of small randomized controlled trials has been reported from one group using sodium selenite 200 μg/kg per day in conjunction with chemotherapy for patients with non-Hodgkin lymphoma[34,35]. While outcomes varied, the Se groups tended to have less toxicity. In the 2007 report, an increased response rate was seen, and a small but statistically significant survival advantage was seen in those achieving CR[35]. Finally, a phase I study from our group has shown that SLM did not significantly impact irinotecan toxicity[19].
Other studies have examined the potential of Se to mitigate radiation-induced toxicity. Muecke et al[36], in a multi-center open-label randomized phase III study with the primary endpoint of improving baseline Se levels, found in 81 post-operative patients with cervical or endometrial cancer a significant reduction in grade ≥ 2 diarrhea (20.5% vs 44.5%, P = 0.04) in the group given selenite 500 μg/d with RT and 300 μg/d on non-RT days compared to controls. Büntzel et al[37] performed a randomized phase II study of 39 patients with advanced stage squamous cell carcinoma of the head and neck (HNSCC) and found less obvious benefit using the same Se regimen as Muecke. There was no statistically significant incidence of severe toxicity overall; however the weekly patient analysis showed a significant reduction of dysphagia in the experimental group during the final week of irradiation (P = 0.05) and overall trends towards prevention of taste loss.
Our study group conducted a phase II, randomized, placebo-controlled study in 18 HNSCC patients undergoing CCRT with cisplatin, in which SLM supplementation at 3600 μg/m2 per day was well-tolerated. While no statistically significant differences were noted in acute CCRT toxicities, nor in patient-reported quality of life measures, a trend was seen for decreased rates of severe mucositis[38].
Trough Se levels rose in all patients for whom baseline plasma Se values were available. No association was seen between baseline Se levels and toxicity in this cohort. A recent review of Se supplementation highlighted the tendency of serum Se levels to fall during the course of radiotherapy[39]. This fact suggests that there may be a correlation between toxicity and Se levels. A report from Eroglu et al[40], however, found no correlation between Se levels and radiation toxicity. This cohort was found to have plasma Se levels between 56-58 ng/mL, which is below the reported levels seen in those undergoing supplementation[19]. The association of plasma Se levels and incidence of radiation of chemotherapy induced toxicity remains unclear.
Our study is limited by a number of factors that require attention. First, the early closure due to poor accrual resulted in a smaller than intended cohort. This calls into question the observed decreased rate of myelosuppression (albeit a significant one), given small patient numbers. These results may be due to other factors, and their influence can’t be assessed without a placebo group. Second, the 35% benchmark set for grade ≥ 3 esophageal toxicity in this patient population may need to be reconsidered in light of newer radiation techniques, including the shift towards IFRT as opposed to ENI. The true rate of severe esophagitis in this setting should perhaps be closer to 20%. Nevertheless, we did see a decrease relative to the most closely-matched cohort.
In conclusion, SLM 4800 μg/d was safe and well tolerated when combined with CCRT in patients with inoperable stage III NSCLC in this multicenter, international, phase II trial. The data suggests the feasibility of investigating SLM to reduce rates of myelosuppresion. Response rates were slightly less than expected when compared to the aforementioned controls. Survival rates are comparable when considering those treated with similar radiation techniques. Treatment-induced toxicity continues to be a significant issue, thus there may be some role for future investigation of Se as a protector from chemotherapy related toxicity, and possibly from radiotherapy-related toxicity in NSCLC.
Concurrent chemoradiation (CCRT) is the standard of care for advanced stage, inoperable non-small cell lung cancer (NSCLC). The use of CCRT has been shown to improve survival, but can lead to significant treatment-related toxicity. Selenium compounds have shown promise in their ability to confer protection on normal tissues during treatment with radiotherapy and/or chemotherapy. The current trial was designed to evaluate the tolerability of selenomethionine (SLM) and its potential to reduce the incidence/severity of treatment-related toxicity during CCRT.
Outcomes of patients treated with CCRT are improving, and there is increasing focus on ways to minimize toxicity during cancer treatment. In this study, there is suggestion that SLM may reduce rates of myelosuppression compared to similarly-treated historical controls.
