Published online Dec 10, 2014. doi: 10.5306/wjco.v5.i5.865
Revised: February 10, 2014
Accepted: April 16, 2014
Published online: December 10, 2014
Processing time: 348 Days and 14.5 Hours
Lung cancer is the leading cause of cancer-related mortality throughout the world. Non-small cell lung cancer (NSCLC) accounts for 85% of all diagnosed lung cancers. Despite considerable progress in the diagnosis and treatment of the disease, the overall 5-year survival rate of NSCLC patients remains lower than 15%. The most common causes of death in lung cancer patients are treatment failure and metastasis. Therefore, developing novel strategies that target both tumour growth and metastasis is an important and urgent mission for the next generation of anticancer therapy research. Heat shock proteins (HSPs), which are involved in the fundamental defence mechanism for maintaining cellular viability, are markedly activated during environmental or pathogenic stress. HSPs facilitate rapid cell division, metastasis, and the evasion of apoptosis in cancer development. These proteins are essential players in the development of cancer and are prime therapeutic targets. In this review, we focus on the current understanding of the molecular mechanisms responsible for HLJ1’s role in lung cancer carcinogenesis and progression. HLJ1, a member of the human HSP 40 family, has been characterised as a tumour suppressor. Research studies have also reported that HLJ1 shows promising dual anticancer effects, inhibiting both tumour growth and metastasis in NSCLC. The accumulated evidence suggests that HLJ1 is a potential biomarker and treatment target for NSCLC.
Core tip: HLJ1, a member of the human heat shock proteins 40 family, has been characterised as a tumour suppressor. Research studies have reported that HLJ1 shows promising dual anticancer effects, inhibiting both tumour growth and metastasis in non-small cell lung cancer (NSCLC). The accumulated evidence suggests that HLJ1 is a potential biomarker and treatment target for NSCLC. We propose a hypothetical model for the roles of HLJ1 stimulator in suppressing lung cancer tumourigenesis. Investigating the integrated and coordinated molecular mechanisms of HLJ1 may shed new light on the treatment of lung cancer. The development of drug targeting HLJ1 may be an effective approach for lung cancer therapy.
- Citation: Tsai MF, Wang CC, Chen JJ. Tumour suppressor HLJ1: A potential diagnostic, preventive and therapeutic target in non-small cell lung cancer. World J Clin Oncol 2014; 5(5): 865-873
- URL: https://www.wjgnet.com/2218-4333/full/v5/i5/865.htm
- DOI: https://dx.doi.org/10.5306/wjco.v5.i5.865
Lung cancer is the most common cause of cancer death in the world, accounting for 17% of all cancer deaths[1,2]. Non-small cell lung cancer (NSCLC) is the predominant type of lung cancer[3]. The three major types of NSCLC are squamous cell carcinoma, large cell carcinoma, and adenocarcinoma, but several other types occur less frequently[4]. Traditional therapeutic strategies (chemotherapy and radiotherapy) are often associated with unsatisfactory outcomes in lung cancer patients, and the problem is exacerbated by early detection difficulties[5]. A minority of patients (approximately 30%) with NSCLC present with an early stage of the disease and receive curative surgery. However, even in those patients, up to 40% will subsequently relapse within 5 years[6,7]. Targeted molecular therapy has become an important part of therapeutic strategies for treating lung cancer. However, the major challenges confronting targeted cancer therapies are variable responsiveness and the development of drug resistance[8]. There have been significant advances in cancer treatments owing to our knowledge of the mechanisms underlying cell signalling pathways; however, the prognosis for patients with locally advanced or metastatic disease is still ominous.
If lung cancer is diagnosed and treated before it metastasizes, the 5-year survival rate is approximately 50%-70%. Once metastasis has occurred, the 5-year survival rate drops to < 5%[9]. Therefore, metastasis is the most critical parameter determining survival in lung cancer patients[10]. Improving survival in lung cancer is a major challenge for modern oncology, particularly considering the 5-year survival rate remains under 15% across all stages of disease[11]. Metastasis, the spread of tumour cells from their primary sites to secondary sites within the body, is a multiple-step process that requires the accumulation of altered expression of many different genes. This complex process involves cell adhesion, degradation of the surrounding extracellular matrix, migration, proliferation at a secondary site, and stimulation of angiogenesis[12,13]. Many studies on cancer metastasis have been conducted, and several molecules that participate in tumour cell invasion and metastasis, such as SLUG, NM23, CD44, MTA1, MMPs, TIMPs, KAI1, E-cadherin, KISS1 and CRMP1, have been identified in various cancer types[14-18]. There is still no effective strategy for preventing or combating these metastatic processes. Therefore, it is important to develop novel strategies that target both tumour growth and metastasis in NSCLC.
In this review, we focus on the current understanding of the molecular mechanisms responsible for the effects of HLJ1 in lung cancer. The role of HLJ1, a member of the human heat shock protein 40 (HSP40) family, in lung cancer development and progression has been extensively investigated[19-21]. HLJ1 has been characterised as a tumour suppressor, and research studies have identified the protein’s promising dual anticancer effects in NSCLC, inhibiting both tumour growth and metastasis[19,22,23]. The accumulated evidence suggests that HLJ1 is a potential biomarker for NSCLC and a potential target of drug development.
The heat shock response was first described in 1962[24], and a number of investigations have revealed that the process is an essential defence mechanism for cellular viability. HSPs are named for their increased synthesis after heat shock. In addition to elevated temperature, HSPs are markedly induced by nutrient deprivation, oxidative stress, heavy metals, radiation, pathogen infection and other stress factors[25]. Under normal conditions, HSPs perform essential biological functions such as modulating protein activity by changing protein conformation, serving as molecular chaperones in protein transport between cell organelles, promoting multiprotein complex assembly/disassembly, and ensuring proper folding of nascent and altered proteins[25,26]. Many other more specific functions have been identified for particular HSP types and include roles in immunological processes, cell cycle regulation, transcriptional activation and signal transduction[27-29].
