Published online Dec 10, 2014. doi: 10.5306/wjco.v5.i5.1088
Revised: April 30, 2014
Accepted: July 12, 2014
Published online: December 10, 2014
Processing time: 354 Days and 8 Hours
AIM: To show a new paradigm of simultaneously testing whether breast cancer therapies impact other causes of death.
METHODS: MA.14 allocated 667 postmenopausal women to 5 years of tamoxifen 20 mg/daily ± 2 years of octreotide 90 mg, given by depot intramuscular injections monthly. Event-free survival was the primary endpoint of MA.14; at median 7.9 years, the tamoxifen+octreotide and tamoxifen arms had similar event-free survival (P = 0.62). Overall survival was a secondary endpoint, and the two trial arms also had similar overall survival (P = 0.86). We used the median 9.8 years follow-up to examine by intention-to-treat, the multivariate time-to-breast cancer-specific (BrCa) and other cause (OC) mortality with log-normal survival analysis adjusted by treatment and stratification factors. We tested whether baseline factors including Insulin-like growth factor 1 (IGF1), IGF binding protein-3, C-peptide, body mass index, and 25-hydroxy vitamin D were associated with (1) all cause mortality, and if so and (2) cause-specific mortality. We also fit step-wise forward cause-specific adjusted models.
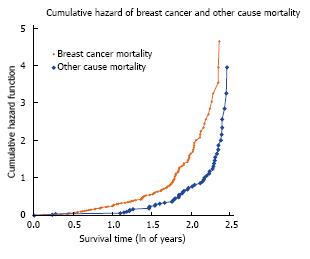
RESULTS: The analyses were performed on 329 patients allocated tamoxifen and 329 allocated tamoxifen+octreotide. The median age of MA.14 patients was 60.1 years: 447 (82%) < 70 years and 120 (18%) ≥ 70 years. There were 170 deaths: 106 (62.3%) BrCa; 55 (32.4%) OC, of which 24 were other malignancies, 31 other causes of death; 9 (5.3%) patients with unknown cause of death were excluded from competing risk assessments. BrCa and OC deaths were not significantly different by treatment arm (P = 0.40): tamoxifen patients experienced 50 BrCa and 32 OC deaths, while tamoxifen + octreotide patients experienced 56 BrCa and 23 OC deaths. Proportionately more deaths (P = 0.004) were from BrCa for patients < 70 years, where 70% of deaths were due to BrCa, compared to 54% for those ≥ 70 years of age. The proportion of deaths from OC increased with increasing body mass index (BMI) (P = 0.02). Higher pathologic T and N were associated with more BrCa deaths (P < 0.0001 and 0.002, respectively). The cumulative hazard plot for BrCa and OC mortality indicated the concurrent accrual of both types of death throughout follow-up, that is the existence of competing risks of mortality. MA.14 therapy did not impact mortality (P = 0.77). Three baseline patient and tumor characteristics were differentially associated with cause of death: older patients experienced more OC (P = 0.01) mortality; patients with T1 tumors and hormone receptor positive tumors had less BrCa mortality (respectively, P = 0.01, P = 0.06). Additionally, step-wise cause-specific models indicated that patients with node negative disease experienced less BrCa mortality (P = 0.002); there was weak evidence that, lower C-peptide (P = 0.08) was associated with less BrCa mortality, while higher BMI (P = 0.01) was associated with worse OC mortality.
CONCLUSION: We demonstrate here a new paradigm of simultaneous testing of therapeutics directed at multiple diseases for which postmenopausal women are concurrently at risk. Octreotide LAR did not significantly impact breast cancer or other cause mortality, although different baseline factors influenced type of death.
Core tip: With earlier detection and improved therapies, patients with early breast cancer simultaneously face multiple health risks; 54% of women ≥ 70 years at diagnosis died from other causes. Octreotide LAR, an early drug targeting the insulin pathway, might have affected both breast and other cause mortality. We demonstrated a method of jointly assessing the impact of therapy and baseline patient characteristics on multiple causes of death. Older patients with higher body mass index experienced more other cause mortality, while women with smaller hormone receptor positive tumours and less lymph node involvement were less likely to die from breast cancer.
