Published online Aug 10, 2015. doi: 10.5306/wjco.v6.i4.64
Peer-review started: October 28, 2014
First decision: December 26, 2014
Revised: May 18, 2015
Accepted: June 4, 2015
Article in press: June 8, 2015
Published online: August 10, 2015
Processing time: 294 Days and 17.2 Hours
AIM: To study the efficacy and safety of abiraterone in patients with and without prior chemotherapy.
METHODS: The databases including PubMed and abstracts presented at the American Society of Clinical Oncology meetings up to April 2014 were systematically searched. Eligible studies included randomized controlled trials (RCTs) in which abiraterone plus prednisone was compared to placebo plus prednisone in metastatic castration-resistant prostate cancer (CRPC) patients. The summary incidence, relative risk, hazard ratio and 95%CI were calculated using random or fixed-effects models. Heterogeneity test was performed to test between-study differences in efficacy and toxicity.
RESULTS: A total of two phase III RCTs were included in our analysis, with metastatic CPRC patients before (n = 1088) and after chemotherapy (n = 1195). Prior chemotherapy did not significantly alter the effect of abiraterone on overall survival (P = 0.92) and prostate-specific antigen (PSA) progression-free survival (P = 0.13), but reduced its effect on radiographic-progression-free survival (P = 0.04), objective response rate (P < 0.001), and PSA response rate (P < 0.001). Prior chemotherapy significantly increased the specific risk of fluid retention and edema (P < 0.001) and hypokalemia (P < 0.001), but decreased the risk of all-grade hypertension (P < 0.001) attributable to abiraterone. There was no significant difference of cardiac disorders associated with abiraterone between the two settings (P = 0.58).
CONCLUSION: Prior chemotherapy may reduce the effectiveness of abiraterone in patients with metastatic CRPC.
Core tip: Our meta-analysis has demonstrated that pre-chemotherapy may affect the efficacy and toxicity of abiraterone treatment in patients with metastatic castration-resistant prostate cancer. Abiraterone was associated with significantly increased radiographic-progression-free survival, objective response rate, and prostate-specific antigen response rate in the pre-chemotherapy setting when compared to the post-chemotherapy setting. In addition, abiraterone in the pre-chemotherapy setting had a significant lower risk of all-grade fluid retention and edema (P < 0.001), and hypokalemia (P < 0.001), but had a higher risk of all-grade hypertension (P < 0.001) when compared to post-chemotherapy.
- Citation: Shameem R, Hamid MS, Xu KY, Wu S. Comparative analysis of the effectiveness of abiraterone before and after docetaxel in patients with metastatic castration-resistant prostate cancer. World J Clin Oncol 2015; 6(4): 64-72
- URL: https://www.wjgnet.com/2218-4333/full/v6/i4/64.htm
- DOI: https://dx.doi.org/10.5306/wjco.v6.i4.64
Metastatic castration-resistant prostate cancer (CRPC) is defined as disease progression despite the castrate levels of testosterone (≤ 50 ng/dL) after medical or surgical castration[1-3]. Tumor growth in this setting may result from aberrant androgen receptor (AR) signaling, up-regulation of androgen synthesis enzymes, and persistent conversion of androgens to testosterone and dihydrotestosterone (DHT)[1,4-6]. Docetaxel as a chemotherapy taxane has shown survival benefit based on randomized controlled trials (RCTs) and has been a standard therapy in patients with metastatic CRPC since 2004[7,8]. However, the treatment of disease relapse on docetaxel had been challenging due to a former nonexistence of secondary effective options[4,7]. Fortunately, in the recent years there has been an improved understanding of metastatic CRPC pathophysiology with the rapid introduction of new agents such as abiraterone to the metastatic CRPC armamentarium[9-11].
Abiraterone acetate (AA) is a pro-drug pregnenolone derivative that is administered orally. Its active metabolite, abiraterone is an irreversible inhibitor of CYP-17, 20 lyase and 17-alpha-hydroxylase, essential enzymes for gonadal and extra-gonadal androgen synthesis[1,9,12,13]. In combination with prednisone, abiraterone has demonstrated favorable outcomes in metastatic CRPC patients who have progressed with docetaxel[14] and in the pre-chemotherapy setting as well[15].
However, as of today the optimal sequence for abiraterone in relationship to chemotherapy is unknown, with a clear absence of evidence-based guidance[16]. We conducted a meta-analysis of RCTs to compare the efficacy and safety of abiraterone in the pre- and post-chemotherapy settings.
The PubMed database (http://www.pubmed.gov) was independently searched from January 1st 2008 to March 31st 2014 using the key words “abiraterone” and “metastatic castrate resistant prostate cancer” or “metastatic castration resistant prostate cancer”. In addition, we searched abstracts presented at the American Society of Clinical Oncology annual conferences from 2010 to 2014 using the same keywords. Selected abstracts and publications were reviewed for complete safety and efficacy information, and verified so that the most recent and up to date version was identified.