Mammalian HSPs have been classified into the following six families according to molecular size: HSP100, HSP90, HSP70, HSP60, HSP40 and small HSPs[30]. HSPs play fundamental roles in the processes of signal transduction, cell proliferation and survival, cell cycle progression and apoptosis, and in other features of malignant cells, including invasion, tumour angiogenesis and metastasis[28,29]. In recent years, research studies have identified several different HSPs, in a variety of tumour types, that may be putative clinical biomarkers or molecular targets for cancer therapy. The dependence of cancer cells on HSP90 has been successfully exploited in therapeutics[30,31]. While HSP40 therapies are in the nascent stages, HSP27 and HSP70 therapies have been successfully used with HSP90 inhibitors as part of dual inhibition treatments and with antineoplastic drugs in combinational therapy[32].
The HSP40 family, also known as DNAJ proteins, constitutes the largest and most diverse sub-group of HSPs[33]. All DNAJ proteins contain the 70 amino acid J domain, which is essential for interaction with HSP70. The DNAJ proteins that have been identified in humans are divided into three subclasses. Based on the presence or absence of conserved domains, DNAJ proteins are classified as type I (DNAJA, 4 members), type II (DNAJB, 13 members) or type III (DNAJC, 32 members)[34]. DNAJ proteins are widely recognised as regulators of HSP70 function, but they also have roles as co-chaperones in the HSP90 multi-chaperone complex[35]. The function and role of DNAJ proteins is still poorly understood. Current research is focused on understanding the role of HSP40 isoforms in regulating HSP70 and their subsequent involvement in disease progression.
The DNAJ proteins are differentially expressed in human tissues and can act as both tumour suppressors and onco-proteins[19,36,37]. There are approximately 49 genes that encode DNAJ proteins, and several of these genes encode for multiple splice variants that may have different biological functions and cellular locations[34]. Tid1 (DNAJA3) has 2 isoforms, Tid1-L and Tid1-S, which may function differently. Tid1-L acts as a tumour suppressor by negatively regulating cell proliferation, cell invasion, and tumorigenicity in breast cancer, lung cancer, and head and neck squamous cell carcinoma[36,38,39]. Tid1-L overexpression in lung cancer cell attenuates epidermal growth factor receptor (EGFR) signalling and inhibits cell proliferation, colony formation, and in vivo tumour growth. Low Tid1-L/high EGFR expression predicts poor overall survival in patients with lung adenocarcinoma. Tid1-L has been shown to act as a tumour suppressor (though Tid1-S has not) by inhibiting EGFR signalling through interaction with EGFR/HSP70/HSP90 and by enhancing EGFR ubiquitinylation and degradation[36]. Research studies have also suggested that Tid1-L is a novel regulator of p53-mediated apoptosis and that the use of the enhanced Tid1-L function to promote mitochondrial localisation of p53 could be an effective therapy in many cancers[40]. In contrast, overexpression of Tid1-S in renal clear cell carcinomas enhances MetR kinase activity, leading to an increase in hepatocyte growth factor-mediated cell migration. The binding of Tid1-S to MetR may stabilise the receptor in a ligand-competent state, and this stabilising function may influence conformational changes that take place during the catalytic cycle to promote kinase activation. Targeted inhibition of Tid1-S may be a useful therapeutic tool in the management of MetR-dependent malignancies[37].
MRJ (DNAJB6) was found to reduce tumorigenicity and metastasis of melanoma and breast cancer cells[41]. MRJ induces β-catenin degradation and may play a role in maintaining an epithelial phenotype[42]. Furthermore, researchers have shown that loss of MCJ (DNAJC15) expression confers resistance to specific chemotherapeutics in ovarian cancer cells[43,44]. In clinical ovarian cancer patients, low MCJ expression was correlated with poor prognosis and resistance to chemotherapy. ABCB1 (drug transporter) gene expression is mediated by increased levels of the c-Jun transcription factor in the absence of MCJ[43,45]. In contrast to the data on HSP70 and HSP90, there is limited information available on the expression and function of most DNAJ proteins in cancer. However, it is evident from the specific DNAJ proteins that have been identified that these proteins may play an important role in affecting cancer properties.
HLJ1, consisting 337 amino acids, was first identified from the human liver cDNA library and was classified as a member of the HSP40 family[46]. HLJ1, also known as DNAJB4, belongs to the type II homologues of the Hsp40 family and comprises the following four conserved functional domains: a highly conserved J domain, a glycine/phenylalanine-rich region, a cysteine-rich region, and a COOH terminal domain[47]. Homology analysis showed that the amino acid sequence of HLJ1 has 84% similarity with HDJ1 (DNAJB1) isolated from human placenta[48]. HLJ1 mRNA is highly expressed in skeletal muscle and in the heart, pancreas, brain, lung, and other organs but is weakly expressed in the liver and kidney. Although the HLJ1 gene was cloned from the liver cDNA library, the expression of HLJ1 in liver tissue is relatively low[46].
Gene expression profiles have been used to identify possible associations with lung cancer behaviour or clinical outcome to better predict patient prognosis. We have previously established a panel of lung adenocarcinoma cell lines with increased invasiveness (CL1-0, CL1-1, CL1-5 and CL1-5-F4) that were derived from a parent lung adenocarcinoma cell line by repeatedly selecting for more invasive cells[49]. These selected sublines have shown greater invasive and metastatic potential than the parental cells. By using a complementary DNA microarray, we identified metastasis-associated genes on a genome-wide scale in model lung cancer cell lines[50]. Cluster analysis of the complementary DNA microarray data revealed that 589 (6.1%) genes were positively or negatively associated with cancer cell invasiveness. Moreover, most of these genes were involved in angiogenesis, cell motility, adhesion and proliferation. HLJ1, one of the metastasis-associated genes identified by that study, has been characterised as a novel tumour suppressor, and high HLJ1 expression is associated with reduced cancer recurrence and prolonged survival in NSCLC patients[19].