- Citation: Chapman JAW, Pritchard KI, Goss PE, Ingle JN, Muss HB, Dent SF, Vandenberg TA, Findlay B, Gelmon KA, Wilson CF, Shepherd LE, Pollak MN. Competing risks of death in younger and older postmenopausal breast cancer patients. World J Clin Oncol 2014; 5(5): 1088-1096
- URL: https://www.wjgnet.com/2218-4333/full/v5/i5/1088.htm
- DOI: https://dx.doi.org/10.5306/wjco.v5.i5.1088
The focus of decades of breast cancer research has been to reduce breast cancer mortality. In the United Kingdom in the early 1970s, 52% of women survived breast cancer for five years; five-year survival improved to 85% in 2005-2009[1]. The US had corresponding improvements with about 75% of women diagnosed during 1975-1977 surviving 5 years, while 89% survived 5 years in 2005-2008[2].
The early 1970s were characterized by women with larger primary breast cancer tumours with greater involvement of lymph nodes and higher risk of systemic disease at diagnosis. Systemic therapy was with less efficacious chemotherapy drugs and regimens; endocrine therapy, when administered, was tamoxifen. We lacked biologic therapy, particularly anti-human epidermal growth factor receptor 2 treatment, and there was an absence of tumour molecular assessments such as intrinsic subtyping and genetic profiling for familial cancer, which precluded our current increasing interest in targeted delivery of therapy. Radiotherapy regimens were more toxic causing more morbidity and therapeutic mortality
Factors contributing to improvements in survival included: the routine use of screening mammography to detect invasive breast cancer earlier and increase detection of non-invasive breast cancer; use of systemic agents to prevent invasive breast cancer in high risk women; and improved management of invasive breast cancer.
Earlier detection of breast cancer has resulted in a decrease in the median age of diagnosis for invasive breast cancer: 65 years at the time Adjuvant! Online was developed[3], to 61 years in the US in 2011[4]. Recent studies have shown that more than fifty percent of women may now be expected to die from a cause other than breast cancer, with an increasing proportion of non-breast cancers occurring at older ages[3,5,6].
Adjuvant! Online was established from the large prospectively and uniformly collected US Surveillance Epidemiology and Endpoints Results data and the Oxford overviews of breast cancer clinical trial meta-analyses[3]. A patient at age 65 who was ER-positive, node negative with 1- to 2-cm tumour without adjuvant therapy would have a 10% chance of dying of breast cancer and 10% chance of dying of other causes in the next 10 years. The Adjuvant! Online website permits clinicians the opportunity to explore trade-offs in risk-benefits for different patient management options for different ages, taking into account the severity of presenting disease, and differing co-morbidity states[4].
Concurrently, experience was reported from a smaller prospectively accrued single institution phase IV cohort at Women’s College Hospital, where routine annual mammography starting at age 35 was employed in the 1960’s, following early scientific reports of its effectiveness[7,8]. This experience was from an era before both routine adjuvant radiotherapy and systemic therapy, and illustrated the effects of early detection[7,8]. At a median 8.2 years follow-up, 20% of those older than 65% and 3% of those under 65 died of other causes (P < 0.001).
We previously reported the effects of competing risks in the NCIC Clinical Trials Group (CTG) MA.17 international phase III randomized placebo-controlled clinical trial of letrozole in post-menopausal hormone-receptor positive breast cancer in women who recently received 5 years of tamoxifen[6]. MA.17 patients had a median age of 62 years. Non-breast cancer deaths accounted for 60% of the known causes of death for all patients, 72% of deaths for those 70 or older.
We face the prospect that older women may simultaneously face dual health risks, increasingly presenting with concomitant competing risks from which at least half of them are likely to die. Women have reaped survival benefits from decades of multi-modality transdisciplinary research that lead us to a new paradigm for early breast cancer management[9]. In this context, it will be important to consider therapeutic regimens which may affect several disease outcomes.
We examine competing risks of mortality in NCIC CTG MA.14 which investigated whether octreotide LAR would affect insulin-like growth factors and subsequent clinical outcome in postmenopausal women with hormone-positive breast cancer[10]. Octreotide was postulated to target the insulin-growth factor pathway so patients were assessed for insulin-growth factor 1 (IGF-1), insulin-growth factor binding protein 3 (IGFBP-3), and C-peptide, a byproduct created when the hormone insulin is produced. Octreotide significantly lowered IGF-1, IGFBP-3, and C-peptide (P < 0.001). High levels of C-peptide and body mass index (BMI) were associated with poor outcome, but octreotide did not significantly affect event-free survival (P = 0.62).
