Published online Dec 10, 2014. doi: 10.5306/wjco.v5.i5.1107
Revised: September 2, 2014
Accepted: October 31, 2014
Published online: December 10, 2014
Processing time: 265 Days and 6.7 Hours
Lymphoepithelioma-like carcinoma (LELC) is a rare type of neoplasm in which only twenty cases have been reported in the breast. This type of tumor can be difficult to distinguish from other breast tumors particularly medullary carcinoma and lymphoma in the breast. We present a case of LELC of the breast presenting as an abscess along with a review of the literature. This is the 21st reported case of LELC of the breast and the first case to present as an abscess. Her clinical picture could have been mistaken for other infectious or inflammatory diseases. Given the potential for favorable outcome, early detection and general knowledge of this neoplasm are essential to expedite treatment for this rare tumor type.
Core tip: We present a case of lymphoepithelioma-like carcinoma (LELC) of the breast, which is a rare tumor type that can be difficult to diagnose. This particular case is the first case of LELC of the breast presenting as an abscess with radiologic and histologic studies as well as literature review of this rare tumor.
- Citation: Suzuki I, Chakkabat P, Goicochea L, Campassi C, Chumsri S. Lymphoepithelioma-like carcinoma of the breast presenting as breast abscess. World J Clin Oncol 2014; 5(5): 1107-1112
- URL: https://www.wjgnet.com/2218-4333/full/v5/i5/1107.htm
- DOI: https://dx.doi.org/10.5306/wjco.v5.i5.1107
Lymphoepithelioma-like carcinoma (LELC) is an undifferentiated carcinoma composed of malignant epithelial cells with a lymphocytic background, which was first described in the nasopharynx by Regaud, Reverchon and Schminke. These cells have been described in other sites including the stomach, salivary gland, lung, thymus, skin and cervix[1]. LELC in the breast was first described by Kumar et al[2]. LELC of the breast is a rare disease with only 20 reported cases described in the literature[2-15]. Distinguishing LELC from medullary carcinoma and certain types of lymphoma has been a diagnostic challenge[3,14,15]. Making this distinction has profound impact on therapy and overall prognosis.
In this paper, we present a case of LELC of the breast in a 64-year-old female with an unusual presentation and clinical course.
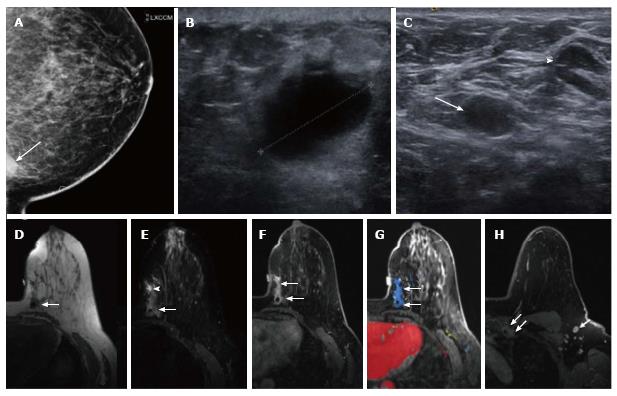
A 64-year-old African American woman presented with a painful palpable lump in her left breast for four weeks. She was initially seen by her primary care physician who prescribed antibiotics without any improvement. She subsequently underwent a mammogram, which demonstrated the anterior aspect of an ill-defined density located in the most posterior medial aspect of the left breast best seen on exaggerated cranio-caudal view (Figure 1A). This mass showed no associated focal architectural distortion or cluster of microcalcification. Her prior annual mammograms were all negative 4 years in a row. Ultrasound revealed an irregular markedly hypoechoic mass with indistinct margins at the 9 o’clock position of the left breast measuring approximately 2.1 cm in maximal diameter (Figure 1B). On color Doppler, no internal vascularity was detected. There were multiple enlarged left axillary lymph nodes detected on both mammogram and ultrasound (Figure 1C). The mass was classified as highly suggestive of malignancy according to the American College of Radiology Breast Imaging Reporting and Data System (ACR BI-RADS: 5). The patient subsequently underwent ultrasound-guided core needle biopsy of the left breast mass and a fine needle aspiration of the two dominant left axillary lymph nodes. Her initial biopsy specimen was described as an abscess-like necrotic tissue but there were atypical cells that were highly suspicious for necrotic malignancy. The fine needle aspirations of the two enlarging axillary lymph nodes were negative for malignancy. This suspicious mass demonstrated low T1 and high T2 signal intensity on the pre-contrast imaging on a subsequent breast MRI (magnetic resonance imaging) with the susceptibility artifact from a biopsy clip (Figure 1D-F). After intravenous administration of gadolinium (Omniscan, GE), the mass showed irregular margins and enhancement, initially rapid and subsequently persisted (type III curve) (Figure 1F-H). Additional linear non mass-like enhancement with similar kinetic pattern was noted anterior to the mass, near the palpable marker, which could represent both tumor extension or post biopsy change. Her right breast showed no suspicious finding. Multiple abnormal enlarged and enhancing level 1 and level 2 left axillary lymph nodes and left inter-pectoral lymph nodes were detected, measuring up to 1.4 cm. There was also a 3.0 cm enhancing soft tissue mass with irregular margins in the left parasternal region, suggestive of left internal mammary adenopathy.