NSCLC is a heterogeneous disease, and patients with similar clinical-pathologic features may have a broad range of outcomes[51,52]. It is important to identify a prognostic marker that can predict clinical outcomes in NSCLC patients. We have demonstrated that HLJ1 is a novel tumour suppressor in NSCLC. Primary cancer specimens from 71 patients with histologically confirmed NSCLC were analysed using reverse transcription-polymerase chain reaction, and the HLJ1 expression in tumour tissue was lower than in adjacent normal tissue in 55/71 (77%) of the patients. Moreover, in 49% of the samples, the tumour tissue showed at least a 2-fold decrease in HLJ1 compared to adjacent normal tissue. NSCLC patients with high HLJ1 expression had significantly longer overall survival and disease-free survival times compared to those with low HLJ1 expression. Clinically, HLJ1 is a significant, independent prognostic predictor of recurrence and overall survival in NSCLC patients[19]. Tumour suppressor genes are frequently deactivated by genetic alterations, such as chromosomal deletions and loss-of-function mutations. The HLJ1 gene is located on human chromosome 1P31.1. Loss of heterozygosity (LOH) on the short arm of chromosome 1 has been reported in many types of cancer[53]. High frequencies of LOH and microsatellite instability in the HLJ1 region have been detected in NSCLC patients[19].
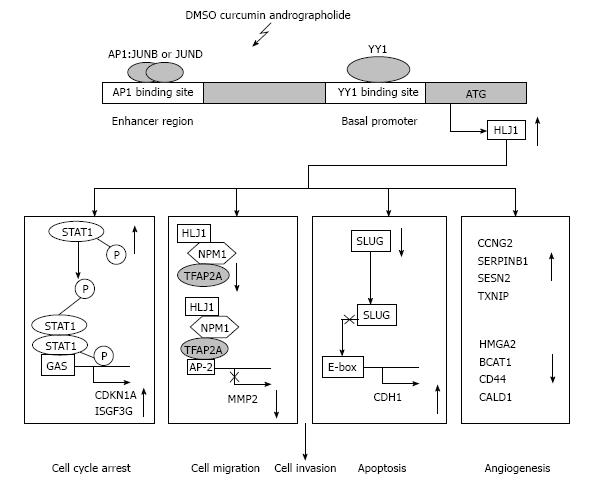
Microarray analysis suggests that HLJ1 expression is negatively correlated with cancer invasiveness. HLJ1 is reportedly up-regulated in less-invasive cell lines than in highly invasive cell lines[20,50]. Restoration of HLJ1 expression in NSCLC cells inhibited cell proliferation, anchorage-independent growth, cell motility, invasion and tumourigenesis. HLJ1 slows cell cycle progression by increasing STAT1 and p21WAF1 expression and decreasing cyclin D1 expression. The same study also suggested that HLJ1 can affect the expression of many genes downstream in the STAT1 pathway, including p21WAF1, ISGF3G, IFIT1, IFITM1, OAS3, and GIP2 and that HLJ1 can increase p21WAF1 expression by affecting a p53-independent pathway[19,20].
E-cadherin is a well-known invasion suppressor in several types of carcinoma[54]. In a previous microarray analysis, we found that expression of the Slug gene positively correlated with invasive ability. Overexpression of Slug suppressed E-cadherin expression and increased invasive ability. The study showed that Slug promoted metastasis in lung cancer by down-regulating E-cadherin, up-regulating matrix metalloproteinase 2 (MMP2) and enhancing angiogenesis[17]. We also found that HLJ1 indirectly up-regulated E-cadherin expression by inhibiting the repressive effect of the Slug gene on the E-cadherin proximal promoter[20]. Recently, research studies have also indicated that HLJ1 acts as a molecular chaperone of E-cadherin that is able to sense E-cadherin folding, providing stabilisation of native folded E-cadherin in the plasma membrane or degradation of an unfolded counterpart. Post-translational regulation of E-cadherin by the HLJ1 molecular chaperone is sufficient to influence gastric cell invasion. HLJ1 is a sensor of E-cadherin structure features that might contribute to gastric cancer progression. Additionally, the expression of HLJ1 and E-cadherin is concomitantly decreased in patients with human gastric carcinoma[55].
Nucleophosmin (NPM1) is a nucleolar phosphoprotein that localises in granular regions of the nucleolus and is highly expressed in malignant and actively dividing cells[56]. NPM1 shuttles continuously between the nucleus and cytoplasm and acts as a multifunctional protein that plays an important role in the increased nucleolar activity needed for cell proliferation[56,57]. Studies have proposed that the NPM1 protein has both oncogenic and tumour-suppressing effects, depending on its level of expression and cellular localisation[58]. HLJ1 modulates NPM1 oligomerisation and NPM1-AP-2α multi-protein complex formation, which alter AP-2α transcriptional activity. These changes then suppress the expression of downstream genes such as MMP2 and, as a result, decrease lung cancer cell invasiveness[22].
Evasion of apoptosis is a hallmark of most cancers[59]. Therefore, it is important to identify genes that promote apoptosis in cancer cells, either under normal or stressful conditions, such as radiotherapy and chemotherapy. Several recent studies indicate that HSPs play important cytoprotective roles and are involved in regulating the apoptosis pathway[60]. HLJ1 can promote cancer cell sensitivity to ultraviolet (UV) stress-induced apoptosis by enhancing c-Jun N-terminal kinase (JNK) activation and caspase activity[61]. Moreover, the HLJ1 protein is a novel substrate of caspase-3, which is followed by protein degradation during the apoptotic process. HLJ1 appears to play an important role in apoptosis. However, further studies are necessary to determine the underlying mechanism of HLJ1 in UV-induced apoptosis and the effects of reduced HLJ1 in late apoptosis[61].