The goal of this study was to determine the impact of prior chemotherapy on the efficacy and toxicity of abiraterone in the treatment of metastatic CRPC. Therefore, we have included RCTs in which abiraterone plus prednisone was compared to placebo plus prednisone in patients with and without prior chemotherapy. All non-RCTs including phase 1 trials and single-arm phase 2 trials were excluded. Trials that met the following criteria were chosen for analysis: phase II or III prospective RCTs, patients with metastatic CRPC, and patients assigned to treatment with abiraterone plus prednisone or placebo plus prednisone. To assess study quality, the 7-item scale (score 0-5) Jadad Score was used for each included clinical trial[17].
Efficacy end points included overall survival (OS), defined from the date of randomization to the date of death from all causes, prostate-specific antigen (PSA) progression-free survival (PFS), and Radiographic-PFS. Radiographic-PFS was defined as progression on bone scanning defined by the Prostate Cancer Working Group 2 (PCWG2) criteria[18] or progressive soft-tissue lesions measured with the use of computed tomography or magnetic resonance imaging according to the modified Response Evaluation Criteria in Solid Tumors (RECIST) criteria[19]. PSA progression was defined by the PCWG2 criteria[18]. Additional clinical endpoints included PSA response rate (≥ 50% decline in PSA level from baseline) and rate of objective response based on the RECIST criteria[18,19].
Safety endpoints were based on the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 3.0. Hypokalemia was categorized as grade 1: < 3.5 mmol/L, grade 3: < 3.0-2.5 mmol/L and, grade 4: < 2.5 mmol/L. Hypertension was categorized grade 1; asymptomatic, transient (< 24 h) increase by > 20 mmHg (diastolic) or to > 150/100 if previously within normal limit with no need for intervention, grade 2; recurrent or persistent (≥ 24 h) or symptomatic increase by > 20 mmHg (diastolic) or to > 150/100 if previously within normal limit; with monotherapy may be indicated, grade 3; requiring more than one drug or more intensive therapy than previously, and grade 4; life-threatening consequences such as hypertensive crisis. We also extracted safety data for various cardiac disorders: ischemic heart disease, myocardial infarction, supra-ventricular arrhythmia, ventricular tachyarrhythmias, cardiac failure, signs and symptoms. Edema and fluid retention was also included and was derived from edema-related adverse effects from sites such head and neck, trunk and genitals, limbs, and viscera.
All statistical analyses were performed using Comprehensive MetaAnalysis program version 2.0 (Biostat, Englewood, New Jersey, United States). Hazard ratios (HR), the median time of OS, time to PSA-PFS, time to Radiographic-PFS of patients in months, along with the proportion of patients showing PSA response rate and rate of objective response in both arms were extracted from the included clinical trials. Safety data-were collected included the number of patients with: all-grade and high-grade cardiac disorders, all-grade and high-grade hypertension, all-grade and high-grade fluid retention and edema, all-grade and high-grade hypokalemia respectively. Collected data were entered into a Microsoft Excel sheet. For each study, the proportion of patients, the relative risk (RR) was calculated and the 95%CI was derived. Because the two clinical trials were designed to have a control arm, the relative risk of safety outcome among patients assigned to abiraterone was calculated and compared to patients assigned to the control arm; in addition, the specific effect of abiraterone was calculated based on the incidence or response rate difference between abiraterone plus prednisone and placebo plus prednisone. For meta-analysis, both fixed-effects (weighted with inverse variance) and random-effects model were considered. Prior to the meta-analysis, Cochran’s Q statistic and I2 were calculated to assess the heterogeneity among the proportions of the included trials. For a P value of < 0.1, the assumption of homogeneity was considered invalid. If invalid the random-effects model was used. If the assumption of homogeneity was valid both the fixed-effects and random-effects model results were reported. A two-tailed P value of < 0.05 was considered to be statistically significant.