Nevertheless, the MA.14 trial illustrates the new paradigm we propose, that trials simultaneously target and assess more than one chronic disease. Octreotide’s action could affect both breast cancer and insulin-related components of cardiovascular disease. Thus, its efficacy would be better assessed concurrently for multiple disease components of overall survival, rather than examined in totality to avoid confounding therapeutic benefits and trade-offs.
Between 1996 and 2000, NCIC CTG MA.14 (ClinicalTrials.gov Identifier: NCT00002864) enrolled 667 postmenopausal women with histologically proven adenocarcinoma of the breast who underwent lumpectomy or total mastectomy[10]. Patients were to have no previous or concurrent malignancies except adequately treated carcinoma of the skin (basal cell), cervix, endometrium, colon, or thyroid, treated more than 5 years before study entry, and were presumed cured, and had no inter-current illness expected to reduce life expectancy to less than 5 years from the date of surgery. Baseline serum was assessed for IGF-1, IGFBP-3, and C-peptide for 646 patients (96.9%), and 25-hydroxy (OH) vitamin D was centrally assessed for 607 of the MA.14 patients (91%)[10]. More detailed descriptions about patient eligibility and trial conduct are available at ClinicalTrials.gov and in the main trial paper[10].
Patients were randomly assigned to tamoxifen 20 mg orally once daily for 5 years or tamoxifen 20 mg orally once daily for 5 years plus octreotide long-acting release 90 mg intramuscularly monthly for 5 years by minimization (tamoxifen+octreotide). In July 2000, the duration of octreotide was reduced to 2 years because of a greater incidence of gallbladder toxicity in the octreotide arm of National Surgical Adjuvant Breast and Bowel Project (NSABP) B-29.
Patients were stratified by adjuvant chemotherapy (none, concurrent, or sequential), nodal status (none, one to three, four or more, or unknown), and receptor status (ER and/or PgR positive, ER and PgR negative, or ER and PgR unknown).
The primary end point of the trial was event-free survival (EFS); events included recurrence of disease, second malignancy, or death as a result of any cause. Overall survival (OS) was a secondary end point. The effects of insulin-like growth factor physiology on outcome were another secondary objective.
MA.14 investigations were by the intention-to-treat principle. Event-free survival was the primary endpoint of MA.14; at the final analysis at median 7.9 years, the tamoxifen+octreotide and tamoxifen arms had similar event-free survival (P = 0.62). Overall survival was a secondary endpoint, and the two trial arms also had similar overall survival (P = 0.86)[10]. We used the median 9.8 years follow-up in intention-to-treat analyses here.
The primary objective of this investigation was to examine whether there was evidence of competing risks operative in the MA.14 trial, by way of whether there were significantly different factor effects indicated for different causes of death. The Lagakos method was used for this purpose[11]. We assumed independent cause-specific risks for death with or from breast cancer (BrCa) and death from other causes (OC). Fuller details about the method used are reported elsewhere[6].
We tested 3 hypotheses (H1, H2, and H3): (1) H1: A factor does not affect type or time to death, βBrCa = βOC = 0, tested with a likelihood ratio criterion (-2logR) [-χ2(2)], where βBrCa and βOC are the cause-specific effects of the factor. With rejection of H1, H2 was tested; (2) H2: A factor has the same effect for both types of death, βBrCa = βOC , tested with -2logR [-χ2(1)]. With rejection of H2, a factor was differentially associated with type of death so was assessed separately for cause-specific mortality: βBrCa (βOC). When H2 is not rejected, there is a common effect, β, on all cause mortality, which was tested by H3[11]; and (3) H3: A factor is not associated with time to all cause mortality (overall survival), β = 0, tested with -2logR [-χ2(1)], which should produce a significant result due to rejection of H1.
Cumulative hazard plots for breast cancer and other cause mortality were used to examine the presence of substantive competing risks, that is overlapping time periods for different types of death. We then used the Lagakos method described above. Factor effects were examined in models that included all of the factors (“full-factor” models).
We examined baseline MA.14 factors: (1) treatment (tamoxifen vs tamoxifen + octreotide); (2) age (in years); (3) race (white, other); (4) ECOG performance status (0, other); (5) pathologic T (1, other); (6) pathologic N (0, other); (7) breast surgery (segmental mastectomy, other); (8) IGF-1 (continuous); (9) IGFBP-3 (continuous); (10) C-peptide (continuous); (11) weight (kg), (12) body mass index (BMI; continuous); (13) vitamin D (continuous), and baseline values of the stratification factors; (14) number of positive nodes (0, Nx, 1-3 +, ≥ 4 +); (15) hormone receptor status (ER-PR-, unknown, ER+ and/or PR +); and (16) adjuvant chemotherapy (no, yes).