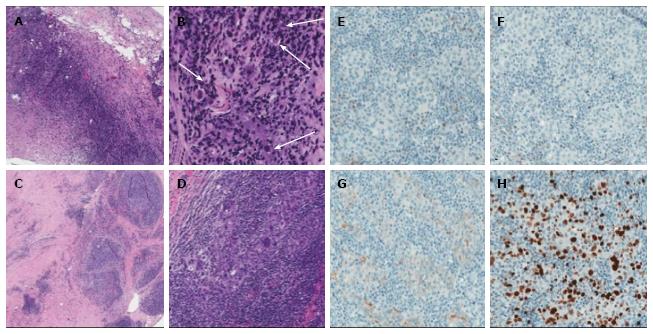
Subsequently, the patient underwent left lumpectomy with axillary node dissection. Positron emission tomograph scan showed no evidence of distant metastases. Her final pathology was consistent with stage IIA (pT1c, N1a, M0) lymphoepithelioma-like carcinoma (Figure 2A-D) with three out of twenty-three lymph nodes involved along with a small focus of extracapsular extension. Additional immunohistochemistry staining (Figure 2E-H) demonstrated that the tumor was negative for both estrogen-receptor and progesterone-receptor (ER 0%, PR 0%) and there was no overexpression of human epidermal growth factor receptor 2 (1+). The staining for proliferative index or Ki67 was 27%. The patient received adjuvant chemotherapy with dose dense doxorubicin and cyclophosphamide followed by weekly paclitaxel and a course of adjuvant radiation therapy.
Her clinical course was complicated by persistent inflammation and serosanguinous drainage from the surgical site. She was given antibiotics on multiple occasions for presumed cellulitis with minimal improvement. Follow-up punch skin biopsy and imaging done of the area showed no evidence of recurrence. These skin changes at the surgical site were thought to be secondary to post-surgical changes and radiation treatments. At her 3-year follow-up, the patient was doing well with no evidence of recurrent disease.
Lymphoepithelioma-like carcinoma of the breast is a rare tumor type characterized by epithelial neoplastic cells with a background of lymphocytic cells. Only twenty cases of this type of breast neoplasm have been described in the literature. The reported cases are summarized in Table 1. Making the diagnosis of LELC can pose a diagnostic challenge due to its morphologic similarities with medullary carcinoma and certain types of lymphoma on pathologic examination[5,14,15]. Making the distinction between LELC-B and other histologically similar tumors has significant impact on therapy and prognosis. Medullary carcinoma of the breast as described by Rapin and Ridolfi includes the following features: syncytial growth pattern > 75%, complete circumscription, diffuse mononuclear stromal infiltrate, moderate to marked nuclear pleomorphism and absence of microglandular features[16,17]. LELC of the breast has similar features, but specifically obscures the neoplastic cells[13]. Though poorly differentiated carcinomas, both medullary carcinomas and LELC-B consistently express cytokeratin markers[12,18]. In our case, the neoplastic cells expressed CK7 and CK5/6, which differentiate these entities from a large cell lymphoma, which may also be on the histologic differential. Additionally, the extensive lymphoplasmacytic infiltrate associated with the epithelial cells may raise concern for a lymphoepithelial lesion, indicating a small cell lymphoma. Special studies for kappa and lambda expression by the B lymphocytes and plasma cells associated with medullary carcinoma and LELC-B show polytypic cell population with expression of both kappa and lambda light chains. In situ hybridization for kappa and lambda light chains was performed in our case, showing a kappa-to-lambda ratio of approximately 2:1, ruling out a monotypic B cell population.