Our literature search generated a total of 178 potentially relevant studies of abiraterone and metastatic CRPC. From these studies a total of 7 RCTs were identified, where 5 of them were excluded due to concomitant treatment with other agents [OGX-427, Sipuleucel-T, Trebanib (AMG-386), GDC-0068/GDC-0980]. Overall, two phase III RCTs were included in our final analysis[14,15] (Figure 1). Both trials included patients with metastatic CRPC; histologically or cytologically confirmed prostate cancer with disease progression based on PCWG2 criteria and ongoing androgen deprivation, with a serum testosterone level of ≤ 50 ng/dL[14,15]. In the COU-AA-301 trial, included patients had Eastern Cooperative Oncology Group (ECOG) performance status scores of ≤ 2 with established disease progression after receiving docetaxel (Table 1). COU-AA-302 included chemotherapy-naïve patients with disease progression, with good performance status (ECOG status ≤ 1) and Brief Pain Inventory-Short Form (BPI-SF) score of 0-3 (n = 1088). In an independent review, the Jadad Score was calculated for each included trial (Table 1). The COU-AA-301 study was given the highest Jadad score of 5, while COU-AA-302 was given a score of 4, based on the 7-item scale. In the COU-AA-302 trial, radiographic progression-free survival was measured by the study investigators, rather than by a blinded review[15].
| Clinical trial characteristics | COU-AA-302[15] | COU-AA-301[14] |
| Inclusion criteria | (1) Age ≥ 18 yr; (2) Confirmed metastatic disease; (3) Histologically or cytologically confirmed Prostate Adeno-CA; (4) PSA progression (PCWG2) or radiograph progression w/ or w/o PSA progression; (5) Testosterone < 50 ng/dL; (6) ECOG 0 or 1; (7) BPI-SF 0-3 | (1) Confirmed metastatic disease; (2) Histologically or cytologically confirmed Prostate Adeno-CA; (3) PSA progression (PCWG2) or radiograph progression w/ or w/o PSA progression; (4) Testosterone < 50 ng/dL; (5) ECOG ≤ 2; (6) previous therapy with docetaxel |
| Exclusion criteria | (1) Visceral metastasis; (2) Previous use of ketoconazole lasting > 7 d | (1) Elevated LFT (> 2.5 ULN) (2) Previous ketoconazole therapy; (3) Viral hepatitis; (4) Chronic liver disease; (5) Uncontrolled HTN; (6) Pituitary or adrenal dysfunction |
| Eligible patients | 546 (abiraterone vs control) | 797 |
| Study arm medication and dose | Abiraterone Acetate 1 gm OD + Prednisone 5 mg BID | |
| Jadad Score | 4 | 5 |
| Median overall survival: Abiraterone vs Placebo (mo) | Not reached vs 27.2 | 15.8 vs 11.2 |
| Median time to PSA PFS: Abiraterone vs Placebo (mo) | 11.1 vs 5.6 | 8.5 vs 6.6 |
| Median time to Radiographic PFS: Abiraterone vs Placebo (mo) | 16.5 vs 8.3 | 5.6 vs 3.6 |
| Median follow up time: Abiraterone vs Placebo (mo) | 22.2 | 12.8 |
A total of 2283 patients diagnosed with metastatic CRPC from the two RCTs were available for the analysis. A total of 1343 (58.3%) patients received the approved United States Food and Drug Administration abiraterone oral dose of 1 g daily with 5 mg of prednisone twice daily by mouth. Finally, 546 patients in the pre-chemotherapy and 797 in the post-chemotherapy arms were included in the meta-analysis (Table 2). In the post-chemotherapy trial, 70% in the abiraterone arm and 69% in the control arm received one previous cytotoxic chemotherapy regimen and approximately 30% in the abiraterone arm and 31% in the control arm received two distinct previous regimens[14]. All included patients received at least one previous cytotoxic chemotherapy regimen containing docetaxel: docetaxel after a treatment break, single therapy with docetaxel, or docetaxel in combination with other agents[14].
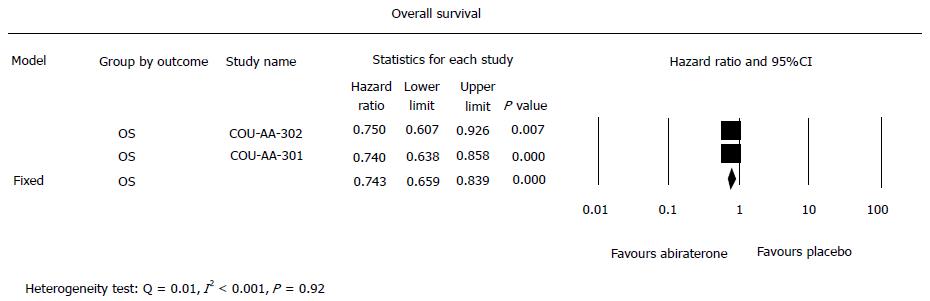
Based on the meta-analysis of the two trials (Figure 2), the summary hazard ratio of OS associated with abiraterone acetate in comparison with the placebo yielded an increase in the OS (HR = 0.74, 95%CI: 0.66-0.84, P≤ 0.001). An increase in OS was also seen in the pre-chemotherapy study (HR = 0.75, 95%CI: 0.61-0.93, P < 0.01) and post-chemotherapy study (HR = 0.65, 95%CI: 0.54-0.78, P < 0.001) respectively. However there was no significant difference in HR between the two settings (Heterogeneity test: Q = 0.01, I2 < 0.001, P = 0.92).