The literature suggests a benefit for selenium in protection from radiation and chemotherapy induced toxicity. The current trial adds to that literature, with the suggestion of decreased rates of myelosuppression with the addition of SLM to CCRT in locally advanced NSCLC.
This study serves as additional evidence supporting the investigation of selenium’s potential role in mitigating chemotherapy and radiotherapy toxicity.
SLM: A naturally occurring amino acid containing selenium, found in certain nuts, beans, and legumes. Myelosuppression: The decrease in production of blood cells that compose the immune system (leukocytes), delivering oxygen to tissues (erythrocytes), and/or those responsible for blood clotting (thrombocytes).
The authors have performed a good study, the manuscript is interesting.
P- Reviewer: Bhawal UK, Lim SM, Reim D, Vetvicka V, Voortman J S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK
| 1. | Ramnath N, Dilling TJ, Harris LJ, Kim AW, Michaud GC, Balekian AA, Diekemper R, Detterbeck FC, Arenberg DA. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e314S-e340S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 329] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 2. | Vokes EE, Herndon JE, Kelley MJ, Cicchetti MG, Ramnath N, Neill H, Atkins JN, Watson DM, Akerley W, Green MR. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol. 2007;25:1698-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 348] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 3. | Aupérin A, Le Péchoux C, Rolland E, Curran WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus R. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1439] [Cited by in RCA: 1366] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 4. | Belani CP, Choy H, Bonomi P, Scott C, Travis P, Haluschak J, Curran WJ. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005;23:5883-5891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 323] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 5. | Hoang T, Dahlberg SE, Schiller JH, Mehta MP, Fitzgerald TJ, Belinsky SA, Johnson DH. Randomized phase III study of thoracic radiation in combination with paclitaxel and carboplatin with or without thalidomide in patients with stage III non-small-cell lung cancer: the ECOG 3598 study. J Clin Oncol. 2012;30:616-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Movsas B. Exploring the role of the radioprotector amifostine in locally advanced non-small cell lung cancer: Radiation Therapy Oncology Group trial 98-01. Semin Radiat Oncol. 2002;12:40-45. [PubMed] |
| 7. | Movsas B, Scott C, Langer C, Werner-Wasik M, Nicolaou N, Komaki R, Machtay M, Smith C, Axelrod R, Sarna L. Randomized trial of amifostine in locally advanced non-small-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: radiation therapy oncology group trial 98-01. J Clin Oncol. 2005;23:2145-2154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Altundag K, Silay YS, Altundag O, Yigitbasi OG, Gundeslioglu O, Gunduz M. Selenium supplementation may increase the efficacy of cetuximab in metastatic colorectal cancer patients. Med Hypotheses. 2005;64:1162-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Cao S, Durrani FA, Rustum YM. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin Cancer Res. 2004;10:2561-2569. [PubMed] |
| 10. | Cao S, Durrani FA, Tóth K, Rustum YM. Se-methylselenocysteine offers selective protection against toxicity and potentiates the antitumour activity of anticancer drugs in preclinical animal models. Br J Cancer. 2014;110:1733-1743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Chintala S, Najrana T, Toth K, Cao S, Durrani FA, Pili R, Rustum YM. Prolyl hydroxylase 2 dependent and Von-Hippel-Lindau independent degradation of Hypoxia-inducible factor 1 and 2 alpha by selenium in clear cell renal cell carcinoma leads to tumor growth inhibition. BMC Cancer. 2012;12:293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Fischer JL, Lancia JK, Mathur A, Smith ML. Selenium protection from DNA damage involves a Ref1/p53/Brca1 protein complex. Anticancer Res. 2006;26:899-904. [PubMed] |