Elucidation of the roles and the regulatory mechanism of tumour suppressors may facilitate the development of rational therapeutic targets that inhibit cancer cell proliferation, angiogenesis and metastasis. YY1 is a 65-kDa multifunctional zinc-finger transcription factor belonging to the human GLI-Kruppel family of nuclear proteins[62]. It can bind to a specific DNA consensus sequence, 5’-CGCCATNTT-3’, which is present in many promoters. YY1 can either activate or repress the target genes, depending on the cofactors that it recruits[62,63]. YY1 is a complex protein that plays pivotal roles in cell development, differentiation, proliferation, and apoptosis[64,65]. Because the expression and function of YY1 are intimately associated with cell cycle progression, its physiologic significance has recently been applied to models of cancer biology[64]. YY1 overexpression has been demonstrated in several human cancers such as breast cancer, prostate cancer, cervical cancer, brain cancer and colon cancer[65,66]. However, functional and clinical analysis of the YY1 in NSCLC remains unclear. As a transcription factor, YY1 regulates the expression of numerous genes that are mostly involved in tumorigenesis. The HLJ1 promoter contains four YY1-binding sites that positively regulate HLJ1 expression. When compared with the WT construct, the mutants of these four potential YY1-binding sites resulted in different levels of reduction in HLJ1 promoter activity, from 17% to 34%, respectively[20]. However, deletion of all four YY1-binding sites reduced the HLJ1 promoter activity by 93%, indicating that YY1 regulation plays an important role in HLJ1 expression. Overexpression of YY1 in NSCLC cells indicated that up-regulates the HLJ1 expression by directly binding to the promoter region, thus inhibiting cancer cell invasion[20]. In addition, an enhancer segment was identified in the HLJ1 gene at -2125 to -1039 bp upstream of the transcription start site, which includes the activator protein 1 (AP-1) site. The activation and synergistic up-regulation of the tumour suppressor HLJ1 is the result of the binding of the transcription factors AP-1 and YY1 to the gene’s enhancer and promoter regions, respectively[21].
Hepatitis B virus (HBV) is a major cause of human hepatocellular carcinoma (HCC). HBV proteins promote migration-related factors such as MT1-MMP, MMP9, and hypoxia-inducible factor 1-α and contribute to the HCC metastatic process[67-69]. However, HBV could also promote HLJ1 expression in HCC cells by up-regulating the transcription factor YY1. The role of the HLJ1 in HCC cells still unclear[70].
Curcumin (diferuloylmethane), a natural compound derived from the spice turmeric (Curcuma longa), has been used in the treatment of various inflammatory diseases in traditional Indian and Chinese medicine[71]. The anticancer or chemopreventive effects of curcumin are the result of a variety of molecular mechanisms, including its direct or indirect interaction with various transcription factors, regulatory proteins and enzymes that all play a central role in key cancer-related processes such as inflammation, proliferation, survival, migration, angiogenesis, invasion and metastasis[72,73]. Curcumin may have potential as a multi-target drug in anticancer therapy[74-76]. Previous evidence has shown that AP-1 complexes enriched with c-Jun and JunB may result in morphologic alterations and anchorage-independent cell growth, whereas complexes enriched with JunD showed anti-proliferative effects. It has been suggested that curcumin inhibits tumour growth by inhibiting AP-1 activation[77,78]. Curcumin increases JunD expression, stimulates HLJ1 enhancer activity, and triggers HLJ1 expression, which may subsequently reduce filopodia formation and up-regulate E-cadherin expression. Curcumin may inhibit cancer cell migration and invasion not only by inhibiting MMP2, MMP9 and MMP14 but also through HLJ1/E-cadherin induction[23,74]. Curcumin may be a template for new antitumor drug developments that target the tumour suppressor HLJ1[23].
Dimethyl sulfoxide (DMSO) is an amphipathic molecule that has a highly polar domain and two apolar methyl groups, making it soluble in both aqueous and organic media[79]. DMSO is commonly used as a very efficient solvent for water-insoluble compounds in biological studies and as a cryoprotectant for cultured cells[80]. In particular, DMSO has been approved by the United States Food and Drug Administration for the treatment of interstitial cystitis[81]. DMSO was also used to treat leukaemia for several years, based on its ability to induce cellular differentiation, which caused leukaemia cells to lose their proliferative properties[82,83]. DMSO has been found to arrest the cell cycle at the G1 phase in lymphoid cell lines[84]. Additionally, DMSO treatment can modulate AP-1 activity, and DMSO is involved in the suppression of intercellular adhesion molecule 1 expression in a rat model of peritonitis sepsis[85]. Our results suggest that DMSO is an important stimulator of the tumour suppressor protein HLJ1, and DMSO works by activating JunB and JunD in highly invasive lung adenocarcinoma[86]. These efforts will help us to develop not only novel anti-cancer drugs that affect lung cancer progression but also new therapeutic strategies for the disease. For instance, a therapeutic strategy combining both induced expression of HLJ1 by DMSO-derived analogues and irradiation would synergistically increase the efficacy of radiotherapy and prolong lung cancer patient survival.
Recently, several types of herbal compounds were proven to be potential anti-cancer drugs. These compounds, including curcumin from a spice turmeric[74], epigallocatechin-3-gallate from green tea[87] and lycopene from tomato[88], could target important mechanisms in tumour growth and metastasis[89]. Screening drugs from traditional Chinese medicine has been suggested as a shortcut in searching for new leading compounds. Using the HLJ1 promoter and luciferase reporter assays, the HLJ1-targeting drug-screening platform was established to screen and identify traditional Chinese herbs that can target the novel tumour suppressor gene HLJ1. Among the herbal drugs identified, the andrographolide is a promising new anticancer agent that could significantly induced HLJ1 expression and suppress tumour growth and invasion in NSCLC[90]. Andrographolide, a diterpenoid lactone isolated from the Chinese herbal medicine Andrographis paniculata, is known for its wide pharmacological activities, such as its anti-inflammatory, anti-angiogenesis, pro-apoptosis and anticancer activities[91]. Moreover, Andrographis paniculata has long been perceived as safe in traditional Chinese medicine as well as in the traditional medicine in Thailand and India. Andrographis paniculata is genotoxically safe and has been applied to clinical investigations on the treatment and prevention of upper respiratory tract infections[92,93].