With log-normal survival analysis, the natural logarithm of survival time, Y = ln(t), is a linear function, Y = α + Σβj z j + σW , where σ is a scale parameter; W is N(0,1); zj, jth baseline factor; and βj, effect of jth factor.
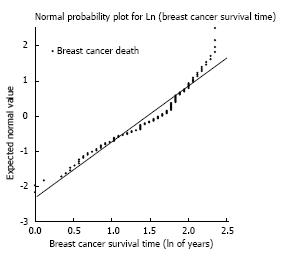
A normal probability plot was utilized to examine the assumption of log-normal survival time. The final model fit was examined with standardized residual plots. Treatment and baseline values of the three stratification factors had forced inclusion in the multivariable models to account for MA.14 design structure. Other factors were considered in stepwise forward regression analyses, including a factor with P value ≤ 0.05 by -2logR [-χ2(1)]. Patient experience was depicted with log-normal survivor plots, where factor effects were adjusted for the effects of treatment, and other significant factors. Breast cancer and other cause 5-year and 10-year survival were reported. Royston described the log-normal model as a pragmatic tool that provides a continuous estimate of prognosis[12]. Of note, both Kaplan-Meier and Cox plots present discontinuous estimates of prognosis.
We used the MA.14 median 9.8 years follow-up for our analysis. The median age of MA.14 patients was 60.1 years: 547 (82%) < 70 years and 120 (18%) ≥ 70 years. MA.14 patients experienced 170 deaths: 106 (62.3%) BrCa; 55 (32.4%) OC, of which 24 were other malignancies, 31 other causes of death; 9 (5.3%) patients had unknown cause of death, so were excluded from competing risk assessments.
The analyses were performed on 329 patients allocated tamoxifen and 329 allocated tamoxifen+octreotide (Table 1). There were no significant differences in the number of BrCa and OC deaths by treatment arm (P = 0.40): tamoxifen patients experienced 50 BrCa and 32 OC deaths, while tamoxifen+octreotide patients experienced 56 BrCa and 23 OC deaths. Proportionately more deaths (P = 0.004) were from BrCa for patients < 70 years, where 70% of deaths were due to BrCa, compared to 54% for those ≥ 70 years of age. The proportion of deaths from OC increased with increasing BMI (P = 0.02). Meanwhile, higher pathologic T and N were associated with more BrCa death (P < 0.0001 and 0.002, respectively).
| Factor | Number of patients | Breast cancer | Other cause1 | P-value2 |
| Treatment | ||||
| Tamoxifen | 329 (50) | 50 | 32 | |
| Tamoxifen | ||||
| + Octreotide LAR | 329 (50) | 56 | 23 | 0.40 |
| Age | ||||
| < 70 | 544 (83) | 85 | 37 | |
| ≥ 70 | 114 (17) | 21 | 18 | 0.004 |
| BMI | ||||
| < 25 | 185 (28) | 30 | 7 | |
| 25-30 | 221 (34) | 29 | 20 | |
| > 30 | 213 (32) | 41 | 24 | 0.02 |
| Tumour size | ||||
| T1 | 382 (58) | 38 | 26 | |
| ≥ T2, unknown | 276 (42) | 68 | 29 | < 0.0001 |
| Nodal status | ||||
| N0 | 346 (53) | 39 | 29 | |
| N1, N2, Nx | 312 (47) | 67 | 26 | 0.002 |
The cumulative hazard plot for BrCa and OC mortality (Figure 1) indicates the concurrent accrual of both types of death throughout follow-up, or the existence of competing risks of mortality. Meanwhile, the normal probability plot (Figure 2) supports the assumption of a log normal model up to about 10 years follow-up. We utilized log-normal survival analysis for the competing risks assessments, providing survival experience up to 10 years.
Table 2 indicates the competing risks results. MA.14 therapy was not associated with mortality (P = 0.77). Baseline factors associated with mortality were age (P = 0.02), tumour size (P = 0.01), nodal status (P = 0.002), number of positive nodes (P < 0.0001), and hormone receptor status (P = 0.08). Age (P = 0.03), tumour size (P = 0.04), and hormone receptor status (P = 0.04) were differently associated with BrCa and OC so cause-specific multivariate analyses were used to investigate their effects. Nodal status (P = 0.54) and number of positive nodes (P = 0.89) did not show different associations with BrCa and OC, but were associated with all cause mortality (P < 0.0001).
| Factor | Association with death | Association by type of death | ||
| χ21 | P-value | χ22 | P-value | |
| Treatment (TAM, TAM + OCT) | 0.52 | 0.77 | ||
| Age (yr) | 7.90 | 0.02 | 4.73 | 0.03 |
| Race (white, other) | 1.85 | 0.40 | ||
| ECOG performance status (0, other) | 0.56 | 0.75 | ||
| Tumour size (T1, ≥ T2, unknown) | 10.55 | 0.01 | 4.22 | 0.04 |
| Nodal status (N0, N1, N2, Nx) | 12.79 | 0.002 | 0.38 | 0.543 |
| Surgery (segmental mastectomy, other) | 0.04 | 0.98 | ||
| IGF-1 (continuous) | 1.17 | 0.56 | ||
| IGFBP-3 (continuous) | 0.19 | 0.91 | ||
| C-peptide (continuous) | 3.86 | 0.15 | ||
| Weight (kg) | 0.27 | 0.88 | ||
| BMI (continuous) | 1.38 | 0.50 | ||
| Vitamin D (continuous) | 1.05 | 0.59 | ||
| Number of positive nodes | ||||
| (0, Nx, 1-3+, ≥ 4+) | 19.43 | < 0.0001 | 0.02 | 0.893 |
| Hormone receptor status (ER-PR-, | ||||
| unknown, ER+ and/or PR+) | 5.11 | 0.08 | 4.19 | 0.04 |
| Adjuvant chemotherapy (no, yes) | 2.39 | 0.30 | ||
The step-wise multivariate models are provided for BrCa and OC in Table 3. Treatment did not significantly affect BrCa (P = 1.0) or OC (P = 0.49). The stratification factor adjuvant chemotherapy (P = 0.20) was not significantly associated with BrCa mortality; however, a lower number of positive lymph nodes (P = 0.002) and hormone receptor positivity (P = 0.03) were associated with less BrCa mortality. Larger tumours (P = 0.0003) led to more BrCa mortality. there was weak evidence that, lower C-peptide (P = 0.08) was associated with less BrCa mortality. None of the stratification factors impacted OC mortality. Older patients (P = 0.002) and higher BMI (P = 0.01) were associated with more OC mortality.
| Factor1 | β(SE)2 | 95%CI2 | P-value3 |
| Breast cancer survival4 | |||
| Treatment (Tamoxifen, Tamoxifen + Octreotide LAR) | 0.0008(0.17) | [-0.33, 0.33] | 1.00 |
| Adjuvant chemotherapy (no, yes) | 0.2553 (0.20) | [-0.14, 0.65] | 0.20 |
| Number of positive nodes | |||
| (0, Nx, 1-3, ≥ 4+) | -0.2524 (0.08) | [-0.41,-0.10] | 0.002 |
| Hormone receptor status (ER-PR-, unknown ER/PR, ER+ and/or PR+) | 0.2821 (0.13) | [0.03,0.54] | 0.03 |
| Tumour size (T1, ≥ T2, unknown) | -0.6521 (0.18) | [-1.00,-0.30] | 0.0003 |
| Other Cause Survival | |||
| Treatment (Tamoxifen, Tamoxifen + Octreotide LAR) | 0.1236 (0.18) | [-0.23, 0.48] | 0.49 |