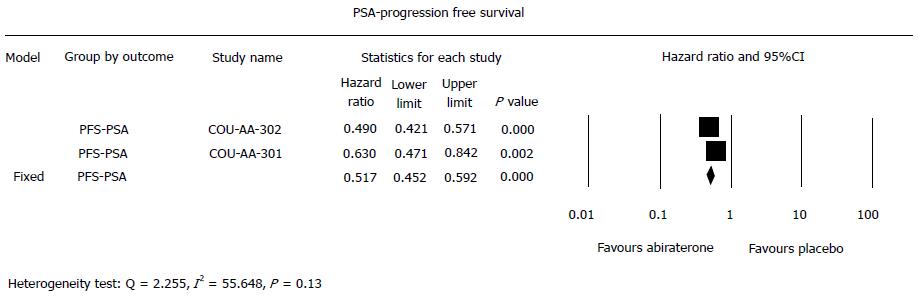
Overall, abiraterone in comparison to placebo significantly reduced the risk of PSA-PFS (HR = 0.52, 95%CI: 0.45-0.59, P < 0.001), which was also seen in the pre-chemotherapy (HR = 0.49, 95%CI: 0.42-0.57, P < 0.001) and post-chemotherapy settings (HR = 0.63, 95%CI: 0.47-0.84, P = 0.002). Even though HR with pre-chemotherapy was lower than post-chemotherapy (Figure 3), there was no significant difference in the risk reduction of PSA-PFS associated with abiraterone between the two settings (Heterogeneity test: Q = 2.255, I2 = 55.648, P = 0.13).
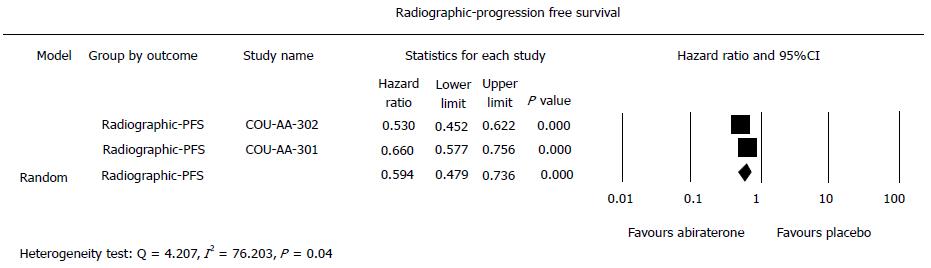
As shown in Figure 4, radiographic-PFS was significantly increased with abiraterone in comparison with placebo (summary HR = 0.60, 95%CI: 0.48-0.75, P < 0.001) in the pre-chemotherapy setting (HR = 0.53, 95%CI: 0.45-0.62, P < 0.001) and the post-chemotherapy setting (HR = 0.67, 95%CI: 0.58-0.78, P < 0.001). There was significant difference between the pre- and post-chemotherapy settings (Heterogeneity test: Q = 4.207, I2 = 76.203, P = 0.035).
Overall, abiraterone compared to placebo significantly increased PSA response rate (summary RR = 3.62, 95%CI: 1.78-7.40, P < 0.001) in the pre-chemotherapy setting (RR = 2.58, 95%CI: 2.19-3.04, P < 0.001) and the post-chemotherapy setting (RR = 5.36, 95%CI: 3.52-8.17, P < 0.001). There was significant difference between the pre-chemotherapy setting and the post-chemotherapy setting (Heterogeneity test: Q = 4.207, I2 = 76.203, P = 0.04).
In addition, we determined the specific effect of abiraterone on PSA response (PSA response rate of abiraterone and prednisone minus placebo and prednisone in the same trial). As shown in Table 2, pre-chemotherapy (38.0%, 95%CI: 34.0%-42.1%) had significantly higher PSA response rate attributable to abiraterone (P < 0.001) than post-chemotherapy (24.0%, 95%CI: 21.2%-27.1%).
The rate of object response with abiraterone in comparison to placebo, was significantly increased (summary RR = 3.02, 95%CI: 1.55-5.90, P < 0.001). The difference between the post-chemotherapy setting (RR = 4.49, 95%CI: 2.57-7.830, P < 0.001) and the pre-chemotherapy setting (RR = 2.25, 95%CI: 1.80-2.81, P < 0.001) significantly favored the pre-chemotherapy setting (Heterogeneity test: Q = 4.207, I2 = 76.203, P = 0.04). In addition, we determined the specific effect of abiraterone on objective response (objective response rate of abiraterone and prednisone minus placebo and prednisone). As shown in Table 2, there was significant difference (P < 0.001) in objective response rate attributable to abiraterone between pre-chemotherapy (20.0%, 95%CI: 16.9%-23.6%) and post-chemotherapy (11.5%, 95%CI: 9.5%-13.9%).