Previously, andrographolide was reported to inhibit colorectal cancer cell invasion and migration by suppressing the activity of c-Fos and c-Jun and thus reducing MMP7 expression[94]. Andrographolide was also reported to suppress invasion and migration in lung cancer cells through attenuation of the PI3K/Akt signalling pathway[95]. The HLJ1-targeting drug-screening platform is useful for screening novel anticancer compounds. Using this platform, we identified andrographolide as a promising new anticancer agent that could suppress tumour growth and invasion in NSCLC. The onco-suppressive effects of andrographolide may be partially mediated by JunB-regulated HLJ1 expression, which modulates the transcription factor AP-2α binding at the MMP2 promoter and represses the expression of MMP2[90]. In addition, silencing HLJ1 partially reverses the inhibition of cancer cell invasion by andrographolide. The results also establish the potential for using andrographolide as a multi-target lead compound in developing anti-cancer therapies.
In the past decade, improved understanding of the molecular mechanism of HLJ1 in NSCLC progression has been greatly appreciated. Accumulated data support HLJ1 as a novel tumour suppressor and a potential druggable target for NSCLC. Restoration of HLJ1 expression in NSCLC cells inhibits cell proliferation, anchorage-independent growth, cell motility, invasion, and tumourigenesis. HLJ1 can inhibit cell cycle progression by increasing the STAT1 and p21WAF1 pathways and by decreasing cyclin D1 expression. The activation of the STAT1 pathway by HLJ1 was independent of p53. We also found that HLJ1 indirectly up-regulates E-cadherin expression through inhibiting the repression effect of the Slug gene on the E-cadherin proximal promoter. Increased HLJ1 expression is associated with prolonged disease-free and overall survival of patients with NSCLC. The endogenous transcriptional expression of HLJ1 is up-regulated through the binding of the enhancer AP-1 to its promoter YY1 with the co-activator p300 and the formation of bending DNA structure. Importantly, HLJ1 was reported to promote UV-induced apoptosis through JNK and caspase-3 activation in NSCLC. HLJ1 is a novel substrate of caspase-3 and is degraded at a late stage of apoptosis[51]. In this review, we summarize the molecular mechanisms of the HLJ1 involved in lung cancer progression and propose a hypothetical model for the roles of HLJ1 stimulator in suppressing lung cancer tumourigenesis (Figure 1). Due to its tumour suppressor properties, HLJ1 is a potential target for anticancer therapy. Targeted induction of HLJ1 is a promising approach for cancer therapy, which also means that curcumin, DMSO, and andrographolide may serve as potential lead compounds or coordinated ligands for the development of novel anti-cancer drugs. Investigating the integrated and coordinated molecular mechanisms of HLJ1 may shed new light on the treatment of lung cancer. The development of drug targeting HLJ1 may be an effective approach for lung cancer therapy.
P- Reviewer: Ou WB, Tamura M S- Editor: Gou SX L- Editor: A E- Editor: Liu SQ
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 2. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9856] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 3. | Meoni G, Cecere FL, Lucherini E, Di Costanzo F. Medical treatment of advanced non-small cell lung cancer in elderly patients: a review of the role of chemotherapy and targeted agents. J Geriatr Oncol. 2013;4:282-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male: female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 562] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 5. | Nakajima T, Yasufuku K. Early lung cancer: methods for detection. Clin Chest Med. 2013;34:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584-594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 571] [Cited by in RCA: 1565] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 7. | Yang P. Epidemiology of lung cancer prognosis: quantity and quality of life. Methods Mol Biol. 2009;471:469-486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Bearz A, Berretta M, Lleshi A, Tirelli U. Target therapies in lung cancer. J Biomed Biotechnol. 2011;2011:921231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Yang P, Allen MS, Aubry MC, Wampfler JA, Marks RS, Edell ES, Thibodeau S, Adjei AA, Jett J, Deschamps C. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128:452-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 329] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 10. | Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: recent developments. Lancet. 2013;382:709-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 576] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 11. | Wao H, Mhaskar R, Kumar A, Miladinovic B, Djulbegovic B. Survival of patients with non-small cell lung cancer without treatment: a systematic review and meta-analysis. Syst Rev. 2013;2:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Meyer T, Hart IR. Mechanisms of tumour metastasis. Eur J Cancer. 1998;34:214-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 124] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Patel LR, Camacho DF, Shiozawa Y, Pienta KJ, Taichman RS. Mechanisms of cancer cell metastasis to the bone: a multistep process. Future Oncol. 2011;7:1285-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Perlikos F, Harrington KJ, Syrigos KN. Key molecular mechanisms in lung cancer invasion and metastasis: a comprehensive review. Crit Rev Oncol Hematol. 2013;87:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 15. | Iiizumi M, Liu W, Pai SK, Furuta E, Watabe K. Drug development against metastasis-related genes and their pathways: a rationale for cancer therapy. Biochim Biophys Acta. 2008;1786:87-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Li DQ, Pakala SB, Nair SS, Eswaran J, Kumar R. Metastasis-associated protein 1/nucleosome remodeling and histone deacetylase complex in cancer. Cancer Res. 2012;72:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 17. | Shih JY, Tsai MF, Chang TH, Chang YL, Yuan A, Yu CJ, Lin SB, Liou GY, Lee ML, Chen JJ. Transcription repressor slug promotes carcinoma invasion and predicts outcome of patients with lung adenocarcinoma. Clin Cancer Res. 2005;11:8070-8078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Pan SH, Chao YC, Hung PF, Chen HY, Yang SC, Chang YL, Wu CT, Chang CC, Wang WL, Chan WK. The ability of LCRMP-1 to promote cancer invasion by enhancing filopodia formation is antagonized by CRMP-1. J Clin Invest. 