| 13. | Fischer JL, Mihelc EM, Pollok KE, Smith ML. Chemotherapeutic selectivity conferred by selenium: a role for p53-dependent DNA repair. Mol Cancer Ther. 2007;6:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Jeong SW, Jung HJ, Rahman MM, Hwang JN, Seo YR. Protective effects of selenomethionine against ionizing radiation under the modulation of p53 tumor suppressor. J Med Food. 2009;12:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Li L, Xie Y, El-Sayed WM, Szakacs JG, Franklin MR, Roberts JC. Chemopreventive activity of selenocysteine prodrugs against tobacco-derived nitrosamine (NNK) induced lung tumors in the A/J mouse. J Biochem Mol Toxicol. 2005;19:396-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Stewart J, Ware J, Fortina P, Breaux J, Gulati S, Kennedy A. L-selenomethionine modulates high LET radiation-induced alterations of gene expression in cultured human thyroid cells. Oncol Rep. 2006;16:569-574. [PubMed] |
| 17. | Yang Y, Huang F, Ren Y, Xing L, Wu Y, Li Z, Pan H, Xu C. The anticancer effects of sodium selenite and selenomethionine on human colorectal carcinoma cell lines in nude mice. Oncol Res. 2009;18:1-8. [PubMed] |
| 18. | Fakih M, Cao S, Durrani FA, Rustum YM. Selenium protects against toxicity induced by anticancer drugs and augments antitumor activity: a highly selective, new, and novel approach for the treatment of solid tumors. Clin Colorectal Cancer. 2005;5:132-135. [PubMed] |
| 19. | Fakih MG, Pendyala L, Brady W, Smith PF, Ross ME, Creaven PJ, Badmaev V, Prey JD, Rustum YM. A Phase I and pharmacokinetic study of selenomethionine in combination with a fixed dose of irinotecan in solid tumors. Cancer Chemother Pharmacol. 2008;62:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Fakih MG, Pendyala L, Smith PF, Creaven PJ, Reid ME, Badmaev V, Azrak RG, Prey JD, Lawrence D, Rustum YM. A phase I and pharmacokinetic study of fixed-dose selenomethionine and irinotecan in solid tumors. Clin Cancer Res. 2006;12:1237-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Jennison C, Turnbull BW. Confidence Intervals for a Binomial Parameter Following a Multistage Test With Application to MIL-STD 105D and Medical Trials. Technometrics. 1983;25:49-58. [RCA] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Fernandes AT, Shen J, Finlay J, Mitra N, Evans T, Stevenson J, Langer C, Lin L, Hahn S, Glatstein E. Elective nodal irradiation (ENI) vs. involved field radiotherapy (IFRT) for locally advanced non-small cell lung cancer (NSCLC): A comparative analysis of toxicities and clinical outcomes. Radiother Oncol. 2010;95:178-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Blumenschein GR, Paulus R, Curran WJ, Robert F, Fossella F, Werner-Wasik M, Herbst RS, Doescher PO, Choy H, Komaki R. Phase II study of cetuximab in combination with chemoradiation in patients with stage IIIA/B non-small-cell lung cancer: RTOG 0324. J Clin Oncol. 2011;29:2312-2318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Poudenx M, Bondiau PY, Chamorey E, Venissac N, Otto J, Pourel N, Castelnau O, Tessier E, De Surmont Salasc B, Berdah JF. Cisplatin-docetaxel induction plus concurrent 3-D conformal radiotherapy and weekly chemotherapy for locally advanced non-small cell lung cancer patients: a phase II trial. Oncology. 2012;83:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Belderbos J, Uitterhoeve L, van Zandwijk N, Belderbos H, Rodrigus P, van de Vaart P, Price A, van Walree N, Legrand C, Dussenne S. Randomised trial of sequential versus concurrent chemo-radiotherapy in patients with inoperable non-small cell lung cancer (EORTC 08972-22973). Eur J Cancer. 2007;43:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Fournel P, Robinet G, Thomas P, Souquet PJ, Léna H, Vergnenégre A, Delhoume JY, Le Treut J, Silvani JA, Dansin E. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie NPC 95-01 Study. J Clin Oncol. 2005;23:5910-5917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 397] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 27. | Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, Katagami N, Ariyoshi Y. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692-2699. [PubMed] |