Overall, abiraterone significantly increased the risk of all-grade cardiac disorders (RR = 1.25, 95%CI: 1.02-1.52, P = 0.03), fluid retention and edema (RR = 1.27, 95%CI: 1.08-1.49, P =0.004), hypertension (RR = 1.58, 95%CI: 1.27-1.98), and hypokalemia (RR = 1.60, 95%CI: 1.06-2.42, P = 0.027). In addition, abiraterone significantly increased the risk of high-grade cardiac disorders (RR = 2.17, 95%CI: 1.37-3.45), but not high-grade fluid retention and edema, hypertension, and hypokalemia (Table 3) based on the summary results of the two trials. Subgroup analyses showed there was significant difference between pre- and post-chemotherapy in the risk of high-grade fluid retention and edema (P = 0.02) and hypokalemia (P = 0.02) associated with abiraterone.
| Adverse events | Pre-chemotherapyincidence (95%CI) | Post-chemotherapyincidence (95%CI) |
| Cardiac disorders all-grade | 3.0% (1.9%-4.8%) | 4.0% (2.8%-5.6%) |
| Fluid retention and edema all-grade | 4.0% (2.6%-6.0%) | 9.0% (7.2%-11.2%)b |
| Hypertension all-grade | 9.0% (6.9%-11.7%) | 3.0% (2.0%-4.4%)b |
| Hypokalemia all-grade | 9.0% (7.2%-11.2%) | 4.0% (2.6%-6.0%)b |
| Hypokalemia high-grade | 0.1% (1.0%-1.5%) | 3.7% (2.6%-5.2%)b |
We further determined the incidence rate attributable to abiraterone (incidence rate of abiraterone and prednisone minus placebo and prednisone). The summary incidence of all-grade fluid retention and edema attributable to abiraterone was 6.1% (95%CI: 2.7%-13.2%). Post-chemotherapy was associated with significantly higher risk of all-grade fluid retention/edema attributable to abiraterone than pre-chemotherapy (9.0% vs 4.0%, P < 0.001). Similarly, post-chemotherapy was associated with significantly higher risk of all-grade hypokalemia attributable to abiraterone than pre-chemotherapy (9.0% vs 4.0%, P < 0.001). In contrast, pre-chemotherapy was associated with significantly higher risk of all-grade hypertension attributable to abiraterone than post-chemotherapy (3.0% vs 9.0%, P < 0.001).
Our meta-analysis has demonstrated that pre-chemotherapy may affect the efficacy and toxicity of abiraterone treatment in patients with metastatic CRPC. Abiraterone was associated with significantly increased radiographic-PFS, objective response rate, and PSA response rate in the pre-chemotherapy setting when compared to the post-chemotherapy setting. However, its effects on OS (P = 0.92) and PSA-PFS (P = 0.13) were not significantly different between the two settings. In addition, abiraterone in the pre-chemotherapy setting had a significant lower risk of all-grade fluid retention and edema (P < 0.001), and hypokalemia (P < 0.001), but had a higher risk of all-grade hypertension (P < 0.001) when compared to post-chemotherapy.
In the setting of metastatic CRPC, the optimal sequence of abiraterone in relation to chemotherapy to achieve the greatest survival benefit is a topic of emerging interest. In a previous meta-analysis of abiraterone for treatment of metastatic CRPC, abiraterone compared to placebo significantly prolonged OS and radiographic-PFS[20]. These results support the efficacy of abiraterone in metastatic CRPC: this however did not ascertain the differences in the pre-chemotherapy and post-chemotherapy settings. This highlights the significance of our results, which provides verification based on well-designed RCTs of the clinical benefits of administering abiraterone early in the metastatic CRPC disease course. Even though, we did not appreciate a significant difference in OS, differences in validated clinical efficacy outcomes were seen. The PCWG2 criteria (used in both COU-AA-301 and COU-AA-302) for disease progression is highly reproducible and associated with OS[18,21]. Radiographic progression is defined as the presence of two or more new lesions on the bone scan, where the first post-treatment bone scan is not used to make treatment decisions to prevent treatment discontinuation due to disease flare as opposed to a true progression[18,21]. Rate of objective response and PSA response rate were also improved in the pre-chemotherapy setting. Definitions for both rate of objective response and PSA response rate were similar in both trials, thus our results are likely meaningful[14,15]. An increase in OS that did not reach statistical significance in our analysis could be explained by the early un-blinding of the COU-AA-302 trial at the time the pre-specified 43% total number of events occurred[15]. A sufficient duration for evaluation of OS likely requires a longer follow-up, highlighted by a lack of survival differences seen in the COU-AA-302 until follow-up of 12 mo[15].
The ease of oral administration, once a day dosing, and favorable safety profile of abiraterone make it an attractive therapy in metastatic CRPC[3]. In the COU-AA-301 trial, abiraterone administered with low-dose prednisone was shown to increase radiographic-PFS with a trend toward improved OS in the post-docetaxel chemotherapy group[14]. In the COU-AA-302 trial, abiraterone administered with prednisone in the metastatic CRPC pre-chemotherapy setting significantly improved OS, and prolonged radiographic-PFS, time to PSA progression, and rate of objective response[15]. Despite favorable results, assurances on when and how to utilize abiraterone in clinical practice is imperfect. Patients with ECOG scores of ≥ 2 comprised only 10% of the COU-AA-301 study population, and were entirely excluded from the COU-AA-302 trial[14,15]. In a retrospective analysis, poor performance status (ECOG ≥ 2) was shown to be associated with poor survival outcomes in both pre-chemotherapy and post-docetaxel patients treated with abiraterone[12]. This highlights the benefit of early initiation of abiraterone prior to deterioration of performance status after administration of taxane chemotherapy.
Cross-resistance, where sensitivity to one compound is impaired by another compound with a similar mechanism of action is a concern with taxanes and abiraterone due to common activity on AR nuclear transport[22]. Docetaxel, a taxane, acts principally through microtubule stabilization but has a unique mechanism of action in metastatic CRPC; AR translocation inhibition in response to androgens and ligand-independent pathways, and down-expression of AR[6,10]. Pre-clinical data has suggested that the efficacy of docetaxel to perform such actions may be diminished by prior abiraterone androgen targeted therapy[10,23]. However, a retrospective analysis of a phase III RCT did not show prior androgen synthesis inhibition with ketoconazole to negatively impact clinical outcomes with subsequent docetaxel chemotherapy[24]. Unfortunately, because the COU-AA-302 trial underwent un-blinding prior to reaching a significant difference in OS, concerns for cross-resistance have not fully been put to rest[14]. However, the significant impact of pre-chemotherapy on the efficacy of abiraterone as shown in this study supported the notion of cross-resistance between docetaxel and abiraterone.
Compared to chemotherapy, abiraterone is less toxic with a relatively low incidence of high-grade adverse effects, and can be tolerated for an extended period in metastatic CRPC patients. The importance of tolerability is especially important for the metastatic CRPC patient population given their advanced age and multiple co-morbidities. In these trials abiraterone was well tolerated with the majority of adverse events being low-grade[14,15]. Abiraterone discontinuation rates in the COU-301 and COU-302 trials were 19% and 10% respectively[14,15].
Abiraterone inhibition of CYP-17 leads to an inhibition of both androgen and glucocorticoid synthesis, where the latter causes an elevation of adrenocorticotropic hormone (ACTH) and excess mineralocorticoid activity[9]. Increased mineralocorticoid activity is the mechanism for many of abiraterone’s adverse effects including hypokalemia, fluid retention and edema, and hypertension. Caution is advised in patients with renal failure, metabolic disturbances, and congestive heart failure[9].
To minimize the incidence of these adverse effects, abiraterone is concomitantly administered with prednisone[9]. Early clinical data has shown that abiraterone can be administered safely without prednisone, and mineralocorticoid adverse effects can be managed with eplerenone, a non-steroidal mineralocorticoid antagonist[11]. Interestingly, our analysis has shown significantly lower incidence of fluid retention and edema and hypokalemia and a higher incidence of hypertension in the pre-chemotherapy setting compared to the post-chemotherapy setting. This is also consistent with lower discontinuation rate of abiraterone in the pre-chemotherapy setting versus post-chemotherapy (10% vs 19%).
To our knowledge, this study is the first to comment on differences in abiraterone toxicity in relation to sequencing with chemotherapy. Both docetaxel and abiraterone can cause fluid retention edema, where post-chemotherapy abiraterone treatment may lead to an additive risk for fluid retention and edema. Differences in the risk of hypokalemia and hypertension with abiraterone in relation to docetaxel that does share the same mineralocorticoid related toxicity profile requires elucidation. It is possible that chemotherapy or cancer progression may decrease food intake leading to increased risk of hypokalemia and reduced risk of hypertension.
In metastatic CRPC, the specific choice of agent and its timing should be made on individual personalized assessment, and should incorporate multiple factors including clinical and symptomatic disease burden, tolerability, side-effect profile, performance status, and physician experience[9,25,26]. For example, patients who are given abiraterone prior to docetaxel may not be able to tolerate subsequent chemotherapy[10]. This prevents patients from receiving chemotherapy that has been proven to have a survival benefit. Abiraterone should be considered as a first-line choice in patients who cannot tolerate chemotherapy or have asymptomatic disease[25]. Opting to use docetaxel chemotherapy prior to abiraterone would be beneficial for patients who have rapid progressive disease such as with visceral metastasis, high Gleason score, rapid PSA doubling time, or with an initial poor response to androgen deprivation therapy[25,27].
This meta-analysis has several limitations. Our findings may be limited by sample size and the accuracy of individual RCTs to assess primary and secondary outcomes that may affect our ability to discern differences in efficacy of abiraterone in the pre-chemotherapy and post-chemotherapy settings. Results regarding differences in toxicity for these two settings may also have been affected by the ability of individual RCTs to correctly grade the severity of adverse events based on NCI-CTCAE version 3.0. Sub-optimal classification could have erroneously estimated the incidence of all-grade and high-grade adverse events in this meta-analysis. Selection bias based on the exclusion of patients with poor baseline ECOG performance status as mentioned earlier may have limited the generalizability and clinical application of our results to the general population. Also mentioned previously, our results may have been effected by the early un-blinding of the COU-AA-301 trial.
In conclusion, our meta-analysis of RCTs has demonstrated that abiraterone is associated with a significantly improved radiographic-PFS, objective response rate, PSA response rate in the pre-chemotherapy setting when compared to the post-chemotherapy setting in patients with metastatic CRPC. This emphasizes that these patients may obtain the greatest clinical benefit with early treatment of androgen synthesis inhibition with abiraterone. Further studies may be necessary to determine the effectiveness of abiraterone in the pre-chemotherapy setting in comparison with other new agents including the androgen signaling pathway inhibitor enzalutamide or vaccines.
Chemotherapy with docetaxel plays an important role in the treatment of metastatic castration-resistant prostate cancer (CRPC). Abiraterone, an androgen biosynthesis inhibitor, has been shown to improve overall survival in metastatic CRPC patients who have not received previous chemotherapy. It is not clear that prior chemotherapy affects the effectiveness of abiraterone. The authors performed a meta-analysis to compare the efficacy and safety of abiraterone in patients with and without prior chemotherapy.
In the setting of metastatic CRPC, the optimal sequence of abiraterone in relation to chemotherapy to achieve the greatest survival benefit is a topic of emerging interest. In a previous meta-analysis of abiraterone for treatment of metastatic CRPC, abiraterone compared to placebo significantly prolonged OS and radiographic-PFS. These results support the efficacy of abiraterone in metastatic CRPC.
This meta-analysis has demonstrated that pre-chemotherapy may affect the efficacy and toxicity of abiraterone treatment in patients with metastatic CRPC. Abiraterone was associated with significantly increased radiographic-PFS, objective response rate, and prostate-specific antigen (PSA) response rate in the pre-chemotherapy setting when compared to the post-chemotherapy setting.
Abiraterone should be considered as a first-line choice in patients who cannot tolerate chemotherapy or have asymptomatic disease. However studies with larger sample size and are required to compare abiraterone in pre and post chemotherapy setting and to compare the efficacy with other new androgen signaling blocking agents.
Radiographic-PFS was defined as progression on bone scanning based on the Prostate Cancer Working Group 2 (PCWG2) criteria or progressive soft-tissue lesions measured with the use of computed tomography or magnetic resonance imaging according to the modified Response Evaluation Criteria in Solid Tumors. PSA progression is defined as a a 25% or greater increase and an absolute increase of 2 ng/mL or more from the nadir is documented and confirmed by a second value obtained 3 or more weeks later, also based on the PCWG2 criteria.
The study was clinically relevant and addressed the important topic of abiraterone sequencing in metastatic castrate resistant prostate cancer. The article provides a succinct yet relevant discussion about the impact of prior chemotherapy in the treatment of this clinical entity.