2011;121:3189-3205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Tsai MF, Wang CC, Chang GC, Chen CY, Chen HY, Cheng CL, Yang YP, Wu CY, Shih FY, Liu CC. A new tumor suppressor DnaJ-like heat shock protein, HLJ1, and survival of patients with non-small-cell lung carcinoma. J Natl Cancer Inst. 2006;98:825-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Wang CC, Tsai MF, Hong TM, Chang GC, Chen CY, Yang WM, Chen JJ, Yang PC. The transcriptional factor YY1 upregulates the novel invasion suppressor HLJ1 expression and inhibits cancer cell invasion. Oncogene. 2005;24:4081-4093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Wang CC, Tsai MF, Dai TH, Hong TM, Chan WK, Chen JJ, Yang PC. Synergistic activation of the tumor suppressor, HLJ1, by the transcription factors YY1 and activator protein 1. Cancer Res. 2007;67:4816-4826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Chang TP, Yu SL, Lin SY, Hsiao YJ, Chang GC, Yang PC, Chen JJ. Tumor suppressor HLJ1 binds and functionally alters nucleophosmin via activating enhancer binding protein 2alpha complex formation. Cancer Res. 2010;70:1656-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Chen HW, Lee JY, Huang JY, Wang CC, Chen WJ, Su SF, Huang CW, Ho CC, Chen JJ, Tsai MF. Curcumin inhibits lung cancer cell invasion and metastasis through the tumor suppressor HLJ1. Cancer Res. 2008;68:7428-7438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 24. | Ritossa F. Discovery of the heat shock response. Cell Stress Chaperones. 1996;1:97-98. [PubMed] |
| 25. | Vabulas RM, Raychaudhuri S, Hayer-Hartl M, Hartl FU. Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb Perspect Biol. 2010;2:a004390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 304] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 26. | Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3762] [Cited by in RCA: 3650] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 27. | Colaco CA, Bailey CR, Walker KB, Keeble J. Heat shock proteins: stimulators of innate and acquired immunity. Biomed Res Int. 2013;2013:461230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Helmbrecht K, Zeise E, Rensing L. Chaperones in cell cycle regulation and mitogenic signal transduction: a review. Cell Prolif. 2000;33:341-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 216] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 29. | Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000;92:1564-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 775] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 30. | Khalil AA, Kabapy NF, Deraz SF, Smith C. Heat shock proteins in oncology: diagnostic biomarkers or therapeutic targets? Biochim Biophys Acta. 2011;1816:89-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Miyata Y, Nakamoto H, Neckers L. The therapeutic target Hsp90 and cancer hallmarks. Curr Pharm Des. 2013;19:347-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | McConnell JR, McAlpine SR. Heat shock proteins 27, 40, and 70 as combinational and dual therapeutic cancer targets. Bioorg Med Chem Lett. 2013;23:1923-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Li J, Qian X, Sha B. Heat shock protein 40: structural studies and their functional implications. Protein Pept Lett. 2009;16:606-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 34. | Sterrenberg JN, Blatch GL, Edkins AL. Human DNAJ in cancer and stem cells. Cancer Lett. 2011;312:129-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Qiu XB, Shao YM, Miao S, Wang L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell Mol Life Sci. 2006;63:2560-2570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 642] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 36. | Chen CY, Jan CI, Lo JF, Yang SC, Chang YL, Pan SH, Wang WL, Hong TM, Yang PC. Tid1-L inhibits EGFR signaling in lung adenocarcinoma by enhancing EGFR Ubiquitinylation and degradation. Cancer Res. 2013;73:4009-4019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Copeland E, Balgobin S, Lee CM, Rozakis-Adcock M. hTID-1 defines a novel regulator of c-Met Receptor signaling in renal cell carcinomas. Oncogene. 2011;30:2252-2263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Chen CY, Chiou SH, Huang CY, Jan CI, Lin SC, Hu WY, Chou SH, Liu CJ, Lo JF. Tid1 functions as a tumour suppressor in head and neck squamous cell carcinoma. J Pathol. 2009;219:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Trentin GA, Yin X, Tahir S, Lhotak S, Farhang-Fallah J, Li Y, Rozakis-Adcock M. A mouse homologue of the Drosophila tumor suppressor l(2)tid gene defines a novel Ras GTPase-activating protein (RasGAP)-binding protein. J Biol Chem. 2001;276:13087-13095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Ahn BY, Trinh DL, Zajchowski LD, Lee B, Elwi AN, Kim SW. Tid1 is a new regulator of p53 mitochondrial translocation and apoptosis in cancer. Oncogene. 2010;29:1155-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Mitra A, Fillmore RA, Metge BJ, Rajesh M, Xi Y, King J, Ju J, Pannell L, Shevde LA, Samant RS. Large isoform of MRJ (DNAJB6) reduces malignant activity of breast cancer. Breast Cancer Res. 2008;10:R22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Mitra A, Menezes ME, Shevde LA, Samant RS. DNAJB6 induces degradation of beta-catenin and causes partial reversal of mesenchymal phenotype. J Biol Chem. 2010;285:24686-24694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Hatle KM, Neveu W, Dienz O, Rymarchyk S, Barrantes R, Hale S, Farley N, Lounsbury KM, Bond JP, Taatjes D. Methylation-controlled J protein promotes c-Jun degradation to prevent ABCB1 transporter expression. Mol Cell Biol. 2007;27:2952-2966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Rein BJ, Gupta S, Dada R, Safi J, Michener C, Agarwal A. Potential markers for detection and monitoring of ovarian cancer. J Oncol. 2011;2011:475983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Witham J, Vidot S, Agarwal R, Kaye SB, Richardson A. Transient ectopic expression as a method to detect genes conferring drug resistance. Int J Cancer. 2008;122:2641-2645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Hoe KL, Won M, Chung KS, Jang YJ, Lee SB, Kim DU, Lee JW, Yun JH, Yoo HS. Isolation of a new member of DnaJ-like heat shock protein 40 (Hsp40) from human liver. Biochim Biophys Acta. 1998;1383:4-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Ohtsuka K, Hata M. Molecular chaperone function of mammalian Hsp70 and Hsp40--a review. Int J Hyperthermia. 2000;16:231-245. [PubMed] |