| 28. | Zatloukal P, Petruzelka L, Zemanova M, Havel L, Janku F, Judas L, Kubik A, Krepela E, Fiala P, Pecen L. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer. 2004;46:87-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 315] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 29. | Bradley JD, Bae K, Graham MV, Byhardt R, Govindan R, Fowler J, Purdy JA, Michalski JM, Gore E, Choy H. Primary analysis of the phase II component of a phase I/II dose intensification study using three-dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non-small-cell lung cancer: RTOG 0117. J Clin Oncol. 2010;28:2475-2480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 30. | Jahangard-Rafsanjani Z, Gholami K, Hadjibabaie M, Shamshiri AR, Alimoghadam K, Sarayani A, Mojtahedzadeh M, Ostadali-Dehaghi M, Ghavamzadeh A. The efficacy of selenium in prevention of oral mucositis in patients undergoing hematopoietic SCT: a randomized clinical trial. Bone Marrow Transplant. 2013;48:832-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Hu YJ, Chen Y, Zhang YQ, Zhou MZ, Song XM, Zhang BZ, Luo L, Xu PM, Zhao YN, Zhao YB. The protective role of selenium on the toxicity of cisplatin-contained chemotherapy regimen in cancer patients. Biol Trace Elem Res. 1997;56:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Sieja K, Talerczyk M. Selenium as an element in the treatment of ovarian cancer in women receiving chemotherapy. Gynecol Oncol. 2004;93:320-327. [PubMed] |
| 33. | Weijl NI, Elsendoorn TJ, Lentjes EG, Hopman GD, Wipkink-Bakker A, Zwinderman AH, Cleton FJ, Osanto S. Supplementation with antioxidant micronutrients and chemotherapy-induced toxicity in cancer patients treated with cisplatin-based chemotherapy: a randomised, double-blind, placebo-controlled study. Eur J Cancer. 2004;40:1713-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 34. | Asfour IA, El-Tehewi MM, Ahmed MH, Abdel-Sattar MA, Moustafa NN, Hegab HM, Fathey OM. High-dose sodium selenite can induce apoptosis of lymphoma cells in adult patients with non-Hodgkin’s lymphoma. Biol Trace Elem Res. 2009;127:200-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Asfour IA, Fayek M, Raouf S, Soliman M, Hegab HM, El-Desoky H, Saleh R, Moussa MA. The impact of high-dose sodium selenite therapy on Bcl-2 expression in adult non-Hodgkin’s lymphoma patients: correlation with response and survival. Biol Trace Elem Res. 2007;120:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Muecke R, Schomburg L, Glatzel M, Berndt-Skorka R, Baaske D, Reichl B, Buentzel J, Kundt G, Prott FJ, Devries A. Multicenter, phase 3 trial comparing selenium supplementation with observation in gynecologic radiation oncology. Int J Radiat Oncol Biol Phys. 2010;78:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Büntzel J, Riesenbeck D, Glatzel M, Berndt-Skorka R, Riedel T, Mücke R, Kisters K, Schönekaes KG, Schäfer U, Bruns F. Limited effects of selenium substitution in the prevention of radiation-associated toxicities. results of a randomized study in head and neck cancer patients. Anticancer Res. 2010;30:1829-1832. [PubMed] |
| 38. | Mix MD, Jameson M, Tills M, Dibaj S, Groman A, Jaggernauth W, Rustum Y, Singh AK. Randomized Double-Blind, Placebo-Controlled, Multicenter Phase 2 Trial of Selenomethionine as a Modulator of Efficacy and Toxicity of Chemoradiation in Locally-Advanced Squamous Cell Carcinoma of the Head and Neck. Int J Radiat Oncol Biol Phy. 2015;87:S466-S467. |
| 39. | Puspitasari IM, Abdulah R, Yamazaki C, Kameo S, Nakano T, Koyama H. Updates on clinical studies of selenium supplementation in radiotherapy. Radiat Oncol. 2014;9:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Eroglu C, Unal D, Cetin A, Orhan O, Sivgin S, Oztürk A. Effect of serum selenium levels on radiotherapy-related toxicity in patients undergoing radiotherapy for head and neck cancer. Anticancer Res. 2012;32:3587-3590. [PubMed] |
| 41. | Socinski MA, Blackstock AW, Bogart JA, Wang X, Munley M, Rosenman J, Gu L, Masters GA, Ungaro P, Sleeper A. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol. 2008;26:2457-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 42. | Curran WJ, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, Movsas B, Wasserman T, Rosenthal SA, Gore E. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 916] [Article Influence: 65.4] [Reference Citation Analysis (1)] |