| Adjuvant Chemotherapy (no, yes) | -0.0703 (0.23) | [-0.52, 0.38] | 0.76 |
| Number of positive nodes | |||
| (0, Nx, 1-3, ≥ 4+) | -0.0723 (0.08) | [-0.23, 0.08] | 0.38 |
| Hormone receptor status (ER-PR-, unknown ER/PR, ER+ and/or PR+) | -0.3852 (0.26) | [-0.89,0.12] | 0.13 |
| Age (years) | -0.0389 (0.01) | [-0.06,-0.02] | 0.002 |
| BMI (continuous) | -0.0395 (0.01) | [-0.06,-0.02] | 0.01 |
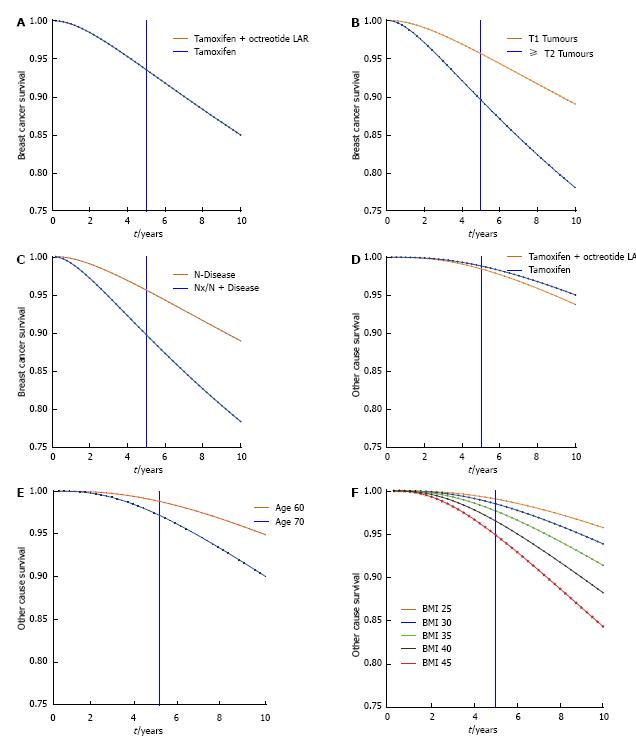
Adjusted 5 and 10 year survival is presented in Table 4 and depicted graphically for BrCa by treatment (Figure 3A), tumour size (Figure 3B), and lymph node involvement (Figure 3C) and for OC by treatment (Figure 3D), age (Figure 3E), and BMI (Figure 3F). In both the Table and plots survival is adjusted (as appropriate) for the effects of treatment, (other) stratification factors, and other significant factors.
| Factor | 5 yr1 | 10 yr1 |
| Breast cancer survival | ||
| Treatment | ||
| Tamoxifen | 0.94 | 0.85 |
| Tamoxifen + Octreotide LAR | 0.94 | 0.85 |
| Tumour size | ||
| T1 | 0.96 | 0.89 |
| ≥ T2, unknown | 0.90 | 0.78 |
| Nodal status | ||
| N0 | 0.96 | 0.89 |
| N1, N2, Nx | 0.90 | 0.78 |
| Other cause survival | ||
| Treatment | ||
| Tamoxifen | 0.98 | 0.94 |
| Tamoxifen + Octreotide LAR | 0.99 | 0.95 |
| Age | ||
| 60 | 0.99 | 0.95 |
| 70 | 0.97 | 0.90 |
| BMI | ||
| 25 | 0.99 | 0.96 |
| 30 | 0.98 | 0.94 |
| 35 | 0.98 | 0.91 |
| 40 | 0.97 | 0.88 |
| 45 | 0.95 | 0.84 |
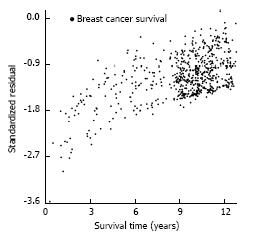
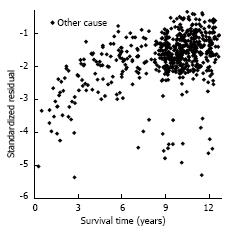
After the log-normal model was fit, each patient’s observed survival time was compared to her expected survival time, based on patient and tumour characteristics. i.e., for patient i, with survival time ti [so, yi = ln(ti)], [yi - (α + Σβj z ij)]/σ] would be expected to have approximately a standard normal distribution with mean 0 and standard deviation 1. A plot of all the patient standardized residuals examines whether departures from the multivariate BrCa (Figure 4) and OC (Figure 5) models appear to be ascribable as random error. The residuals indicate that the yi (ln of survival times) are consistently smaller than they are expected to be; there are explanatory factors for both BrCa and OC that are not included in this investigation.
The addition of octreotide therapy to tamoxifen in MA.14 was an early strategy to test the impact of targeting the insulin-like growth factor pathway for early postmenopausal breast cancer patients. In the main trial analyses, octreotide was not found to impact either the primary endpoint of EFS or OS potentially because the administration of octreotide was reduced to 2 years (from the originally planned 5 years) due to excess gallbladder toxicity[10]. The trial was designed with the expectation that the 5 year EFS rate for patients allocated tamoxifen would be 73%. For patients treated with tamoxifen alone, the 5-year BrCa was 94%, while the 5-year OC experience was 98%. The MA.14 trial was under-powered to detect a significant octreotide effect.
However, the main trial indicated a possible role for the insulin-like growth factor pathway with significant treatment associated changes in the mandatory correlative biomarkers of IGF-1, IGFBP-3, and C-peptide as well as in BMI. Further, higher C-peptide levels, or higher BMI, were significantly associated (P < 0.001) with worse EFS. As EFS involved both breast cancer relapse and all cause mortality, the questions we addressed here were whether the patients simultaneously had substantive risks of both BrCa and OC mortality, and if so, whether there were differential effects of factors on cause-specific mortality.
We found that the early postmenopausal patients, 91% of whom had hormone-receptor positive tumours, faced the risk of both BrCa and OC mortality. The prognostic breast cancer factors of larger tumour size (P = 0.0003), higher number of positive lymph nodes (P = 0.002), and a tumour that was hormone receptor negative (P = 0.03) were associated with more BrCa mortality. Meanwhile, older age (P = 0.002) and higher BMI were associated with more OC mortality. The increase in OC mortality with older age replicates what was found with the NCIC CTG MA.17 trial with extended adjuvant endocrine therapy where patients were randomized to 5 years of letrozole or placebo following 5 years of tamoxifen[6].
Adjusted survival estimates were provided for BrCa by treatment, tumour size, lymph node status, and for OC by age and BMI. Graphical presentations were to 10 years, along with a table of specific 5- and 10-year survival rates.
This study represents a secondary use of a breast cancer trial database. We examined whether prognostic risk factors similarly impacted both BrCa and OC, and found that there was differential association of baseline factors and type of death. Age and higher BMI were associated with OC mortality, while T status, nodal involvement and hormone receptor status were associated with BrCa mortality. Adjuvant chemotherapy was neither mandated nor prohibited; 33% of patients received adjuvant chemotherapy (Pritchard et al[10]). However, in this trial, age was not associated with BrCa death, but OC mortality.
There is a limitation in ability to delineate comorbidity with MA.14 data. Eligibility criteria were that patients have no previous or concurrent other malignancy except for carcinoma of the skin, cervix, endometrium, colon, or thyroid adequately treated 5 or more years before study entry, and had no inter-current illness expected to reduce life expectancy to less than 5 years after surgery. The number of non-breast cancer deaths was too few to refine investigations since with a median 9.8 years follow-up, 24 patients experienced other malignancies and 31 other causes of death; the two classifications were combined. Prospective collection of death type in larger trial populations, over longer follow-up would be needed to improve disease-specific interpretation.
The MA.14 trial with octreotide was an early attempt at targeting the insulin pathway for adjuvant postmenopausal breast cancer patients. octreotide therapy did not impact either BrCa (P = 1.00) or OC (P = 0.49) mortality. However, the methodology demonstrated here may be of interest in eventually assessing the effects of competing risks and the insulin pathway on both BrCa and OC mortality in the much larger metformin placebo-controlled trial, NCIC CTG MA.32.
Alternatively, other joint assessment of chronic diseases is conceptually possible in trials where therapies may be postulated to have dual modes of action. NCIC CTG MA.27 originally had a factorial design with administration of celecoxib that was postulated to also be an anti-breast cancer agent until excess cardiac toxicity led to the closing of the celecoxib arms[13]. The dual modalities for MA.27 would have been breast cancer and possibly musculoskeletal or arthralgic sequelae[14] which could also be targets of celecoxib therapy. The prevention trial, NCIC CTG MAP.3, likewise had to close its celecoxib arms so is not a good venue for this joint testing[15]. We await the results of the REACT trial which was able to accrue its full sample size to celecoxib, and postulate that it will be a good candidate for this modality of joint testing[16].
In summary, the progress for earlier detection and improved management of breast cancer means that women with early breast cancer will increasingly face joint mortality risks of other chronic diseases. This situation raises the opportunity for a new paradigm of simultaneously targeting several chronic diseases, or at least assessing the joint risks of mortality, for a better understanding of predisposition of patients to mortality other than breast cancer. MA.14 was an early trial of the insulin pathway targeted therapy, octreotide, which conceptually may have affected both BrCa and OC mortality, so was used here to demonstrate analyses that might be considered for the new assessment framework. The current work is offered as a proof of principle to demonstrate the relevance of collecting detailed cause-specific mortality data and factors which may be associated with non-breast cancer death.
Improvements in detection and management of breast cancer lead to the prospect that many postmenopausal women will now not die from the disease as they face joint mortality risks of other chronic diseases. Investigators need to determine baseline characteristics differentially associated with breast cancer vs other causes of death for more effective overall patient care. This paper proposes a method that might provide a basis for such discernment. The method is demonstrated for a phase III clinical trial which tested an early target of the insulin pathway that could have impacted both breast cancer and other cause mortality.
Successful development of therapies which would simultaneously target several chronic diseases would improve whole population survival. Additionally, the management of elderly breast cancer patients is currently an important research area given the general increase in proportion of older people worldwide.
The usual process of inferring different factors influencing different causes of death involves qualitative observations. The competing risks method demonstrated here has quantitative formal tests. Older age and higher BMI increased the prospect that a patient would die of other causes than breast cancer, as did patients having smaller, hormone-receptor positive tumours and less lymph node involvement. The authors also reported quantitative patient survival over time by type of death.
This work could be extended to existing uniformly collected databases where there was careful collection of cause of death to better delineate factors affecting a broader range of competing risks of death. The more routine collection of multiple causes of death would assist future work.
Log-normal survival analysis examines the linear effects of factors on the natural logarithm of survival time to provide smooth continuous functions and plots of factor effects.
Well-prepared article offered new insights in breast cancer research and in evaluation of treatment, particularly in postmenopausal women.
P- Reviewer: Vetvicka V S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Cancer survival statistics for common cancers. Cancer Research UK. Available from: http: //www.cancerresearchuk.org/cancer-info/cancerstats/survival/common-cancers/. |
| 2. | Cancer advances in focus. Breast cancer. National Cancer institute at the National Institutes of Health. Available from: http: //www.cancer.gov/cancertopics/factsheet/cancer-advances-in-focus/breast.. |
| 3. | Ravdin PM, Siminoff LA, Davis GJ, Mercer MB, Hewlett J, Gerson N, Parker HL. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Breast cancer facts and figures 2011-2012. American Cancer Society. Available from: http: //www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-030975.pdf. |
| 5. | Fish EB, Chapman JA, Link MA. Competing causes of death for primary breast cancer. Ann Surg Oncol. 1998;5:368-375. [PubMed] |
| 6. | Chapman JA, Meng D, Shepherd L, Parulekar W, Ingle JN, Muss HB, Palmer M, Yu C, Goss PE. Competing causes of death from a randomized trial of extended adjuvant endocrine therapy for breast cancer. J Natl Cancer Inst. 2008;100:252-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Trudeau ME, Pritchard KI, Chapman JA, Hanna WM, Kahn HJ, Murray D, Sawka CA, Mobbs BG, Andrulis I, McCready DR. Prognostic factors affecting the natural history of node-negative breast cancer. Breast Cancer Res Treat. 2005;89:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Sun J, Chapman J, Gordon R, Sivaramakrishna R, Link M, Fish E. Survival from primary breast cancer after routine clinical use of mammography. Breast J. 2002;8:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Giordano SH, Hortobagyi GN. Time to remove the subspecialty blinders: breast cancer does not exist in isolation. J Natl Cancer Inst. 2008;100:230-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Pritchard KI, Shepherd LE, Chapman JA, Norris BD, Cantin J, Goss PE, Dent SF, Walde D, Vandenberg TA, Findlay B. Randomized trial of tamoxifen versus combined tamoxifen and octreotide LAR Therapy in the adjuvant treatment of early-stage breast cancer in postmenopausal women: NCIC CTG MA.14. J Clin Oncol. 2011;29:3869-3876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Lagakos SW. A covariate model for partially censored data subject to competing causes of failure. Appl Stat. 1978;27:235–241. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Royston P. The lognormal distribution as a model for survival time In cancer, with an emphasis on prognostic factors. Stat Neerlandica. 2001;55:89-104. [RCA] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Goss PE, Ingle JN, Pritchard KI, Ellis MJ, Sledge GW, Budd GT, Rabaglio M, Ansari RH, Johnson DB, Tozer R. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27--a randomized controlled phase III trial. J Clin Oncol. 2013;31:1398-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 14. | Ingle JN, Schaid DJ, Goss PE, Liu M, Mushiroda T, Chapman JA, Kubo M, Jenkins GD, Batzler A, Shepherd L. Genome-wide associations and functional genomic studies of musculoskeletal adverse events in women receiving aromatase inhibitors. J Clin Oncol. 2010;28:4674-4682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, McTiernan A, Robbins J, Johnson KC, Martin LW. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 672] [Article Influence: 48.0] [Reference Citation Analysis (0)] |