P- Reviewer: Garfield D, Kabir A, Vetvicka V S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Tan PS, Haaland B, Montero AJ, Kyriakopoulos CE, Lopes G. Hormonal Therapeutics Enzalutamide and Abiraterone Acetate in the Treatment of Metastatic Castration-Resistant Prostate Cancer (mCRPC) Post-docetaxel-an Indirect Comparison. Clin Med Insights Oncol. 2014;8:29-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Ryan CJ, Molina A, Li J, Kheoh T, Small EJ, Haqq CM, Grant RP, de Bono JS, Scher HI. Serum androgens as prognostic biomarkers in castration-resistant prostate cancer: results from an analysis of a randomized phase III trial. J Clin Oncol. 2013;31:2791-2798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Liu JJ, Zhang J. Sequencing systemic therapies in metastatic castration-resistant prostate cancer. Cancer Control. 2013;20:181-187. [PubMed] |
| 4. | Agarwal N, Di Lorenzo G, Sonpavde G, Bellmunt J. New agents for prostate cancer. Ann Oncol. 2014;25:1700-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Huang X, Chau CH, Figg WD. Challenges to improved therapeutics for metastatic castrate resistant prostate cancer: from recent successes and failures. J Hematol Oncol. 2012;5:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Shafi AA, Yen AE, Weigel NL. Androgen receptors in hormone-dependent and castration-resistant prostate cancer. Pharmacol Ther. 2013;140:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 293] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 7. | Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4284] [Cited by in RCA: 4356] [Article Influence: 207.4] [Reference Citation Analysis (0)] |
| 8. | Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2824] [Cited by in RCA: 2815] [Article Influence: 134.0] [Reference Citation Analysis (0)] |
| 9. | Chi KN, Bjartell A, Dearnaley D, Saad F, Schröder FH, Sternberg C, Tombal B, Visakorpi T. Castration-resistant prostate cancer: from new pathophysiology to new treatment targets. Eur Urol. 2009;56:594-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Fitzpatrick JM, de Wit R. Taxane mechanisms of action: potential implications for treatment sequencing in metastatic castration-resistant prostate cancer. Eur Urol. 2014;65:1198-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Zhang TY, Agarwal N, Sonpavde G, DiLorenzo G, Bellmunt J, Vogelzang NJ. Management of castrate resistant prostate cancer-recent advances and optimal sequence of treatments. Curr Urol Rep. 2013;14:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Azad AA, Eigl BJ, Leibowitz-Amit R, Lester R, Kollmannsberger C, Murray N, Clayton R, Heng DY, Joshua AM, Chi KN. Outcomes with abiraterone acetate in metastatic castration-resistant prostate cancer patients who have poor performance status. Eur Urol. 2015;67:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Goyal J, Antonarakis ES. Clinical Evaluation of Abiraterone in the Treatment of Metastatic Prostate Cancer. Clin Med Insights Urol. 2013;2013:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2008] [Cited by in RCA: 2156] [Article Influence: 179.7] [Reference Citation Analysis (0)] |
| 15. | Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, Staffurth JN, North S, Vogelzang NJ, Saad F. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 1053] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 16. | El-Amm J, Aragon-Ching JB. The changing landscape in the treatment of metastatic castration-resistant prostate cancer. Ther Adv Med Oncol. 2013;5:25-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12275] [Cited by in RCA: 12889] [Article Influence: 444.4] [Reference Citation Analysis (1)] |
| 18. | Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148-1159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1845] [Cited by in RCA: 1820] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 19. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21632] [Article Influence: 1352.0] [Reference Citation Analysis (1)] |
| 20. | Zhou ZR, Liu SX, Zhang TS, Xia J, Li B. Abiraterone for treatment of metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:1313-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Morris MJ, Autio KA, Basch EM, Danila DC, Larson S, Scher HI. Monitoring the clinical outcomes in advanced prostate cancer: what imaging modalities and other markers are reliable? Semin Oncol. 2013;40:375-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | van Soest RJ, van Royen ME, de Morrée ES, Moll JM, Teubel W, Wiemer EA, Mathijssen RH, de Wit R, van Weerden WM. Cross-resistance between taxanes and new hormonal agents abiraterone and enzalutamide may affect drug sequence choices in metastatic castration-resistant prostate cancer. Eur J Cancer. 2013;49:3821-3830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 23. | Mezynski J, Pezaro C, Bianchini D, Zivi A, Sandhu S, Thompson E, Hunt J, Sheridan E, Baikady B, Sarvadikar A. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Ann Oncol. 2012;23:2943-2947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 24. | Aggarwal R, Halabi S, Kelly WK, George D, Mahoney JF, Millard F, Stadler WM, Morris MJ, Kantoff P, Monk JP. The effect of prior androgen synthesis inhibition on outcomes of subsequent therapy with docetaxel in patients with metastatic castrate-resistant prostate cancer: results from a retrospective analysis of a randomized phase 3 clinical trial (CALGB 90401) (Alliance). Cancer. 2013;119:3636-3643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Bahl A, Masson S, Birtle A, Chowdhury S, de Bono J. Second-line treatment options in metastatic castration-resistant prostate cancer: a comparison of key trials with recently approved agents. Cancer Treat Rev. 2014;40:170-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Asselah J, Sperlich C. Post-docetaxel options for further survival benefit in metastatic castration-resistant prostate cancer: Questions of choice. Can Urol Assoc J. 2013;7:S11-S17. [PubMed] |
| 27. | Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 960] [Cited by in RCA: 1047] [Article Influence: 87.3] [Reference Citation Analysis (0)] |