| 48. | Uchiyama Y, Takeda N, Mori M, Terada K. Heat shock protein 40/DjB1 is required for thermotolerance in early phase. J Biochem. 2006;140:805-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Chu YW, Yang PC, Yang SC, Shyu YC, Hendrix MJ, Wu R, Wu CW. Selection of invasive and metastatic subpopulations from a human lung adenocarcinoma cell line. Am J Respir Cell Mol Biol. 1997;17:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 362] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 50. | Chen JJ, Peck K, Hong TM, Yang SC, Sher YP, Shih JY, Wu R, Cheng JL, Roffler SR, Wu CW. Global analysis of gene expression in invasion by a lung cancer model. Cancer Res. 2001;61:5223-5230. [PubMed] |
| 51. | Chen HY, Yu SL, Chen CH, Chang GC, Chen CY, Yuan A, Cheng CL, Wang CH, Terng HJ, Kao SF. A five-gene signature and clinical outcome in non-small-cell lung cancer. N Engl J Med. 2007;356:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 686] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 52. | Kim KJ, Ahn YC, Lim do H, Han J, Park K, Park JO, Kim K, Kim J, Shim YM. Analyses on prognostic factors following tri-modality therapy for stage IIIa non-small cell lung cancer. Lung Cancer. 2007;55:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Ragnarsson G, Eiriksdottir G, Johannsdottir JT, Jonasson JG, Egilsson V, Ingvarsson S. Loss of heterozygosity at chromosome 1p in different solid human tumours: association with survival. Br J Cancer. 1999;79:1468-1474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 133] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 54. | Canel M, Serrels A, Frame MC, Brunton VG. E-cadherin-integrin crosstalk in cancer invasion and metastasis. J Cell Sci. 2013;126:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 497] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 55. | Simões-Correia J, Silva DI, Melo S, Figueiredo J, Caldeira J, Pinto MT, Girão H, Pereira P, Seruca R. DNAJB4 molecular chaperone distinguishes WT from mutant E-cadherin, determining their fate in vitro and in vivo. Hum Mol Genet. 2014;23:2094-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Grisendi S, Mecucci C, Falini B, Pandolfi PP. Nucleophosmin and cancer. Nat Rev Cancer. 2006;6:493-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 650] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 57. | Borer RA, Lehner CF, Eppenberger HM, Nigg EA. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989;56:379-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 901] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 58. | Li J, Sejas DP, Burma S, Chen DJ, Pang Q. Nucleophosmin suppresses oncogene-induced apoptosis and senescence and enhances oncogenic cooperation in cells with genomic instability. Carcinogenesis. 2007;28:1163-1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47112] [Article Influence: 3365.1] [Reference Citation Analysis (5)] |
| 60. | Sreedhar AS, Csermely P. Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive review. Pharmacol Ther. 2004;101:227-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 313] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 61. | Lin SY, Hsueh CM, Yu SL, Su CC, Shum WY, Yeh KC, Chang GC, Chen JJ. HLJ1 is a novel caspase-3 substrate and its expression enhances UV-induced apoptosis in non-small cell lung carcinoma. Nucleic Acids Res. 2010;38:6148-6158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Galvin KM, Shi Y. Multiple mechanisms of transcriptional repression by YY1. Mol Cell Biol. 1997;17:3723-3732. [PubMed] |
| 63. | Yao YL, Yang WM, Seto E. Regulation of transcription factor YY1 by acetylation and deacetylation. Mol Cell Biol. 2001;21:5979-5991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 64. | Wang CC, Chen JJ, Yang PC. Multifunctional transcription factor YY1: a therapeutic target in human cancer? Expert Opin Ther Targets. 2006;10:253-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Castellano G, Torrisi E, Ligresti G, Malaponte G, Militello L, Russo AE, McCubrey JA, Canevari S, Libra M. The involvement of the transcription factor Yin Yang 1 in cancer development and progression. Cell Cycle. 2009;8:1367-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 66. | Zhang Q, Stovall DB, Inoue K, Sui G. The oncogenic role of Yin Yang 1. Crit Rev Oncog. 2011;16:163-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 67. | Lara-Pezzi E, Gómez-Gaviro MV, Gálvez BG, Mira E, Iñiguez MA, Fresno M, Martínez-A C, Arroyo AG, López-Cabrera M. The hepatitis B virus X protein promotes tumor cell invasion by inducing membrane-type matrix metalloproteinase-1 and cyclooxygenase-2 expression. J Clin Invest. 2002;110:1831-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Liu LP, Liang HF, Chen XP, Zhang WG, Yang SL, Xu T, Ren L. The role of NF-kappaB in Hepatitis b virus X protein-mediated upregulation of VEGF and MMPs. Cancer Invest. 2010;28:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 69. | Moon EJ, Jeong CH, Jeong JW, Kim KR, Yu DY, Murakami S, Kim CW, Kim KW. Hepatitis B virus X protein induces angiogenesis by stabilizing hypoxia-inducible factor-1alpha. FASEB J. 2004;18:382-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 70. | Zhang L, Cai X, Chen K, Wang Z, Wang L, Ren M, Huang A, Tang H. Hepatitis B virus protein up-regulated HLJ1 expression via the transcription factor YY1 in human hepatocarcinoma cells. Virus Res. 2011;157:76-81. |