| Case | Ref. | Year | Age | Presenting problem | Size(cm) | Lymph node (Y/N) | Surgery | Chemotherapy (Y/N) (agent listed if known) | Radiation therapy (Y/N) | Outcome (mo)1 | ER status | PR status | Her2 status | EBV |
| 1 | Kumar et al[2] | 1994 | 65 | 2 | N | Mastectomy | NR | NR | 7 | + | + | NR | NR | |
| ALND | ||||||||||||||
| 2 | Cristina et al[3] | 2000 | 54 | Mass | 1.5 | N | Quadrantectomy | Y | N | 6 | + | - | - | - |
| ALND | ||||||||||||||
| 3 | Dadmanesh et al[4] | 2001 | 43 | NR | 1.9 | Y | Quadranectomy | N | N | 60 | - | - | - | NR |
| 4 | Dadmanesh et al[4] | 2001 | 53 | NR | 2 | N | NR | N | N | 72 | - | - | - | NR |
| 5 | Dadmanesh et al[4] | 2001 | 49 | NR | 1 | N | Quadranectomy | N | N | 2 | - | - | - | NR |
| 6 | Dadmanesh et al-4[4] | 2001 | 52 | NR | 2.7 | N | Quadranectomy | N | N | 36 | + | - | - | NR |
| 7 | Dadmanesh et al[4] | 2001 | 64 | NR | 2 | N | Mastectomy | N | N | 60 | - | - | - | NR |
| 8 | Dadmanesh et al[4] | 2001 | 69 | NR | 2.3 | N | Mastectomy | N | Y | 48 | - | - | - | NR |
| 9 | Naidoo et al[10] | 2001 | 50 | Mass | 2.5 | Y | Wide local excision | N | N | 3 | NR | NR | NR | - |
| ALND | ||||||||||||||
| 10 | Peştereli et al[13] | 2002 | 56 | Mass | 1.9 | Y | Modified radical mastectomy | Y | N | 12 | + | + | - | - |
| ALND | ||||||||||||||
| 11 | Ilvan et al[6] | 2004 | 59 | Mass | 3.5 | N | Wide local excision | Y (Tamoxifen) | Y | 52 | + | + | NR | - |
| ALND | ||||||||||||||
| 12 | Ilvan et al[6] | 2004 | 67 | Mass | 1.1 | N | Quadrantectomy | N | Y | 46 | + | + | NR | - |
| ALND | ||||||||||||||
| 13 | Sanati et al[15] | 2004 | 62 | Mass | 3 | NR | NR | NR | NR | 36 | + | - | - | |
| 14 | Kurose et al[9] | 2005 | 47 | Mass | 2.8 | Total mastectomy | Y | N | 12 | + | + | + | - | |
| ALND | (CEF) | |||||||||||||
| Tamoxifen | ||||||||||||||
| 15 | Saleh et al[14] | 2005 | 51 | Mass | 2 | Y | Lumpectomy | N | N | NR | - | - | NR | - |
| ALND | ||||||||||||||
| 16 | Kulka et al[8] | 2008 | 42 | Mass | 2.5 | N | Lumpectomy | N | Y | NR | + | - | - | - |
| 17 | O’Sullivan-Meija et al[12] | 2009 | 55 | Abnormal mammo | 2 | N | Mastectomy | Y | Y | 22 | - | - | + | - |
| (Trastuzumab) | ||||||||||||||
| 18 | Jeong et al[7] | 2010 | 37 | Mass | 2.2 | N | Modified mastectomy | Y | N | 23 | - | - | - | - |
| 19 | Dinniwell et al[5] | 2012 | 55 | Mass, tenderness | 4 | N | Excisional biopsy | N | Y | 36 | - | - | - | - |
| 20 | Nio et al[11] | 2012 | 45 | Mass | 3 | N | Quadrantectomy | Y | Y | NR | - | - | - | NR |
| ALND | ||||||||||||||
| 21 | Present case | 2012 | 64 | Mass, tenderness | 2 | Y | Partial mastectomy | Y | Y | 36 | - | - | + | NR |
| ALND | (AC + paclitaxel) |
Although not tested in our patient, Epstein-Barr virus (EBV) and human papilloma virus (HPV) have been cited for their possible association with LELC of the breast. EBV has been linked to Burkitt’s lymphoma and nasopharyngeal carcinoma. To date, EBV has not been shown to be associated with LELC of the breast; however, LELC in other anatomic sites namely salivary glands, sinonasal tract, stomach, thymus and lungs have been associated with EBV positivity[1,19]. In addition, HPV has been associated with two cases of LELC-B[8,11]. Another case has been seen with sclerosing lymphocytic lobulitis[10].
The case presented here, to the best of our knowledge, is the first case of lymphoepithelioma-like carcinoma of the breast to present as an abscess. Many of the prior case reports of LELC of the breast focus on the distinguishing histopathologic features of the disease and how to distinguish it from morphologically similar entities. Our case illustrates an unusual clinical presentation of this rare tumor type. Our patient presented with a painful breast lump and tenderness that could have easily been mistaken for an infection or other inflammatory processes which would have lead down a completely different diagnostic path and ultimately a significant delay in appropriate treatment. The patient’s tumor also appeared to have necrotic and abscess-like features on initial pathologic examination, which has not been described in other cases of LELC. Initial clinical presentations were reported in 13 of the 20 cases. Twelve patients (60%) presented with a palpable mass while only 1 patient had abnormal findings on mammography. Her clinical course was also complicated by recurrent cellulitis and inflammation of the involved site minimally responsive to antimicrobial therapies. These changes may have been secondary to surgery and radiation therapy but did not appear to be consistent with recurrence of her primary disease.
All reported cases of LELC of the breast (Table 1) were found in women, ranging in age from 37 to 69 years. The median age was 54 years at time of presentation. The tumor sizes ranged from 1 to 4 cm and lymph node involvement seen in 25% of the patients including our patient. ER and PR status were evaluated in all but one patient (19 patients). ER was positive in nine patients (47%) and PR was positive in five patients (26%). Her-2 receptor status was reported in fifteen patients and was found to be overexpressed in three patients (20%).
Our patient regularly had annual mammography. Her last screening mammogram was performed six months prior to the detection of the palpable mass which showed no suspicious findings. However, the mass was probably not included in the field of view because of its very posterior location in the medial breast. This type of breast neoplasm appears to have overall favorable prognosis but early detection is imperative for proper treatment. After review of the literature, management of our patient went in line with most of the prior LELC-B cases. Seventeen of the reported cases mentioned some type of surgical intervention including mastectomy (complete or partial) in ten patients (59%) and axillary node dissections in nine patients (53%). Seven patients (34%) received some type of adjuvant chemotherapy and radiation therapy. Our patient underwent partial mastectomy and axillary node dissection followed by adjuvant chemotherapy along with radiation therapy. Disease-free outcome duration ranged from 2 to 72 mo in the cases reviewed while our patient was doing well without evidence of recurrent 36 mo after primary surgery.
In conclusion, this case and other cases of LELC-B demonstrate the diagnostic challenges for this particular type of tumor mainly due to its morphologic similarities with other neoplasms and rarity of the tumor. This is the first reported case of LELC of the breast presented with abscess-like clinical and pathologic features. Early recognition and appropriate diagnostic workup is the key for optimal management for this particular type of tumor.
A 64-year-old African American woman presented with a painful palpable lump.
Palpable mass of left anterior breast.
Breast cancer, breast cellulitis/abscess.
Ultrasound showed an irregular markedly hypoechoic mass with indistinct margins at the 9 o’clock position of the left breast measuring approximately 2.1 cm in maximal diameter.
Her final pathology was consistent with stage IIA (pT1c, N1a, M0) lymphoepithelioma-like carcinoma. Additional immunohistochemistry staining demonstrated that the tumor was negative for both estrogen and progesterone receptors (ER 0%, PR 0%) and there was no overexpression of human epidermal growth factor receptor 2 (1+). The staining for proliferative index or Ki67 was 27%.
The patient received adjuvant chemotherapy with doxorubicin and cyclophosphamide followed by weekly paclitaxel and a course of adjuvant radiation therapy.
Lymphoepithelioma-like carcinoma (LELC) of the breast is a rare tumor type and the case presented is the first case of LELC of the breast presenting as a breast abscess.
LELC is an undifferentiated carcinoma composed of malignant epithelial cells with a lymphocytic background.
This case report emphasizes the importance of having knowledge of rare tumor types, particularly those in which early recognition can have profound impact on treatment outcome.
It is an interesting and rare case of overlapping features where the diagnosis hangs between abscess and a tumour. The diagnosis is critical for appropriate management.
P- Reviewer: Mehdi I S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Iezzoni JC, Gaffey MJ, Weiss LM. The role of Epstein-Barr virus in lymphoepithelioma-like carcinomas. Am J Clin Pathol. 1995;103:308-315. [PubMed] |
| 2. | Kumar S, Kumar D. Lymphoepithelioma-like carcinoma of the breast. Mod Pathol. 1994;7:129-131. [PubMed] |
| 3. | Cristina S, Boldorini R, Brustia F, Monga G. Lymphoepithelioma-like carcinoma of the breast. An unusual pattern of infiltrating lobular carcinoma. Virchows Arch. 2000;437:198-202. [PubMed] |
| 4. | Dadmanesh F, Peterse JL, Sapino A, Fonelli A, Eusebi V. Lymphoepithelioma-like carcinoma of the breast: lack of evidence of Epstein-Barr virus infection. Histopathology. 2001;38:54-61. [PubMed] |
| 5. | Dinniwell R, Hanna WM, Mashhour M, Saad RS, Czarnota GJ. Lymphoepithelioma-like carcinoma of the breast: a diagnostic and therapeutic challenge. Curr Oncol. 2012;19:e177-e183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Ilvan S, Celik V, Ulker Akyildiz E, Senel Bese N, Ramazanoglu R, Calay Z. Lymphoepithelioma-like carcinoma of the breast: is it a distinct entity? Clinicopathological evaluation of two cases and review of the literature. Breast. 2004;13:522-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Jeong AK, Park SB, Kim YM, Ko BK, Yang MJ, Kwon WJ, Lee JH, Weon YC. Lymphoepithelioma-like carcinoma of the breast. J Ultrasound Med. 2010;29:485-488. [PubMed] |
| 8. | Kulka J, Kovalszky I, Svastics E, Berta M, Füle T. Lymphoepithelioma-like carcinoma of the breast: not Epstein-Barr virus-, but human papilloma virus-positive. Hum Pathol. 2008;39:298-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Kurose A, Ichinohasama R, Kanno H, Kobayashi T, Ishida M, Nishinari N, Sawai T. Lymphoepithelioma-like carcinoma of the breast. Report of a case with the first electron microscopic study and review of the literature. Virchows Arch. 2005;447:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Naidoo P, Chetty R. Lymphoepithelioma-like carcinoma of the breast with associated sclerosing lymphocytic lobulitis. Arch Pathol Lab Med. 2001;125:669-672. [PubMed] |
| 11. | Nio Y, Tsuboi K, Tamaoki M, Tamaoki M, Maruyama R. Lymphoepithelioma-like carcinoma of the breast: a case report with a special analysis of an association with human papilloma virus. Anticancer Res. 2012;32:1435-1441. [PubMed] |
| 12. | O'Sullivan-Mejia E, Idowu MO, Davis Masssey H, Cardenosa G, Grimes MM. Lymphoepithelioma-like carcinoma of the breast: diagnosis by core needle biopsy. Breast J. 2009;15:658-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Peştereli HE, Erdogan O, Kaya R, Karaveli FS. Lymphoepithelioma-like carcinoma of the breast. APMIS. 2002;110:447-450. [PubMed] |
| 14. | Saleh R, DaCamara P, Radhi J, Boutross-Tadross O. Lymphoepithelioma-like carcinoma of the breast mimicking nodular sclerosing Hodgkin’s lymphoma. Breast J. 2005;11:353-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Sanati S, Ayala AG, Middleton LP. Lymphoepithelioma-like carcinoma of the breast: report of a case mimicking lymphoma. Ann Diagn Pathol. 2004;8:309-315. [PubMed] |
| 16. | Rapin V, Contesso G, Mouriesse H, Bertin F, Lacombe MJ, Piekarski JD, Travagli JP, Gadenne C, Friedman S. Medullary breast carcinoma. A reevaluation of 95 cases of breast cancer with inflammatory stroma. Cancer. 1988;61:2503-2510. [PubMed] |
| 17. | Ridolfi RL, Rosen PP, Port A, Kinne D, Miké V. Medullary carcinoma of the breast: a clinicopathologic study with 10 year follow-up. Cancer. 1977;40:1365-1385. [PubMed] |
| 18. | Rakha EA, El-Sayed ME, Green AR, Paish EC, Lee AH, Ellis IO. Breast carcinoma with basal differentiation: a proposal for pathology definition based on basal cytokeratin expression. Histopathology. 2007;50:434-438. [PubMed] [DOI] [Full Text] |
| 19. | Chu JS, Chen CC, Chang KJ. In situ detection of Epstein-Barr virus in breast cancer. Cancer Lett. 1998;124:53-57. [PubMed] |