| 71. | Gautam SC, Gao X, Dulchavsky S. Immunomodulation by curcumin. Adv Exp Med Biol. 2007;595:321-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 72. | Goel A, Jhurani S, Aggarwal BB. Multi-targeted therapy by curcumin: how spicy is it? Mol Nutr Food Res. 2008;52:1010-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 73. | Vyas A, Dandawate P, Padhye S, Ahmad A, Sarkar F. Perspectives on new synthetic curcumin analogs and their potential anticancer properties. Curr Pharm Des. 2013;19:2047-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 74. | Chen HW, Yu SL, Chen JJ, Li HN, Lin YC, Yao PL, Chou HY, Chien CT, Chen WJ, Lee YT. Anti-invasive gene expression profile of curcumin in lung adenocarcinoma based on a high throughput microarray analysis. Mol Pharmacol. 2004;65:99-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 75. | Chen A, Xu J, Johnson AC. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006;25:278-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 201] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 76. | Chen QY, Zheng Y, Jiao DM, Chen FY, Hu HZ, Wu YQ, Song J, Yan J, Wu LJ, Lv GY. Curcumin inhibits lung cancer cell migration and invasion through Rac1-dependent signaling pathway. J Nutr Biochem. 2014;25:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 77. | Leaner VD, Kinoshita I, Birrer MJ. AP-1 complexes containing cJun and JunB cause cellular transformation of Rat1a fibroblasts and share transcriptional targets. Oncogene. 2003;22:5619-5629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 78. | Huang TS, Lee SC, Lin JK. Suppression of c-Jun/AP-1 activation by an inhibitor of tumor promotion in mouse fibroblast cells. Proc Natl Acad Sci USA. 1991;88:5292-5296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 275] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 79. | Santos NC, Martins-Silva J, Saldanha C. PTEN “meets” DMSO. Leuk Res. 2005;29:361-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 80. | Pegg DE. Principles of cryopreservation. Methods Mol Biol. 2007;368:39-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 251] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 81. | Parkin J, Shea C, Sant GR. Intravesical dimethyl sulfoxide (DMSO) for interstitial cystitis--a practical approach. Urology. 1997;49:105-107. [PubMed] |
| 82. | Santos NC, Figueira-Coelho J, Martins-Silva J, Saldanha C. Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochem Pharmacol. 2003;65:1035-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 450] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 83. | Collins SJ, Ruscetti FW, Gallagher RE, Gallo RC. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci USA. 1978;75:2458-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 891] [Cited by in RCA: 1015] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 84. | Teraoka H, Mikoshiba M, Takase K, Yamamoto K, Tsukada K. Reversible G1 arrest induced by dimethyl sulfoxide in human lymphoid cell lines: dimethyl sulfoxide inhibits IL-6-induced differentiation of SKW6-CL4 into IgM-secreting plasma cells. Exp Cell Res. 1996;222:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 85. | Chang CK, Albarillo MV, Schumer W. Therapeutic effect of dimethyl sulfoxide on ICAM-1 gene expression and activation of NF-kappaB and AP-1 in septic rats. J Surg Res. 2001;95:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 86. | Wang CC, Lin SY, Lai YH, Liu YJ, Hsu YL, Chen JJ. Dimethyl sulfoxide promotes the multiple functions of the tumor suppressor HLJ1 through activator protein-1 activation in NSCLC cells. PLoS One. 2012;7:e33772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 87. | Sideris DA, Toumanidis ST, Kostis EB, Stagiannis K, Spyropoulos G, Moulopoulos SD. Response of tertiary centres to pressure changes. Is there a mechano-electrical association? Cardiovasc Res. 1990;24:13-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 88. | Tang FY, Cho HJ, Pai MH, Chen YH. Concomitant supplementation of lycopene and eicosapentaenoic acid inhibits the proliferation of human colon cancer cells. J Nutr Biochem. 2009;20:426-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 89. | Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004;215:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 340] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 90. | Lai YH, Yu SL, Chen HY, Wang CC, Chen HW, Chen JJ. The HLJ1-targeting drug screening identified Chinese herb andrographolide that can suppress tumour growth and invasion in non-small-cell lung cancer. Carcinogenesis. 2013;34:1069-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 91. | Sheeja K, Guruvayoorappan C, Kuttan G. Antiangiogenic activity of Andrographis paniculata extract and andrographolide. Int Immunopharmacol. 2007;7:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 92. | Chandrasekaran CV, Thiyagarajan P, Sundarajan K, Goudar KS, Deepak M, Murali B, Allan JJ, Agarwal A. Evaluation of the genotoxic potential and acute oral toxicity of standardized extract of Andrographis paniculata (KalmCold). Food Chem Toxicol. 2009;47:1892-1902. [PubMed] |
| 93. | Poolsup N, Suthisisang C, Prathanturarug S, Asawamekin A, Chanchareon U. Andrographis paniculata in the symptomatic treatment of uncomplicated upper respiratory tract infection: systematic review of randomized controlled trials. J Clin Pharm Ther. 2004;29:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 94. | Shi MD, Lin HH, Chiang TA, Tsai LY, Tsai SM, Lee YC, Chen JH. Andrographolide could inhibit human colorectal carcinoma Lovo cells migration and invasion via down-regulation of MMP-7 expression. Chem Biol Interact. 2009;180:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 95. | Lee YC, Lin HH, Hsu CH, Wang CJ, Chiang TA, Chen JH. Inhibitory effects of andrographolide on migration and invasion in human non-small cell lung cancer A549 cells via down-regulation of PI3K/Akt signaling pathway. Eur J Pharmacol. 2010;632:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |