Published online Dec 10, 2014. doi: 10.5306/wjco.v5.i5.1060
Revised: April 16, 2014
Accepted: June 10, 2014
Published online: December 10, 2014
Processing time: 301 Days and 15.3 Hours
AIM: To analyze experiences to identify treatment outcomes and prognostic factors in a Saudi population.
METHODS: Medical records of patients with brainstem gliomas treated from July 2001 to December 2012 were reviewed to identify treatment outcomes of surgery, radiation therapy and chemotherapy and associated prognostic factors in a Saudi population.
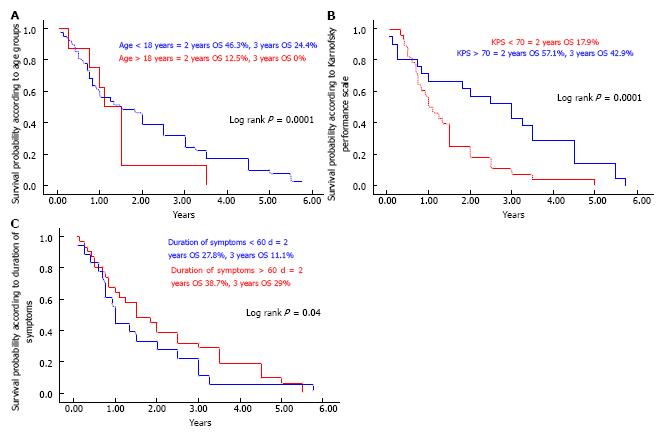
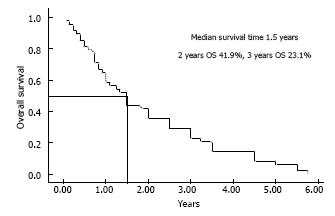
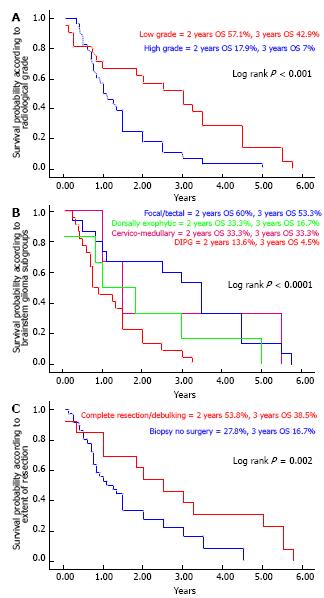
RESULTS: We analyzed 49 brain stem glioma (BSG) patients from July 2001 to December 2012; 31 of them were males (63.3%) with a median age of 12.6 years (range: 8-64 mo). Twenty-two patients (44.9%) had diffuse intrinsic pontine gliomas (DIPG) and 15 (30.6%) presented with focal/tectal BSG. Histopathology was available in 30 patients (61.2%). Median survival time for the whole cohort was 1.5 years. One and two year OS rates were 51.1% and 41.9% respectively. Two year OS rates for focal/tectal, dorsally exophytic, cervicomedullary and DIPG tumors were 60%, 33.3%, 33.3% and 13.6% respectively (P < 0.0001). Significant prognostic factors related to OS were age at diagnosis (worse for > 18 years) P = 0.01, KPS < 70 P = 0.02, duration of symptoms (< 60 d) P = 0.002, histology (better for favorable) P = 0.002, surgery (maximal resection) P = 0.002, and concurrent chemotherapy with radiation therapy in DIPG (better if given) P = 0.01.
CONCLUSION: BSG, especially the DIPG subgroup, had a dismal prognosis, needing more aggressive neurosurgical, radiation and chemotherapy techniques, while focal and tectal tumors were found to have a better prognosis.
Core tip: Brain stem gliomas (BSG) are a heterogeneous group of tumors with a poor prognosis. We analyzed 49 BSG patients from July 2001 to December 2012 with a median age of 12.6 years (range: 8-64 mo). Twenty-two patients (44.9%) had diffuse intrinsic pontine gliomas (DIPG) and 15 (30.6%) presented with focal/tectal BSG. Histopathology was available in 30 patients (61.2%). Median survival time for the whole cohort was 1.5 years. One and two year OS rates were 51.1% and 41.9% respectively. Two year OS rates for focal/tectal, dorsally exophytic, cervicomedullary and DIPG tumors were 60%, 33.3%, 33.3% and 13.6% respectively (P < 0.0001). We concluded that BSG, especially the DIPG subgroup, had a dismal prognosis, needing more aggressive neurosurgical, radiation and chemotherapy techniques, while focal and tectal tumors were found to have a better prognosis.
- Citation: Bayoumi Y, Sabbagh AJ, Mohamed R, ElShokhaiby UM, Maklad AM, Tunio MA, Balbaid AAO. Clinicopathological features and treatment outcomes of brain stem gliomas in Saudi population. World J Clin Oncol 2014; 5(5): 1060-1067
- URL: https://www.wjgnet.com/2218-4333/full/v5/i5/1060.htm
- DOI: https://dx.doi.org/10.5306/wjco.v5.i5.1060
Brain stem gliomas (BSG) account for about 10%-20% of all central nervous system tumors in children and 1%-2% in adults[1,2]. Traditionally, the term “brain stem glioma” was designed as a clinical diagnosis without histological confirmation because the morbidity for surgical intervention within the pons was high and the relevance of a histological diagnosis was low. With the advent of newer diagnostic modalities, BSG are now considered a heterogeneous group of tumors which are mainly divided into three categories according to treatment and prognosis[3]: (1) the dorsally exophytic and cervicomedullary tumors appear to benefit significantly from surgical resection[3]; (2) focal tectum glioma (solid or cystic) may be associated with a long history of symptoms and with neurofibromatosis type I[4]; and (3) the largest subgroup of diffuse intrinsic pontine glioma (DIPG), in contrast, have a poor prognosis[5]. The DIPG subgroup clearly differs from focal, dorsally exophytic and cervicomedullary tumors on various points as DIPG is typically seen with rapidly progressing symptoms and signs comprising multiple erratic cranial nerve palsies, long track deficits, cerebellar symptoms and/or raised intracranial pressure with a median survival of 9 mo[6,7]. Gadolinium enhanced magnetic resonance imaging (MRI) allows easy confirmation of diagnosis for DIPG and high-grade BSG[8].
Surgery is the mainstay of therapy for focal, dorsally exophytic and cervicomedullary BSG; however, for DIPG the radiation therapy remains the standard treatment option[9,10]. Various chemotherapeutic agents investigated as monotherapy neoadjuvant agents (carboplatin or irinotecan) or as combination neoadjuvant chemotherapeutic agents (carboplatin, etoposide and vincristine or cisplatin, cyclophosphamide, etoposide and vincristine) have offered no significant improvements[11,12]. Similarly, concurrent chemotherapeutic agents (etanidazole, topotecan, carboplatin and temozolomide) or high-dose chemotherapy followed by stem cell support have not shown any significant improvements in overall and progression free survival rates[13,14].
Our aim was to evaluate the frequency of BSG and to identify treatment outcomes of surgery, radiation therapy and chemotherapy and associated prognostic factors in a Saudi population.
After formal approval from the institutional ethical committee, medical charts of patients with confirmed brainstem gliomas who were treated in our hospital were reviewed. Patients were selected if they met the following criteria.
Availability of a complete medical record: (1) demographic data [age at diagnosis, gender, main symptoms and duration, performance status according to Karnofsky Performance Scale (KPS) and main neurological signs]; (2) radiological characteristics of the tumors on MRI (T1 and T2-weighted images); and (3) surgical procedures including histopathological characteristics and other treatment modalities (radiation therapy and chemotherapy).
The epicenter (main bulk) of the tumor was located in the brainstem (midbrain, pons and medulla oblongata) and diagnosis was either based on clinical history and characteristic MRI features or histopathological confirmation.
Exclusion criteria were: (1) the epicenter of the tumor was located in the thalamus, cerebellar peduncles or cervical spinal cord; and (2) suspicion of infection could not be ruled out on MRI in the absence of biopsy results.
The National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 2.0 was used to score acute radiation and chemotherapy toxicity (< 90 d from the start of radiation therapy). The Radiation Therapy Oncology Group Late Radiation Morbidity Scoring Criteria was used to score radiation toxicity persisting beyond 90 d from the completion of radiotherapy.
Functional recovery after surgical and other treatment modalities was assessed. Radiological response to radiotherapy and chemotherapy was reported according to Response Evaluation Criteria in Solid Tumors (RECIST): (1) a complete response (CR), i.e., disappearance of all visible tumor; (2) a partial response (PR), i.e., a decrease of > 50% in the axial cross-section of the greatest surface area; (3) progressive disease (PD), i.e., > 25% increase in axial cross-section of the greatest surface area; or (4) stable disease (SD), i.e., all other situations.
The primary endpoints were functional recovery, response rates and the overall survival. Progression-free survival (PFS) was defined as the duration between the completion of treatment and the date of documented disease progression, death resulting from the cancer and/or last follow-up visit (censored). Overall survival (OS) was defined as the duration between the completion of treatment and the date of patient death or last follow-up visit (censored). The probabilities of OS were determined with the Kaplan-Meier method and its 95%CI by the Rothman method. The comparisons for various endpoints were performed using the log-rank test. A P value of 0.05 was considered statistically significant. The multivariate analysis was used to test prognostic factors in multivariate analysis. Results are expressed with relative risk and its 95%CI. Statistical analyses were performed using the computer program SPSS (Statistical Package for the Social Sciences, version 17.0, SPSS Inc., Chicago, IL).
Between July 2001 and December 2012, 49 patients with BSG from the institutional database fulfilled the criteria and were analyzed.
Among forty-nine patients, the majority of the cohort consisted of children and adolescents (81.6%) with a median age of 12.62 years (range: 0.8-64). An age of > 18 years at diagnosis was associated with a significantly shorter OS compared with a younger age (P = 0.0001) (Figure 1A). Median Karnofsky performance status (KPS) at diagnosis was 80 (range: 40-100). KPS < 70 was related to a shorter OS (P = 0.0001) (Figure 1B). Main symptoms at time of diagnosis were headaches (42.8%), diplopia or squint (38.8%), gait disturbance (34.7%), nystagmus (37.7%) and difficulty in swallowing or choking (26.5%). Mean duration of symptoms before diagnosis was 83.4 ± 47.5 d. Patients with short duration of symptoms (< 2 mo) had poor OS (P = 0.04) (Figure 1C). Main neurological signs were cranial nerve palsies, mainly VI, VII, IX, X (65.3%), cerebellar dysfunction (51%), bilateral papilledema (38.8%), nystagmus (37.7%) and motor weakness (28.6%). Histopathological diagnosis was available in 30 patients (61.2%), mainly of astrocytic origin (23/28) and high grade (63.3%) (Table 1).
| Mean age at diagnosis (yr) | 12.62 (0.8-64) SD ± 13.42 | Diffuse astrocytoma grade II | 4/30 (13.3%) |
| Gender | Anaplastic astrocytoma | 10/30 (33.3%) | |
| Male | 31 (63.3%) | Glioblastoma multiforme | 4/30 (13.3%) |
| Female | 18 (36.7%) | Astroblastoma | 1/30 (3.3%) |
| According to age | Nonspecified glioma | 5/30 (16.7%) | |
| Children and adolescents | 40 (81.6%) | No | 19 (38.8%) |
| Adults | 9 (18.4%) | Radiological diagnosis | |
| Duration of symptoms (d) | 83.4, SD ± 47.5 | Focal | 12 (24.5%) |
| Karnofsky Performance Status | 80 (50-100) | Tectal | 3 (6.1%) |
| Symptoms at time of presentation | Dorsally exophytic | 6 (12.2%) | |
| Headache | 21 (42.8%) | Cervicomedullary | 3 (6.1%) |
| Vomiting | 11 (22.4%) | DIPG | 22 (44.9%) |
| Diplopia/ squint | 19 (38.8%) | Others | 3 (6.1%) |
| Unsteady gait | 17 (34.7%) | Surgery | 30/49 (61.2%) |
| Difficulty in swallowing or choking | 13 (26.5%) | Total/maximal resection | 14/30 (36.7%) |
| Motor weakness/ paresis | 10 (20.4%) | Subtotal resection | 14/30 (36.7%) |
| Convulsions | 4 (8.2%) | Biopsy only | 2/30 (6.1%) |
| Dysphonia/ dysarthria | 11 (22.4%) | VP shunt | 19/30 (63.3%) |
| Altered consciousness | 5 (10.2%) | ETV | 4/30 (13.3%) |
| Isolated facial paresis | 6 (12.2%) | EDV | 5/30 (16.7%) |
| Hearing problems | 3 (6.1%) | IONP | 14/30 (36.7%) |
| Fever | 2 (4.1%) | Radiation therapy | 32/49 (65.3%) |
| Failure to thrive | 1 (2.0%) | Postoperative | 14/32 (43.7%) |
| Neurological signs at time of presentation | Radiotherapy alone | 18/32 (56.3%) | |
| Mental status change | 8 (16.3%) | Total dose (Gy) | 50.4-59.4 |
| Cranial nerve palsies | 32 (65.3%) | Fractions | 30-33 |
| Trigeminal | 2 (6.3%) | Duration (wk) | 6-6.5 |
| Abducens | 18 (56.3%) | Technique | |
| Facial | 12 (37.5%) | 3DCRT | 15 (46.9%) |
| Vestibulocochlear | 2 (6.3%) | IMRT | 17 (53.1%) |
| Glossopharyngeal | 12 (37.5%) | Chemotherapy | 23/49 (46.9%) |
| Vagus | 8 (25.0%) | Concurrent | 12/23 (52.2%) |
| Motor deficit | 14 (28.6%) | TMZ | 12/12 (100%) |
| Sensory deficit | 6 (12.2%) | Neoadjuvant | 7/23 (30.4%) |
| Bilateral Babinski sign | 13 (26.5%) | Vincristine + carboplatin | 4/7 (51.1%) |
| Cerebellar signs | 25 (51.0%) | High dose chemotherapy with stem cell rescue | 2/7 (28.7%) |
| Nystagmus | 17 (34.7%) | Cyclophosphamide | 1/7 (14.3%) |
| Bilateral papilledema | 19 (38.8%) | Adjuvant/ salvage | 11/23 (47.8%) |
| Pathological diagnosis | BCNU + procarbazine + vincristine | 5/11(45.7%) | |
| Yes | 30 (61.2%) | Vincristine + carboplatin | 4/11 (36.2%) |
| Pilocytic astrocytoma | 6/30 (20.0%) | Irinotecan + bevacizumab | 2/11 (18.1%) |
The main MRI characteristics are illustrated in Table 2.
| Subgroups | Enhancement enhancing | Non-enhancing | T2W image hyper | Intensity mixed | Character | ||
| Cystic | Solid | Mixed | |||||
| Focal (12) | 17 (100) | - | 10 (83.3) | 2 (16.7) | 3 (25) | 6 (50) | 3 (25) |
| Focal tectal (3) | 2 (66.7) | 1 (33.3) | 1 (33.3) | 2 (66.7) | 3 (100) | ||
| Dorsally exophytic (6) | 4 (66.7) | 2 (33.3) | 3 (50.0) | 3 (50.0) | 4 (667) | 2 (33.3) | |
| Cervicomedullary (3) | 2 (66.7) | 1 (33.3) | - | 3 (100) | 3 (100) | ||
| DIPG (22) | 10 (45.5) | 12 (54.5) | 10(45.4%) | 12 (54.5) | 19 (86.4) | 3 (13.6) | |
On MRI, patterns were identified representing non-enhancing diffusely infiltrative tumors (54.5%), contrast-enhancing localized masses (33.3%) and tectal tumors (33.3%). Presumed necrosis on MRI, defined as a zone of irregularly shaped T1 hyposignal surrounded by contrast enhancement, was found in 5 (10.2%) patients.
Thirty patients (61.2%) had surgery. Complete resection was done in dorsally exophytic (83.3%), focal tectal (66.7%) and focal (50%) tumors. Cerebrospinal fluid (CSF) shunts, including ventriculoperitoneal shunt, endoscopic third ventriculostomy and endoscopic ventricular drain was performed in 28/49 patients (57.1%) to control raised intracranial pressure. Interestingly, 6/22 patients (27.3%) with DIPG underwent surgical debulking (Table 3). Postoperative radiation therapy was given in 14/32 patients (43.7%) and radical radiation therapy with and without chemotherapy was given in 18/32 patients (56.3%). Mean duration of time between surgery and starting radiation therapy was 25 d (range: 21-28). The majority of cases (17/32) were treated with intensity modulated radiation therapy (Table 4). Among all 32 patients who received radiation therapy, the treatment protocol completion rate was 90% (95%CI, 85-100). Chemotherapy in an adjuvant or salvage setting was given mainly for the DIPG subgroup and patients with leptomeningeal dissemination which was seen in 5/49 patients (10.2%) (Table 5).
| Subgroups | Resection | VP shunt | ETV | EVD | IONP | |
| Complete | Incomplete/biopsy | |||||
| Focal (12) | 6 (50.0) | 6 (50.0) | 3 (25.0) | 1 (8.3) | 2 (16.6) | 5 (41.7) |
| Focal tectal (3) | 2 (66.7) | 1 (33.3) | 2 (66.7) | 1 (33.3) | 1 (33.3) | 2 (66.7) |
| Dorsally exophytic (6) | 5 (83.3) | 1 (16.7) | 3 (50.0) | - | 2 (33.3) | 5 (83.3) |
| Cervicomedullary (3) | 1(33.3) | 2 (66.7) | - | - | - | 2 (33.3) |
| DIPG (22) | - | 6 (27.3) | 11 (50) | 2 (9.0) | - | 1 (4.5) |
| Subgroups | Indication | Technique | Total dose (Gy) | ||
| Postoperative | Radical | 3DCRT | IMRT | ||
| Focal (12) | 6 (50) | - | 3 (50) | 3 (50) | 50.4-54 |
| Focal tectal (3) | 1 (33.3) | - | - | 1 (100) | 54 |
| Dorsally exophytic (6) | 1 (16.7) | - | 1 (100) | - | 54 |
| Cervicomedullary (3) | 2 (66.7) | - | 2 (100) | - | 50.4-54 |
| DIPG (22) | 6 (27.3) | 16(72.7) | 9 (40.9) | 13 (59.1) | 54-59.4 |
| Subgroups | Neoadjuvant | Concurrent | Adjuvant/salvage |
| Focal (12) | - | 3 (25.0) | |
| Focal tectal (3) | - | - | - |
| Dorsally exophytic (6) | - | - | - |
| Cervicomedullary (3) | 1 (33.3) | - | 2 (66.6) |
| DIPG (22) | 6 (27.3) | 12 (54.5) | 6 (27.3) |
Common acute grade 2 radiation induced toxicities were nausea and vomiting (30/32) and worsening of weakness (21/32). Grade 3 toxicities were nausea and vomiting (2/32) and worsening of weakness (4/32) and were treated with antiemetics and corticosteroids. Acute grade 2 otitis media was seen in one patient. Late toxicities at time of analysis were minimal and grade 2 skin pigmentation was seen in one patient. Common acute grade 3 chemotherapy induced toxicities were myelosuppression (5/23), thrombocytopenia (2/23), rash (1/23) and febrile neutropenia (5/23), of whom three had repeated episodes. No treatment related death was seen.
Clinical response of radiotherapy ± chemotherapy (defined as regression of cranial nerve palsies or weakness of the limbs or cerebellar symptoms for > 3 mo) was seen in 16/32 (50%) patients, confirmed by a neurologist. Radiological response was also evaluated in all patients and response rates according to RECIST were CR (0/32), PR (16/32), SD (6/32) and PD (11/32). The mean response time was 12 ± 8 mo (range: 7-30). The mean reduction of tumor volume was 50% and clinical benefit (PR + SD) was 68.7% for all patients. Clinical response of adjuvant chemotherapy was seen in 2/11 (18.1%) at the mean time of 5 mo (range: 4-18). Three months after chemotherapy, radiological PR was seen in two patients, SD in five (45.7%) and progressive disease in four cases (36.2%).
Median survival time for the whole cohort was 1.5 years and 1, 2, 3 year OS rates for the whole cohort were 51.1%, 41.9% (29/49 died) and 23.1% (Figure 2). PFS rates at 1 and 2 years were 57.3% and 38.2% respectively.
At 2 years, the OS rate for radiologically low grade (favorable) tumors was clearly high (57.1%) compared to high grade (unfavorable) in which the OS rate was 17.9% (P < 0.001) (Figure 3A). Furthermore, among the subgroups, two year OS rates for focal/tectal, dorsally exophytic, cervicomedullary and DIPG tumors were 60%, 33.3%, 33.3% and 13.6% respectively (P < 0.0001) (Figure 3B).
Two year OS rates for patients (14/30) with complete or maximal resection and patients (16/30) with incomplete resection or biopsy only were 53.8% and 27.8% respectively (P = 0.002) (Figure 3C).
Median time of survival, one and two year OS rates for patients who were treated with postoperative (16/32) or radical radiation therapy (16/32) were 1.09 years, 50% and 17.9% respectively (Figure 4A). Patients treated with chemotherapy (neoadjuvant, concurrent or adjuvant/salvage) had a median survival time of 1.3 years and one and two year OS rates of 56.5% and 21.7% respectively (Figure 4B).
Multivariate analysis showed that for favorable tumors, important prognostic factors were: (1) age at diagnosis (worse for > 18 years); (2) KPS < 70; (3) histopathologically high grade; and (4) incomplete resection. Important prognostic factors for unfavorable tumors including DIPG were: (1) age at diagnosis (worse for > 18 years); (2) KPS < 70; (3) histopathologically high grade; (4) radiological high grade (necrosis on MRI); and (5) no concurrent chemotherapy, as shown in Table 6.
| Variables | RR (95%CI) | P value |
| Age at diagnosis (> 18 yr) | 3.0 (1.8-6.0) | 0.01 |
| KPS < 80 | 3.3 (1.7–5.3) | 0.02 |
| Duration of symptoms (< 60 d) | 6.7 (4.3-9.4) | 0.002 |
| Histopathology (high grade) | 6.1 (3.5-10.2) | 0.002 |
| MRI characteristics (presence of necrosis) | 3.0 (1.9-5.9) | 0.01 |
| Incomplete resection for favorable tumors | 6.6 (3.9-12.2) | 0.002 |
| No concurrent chemotherapy with RT | 3.1 (2.2- 8.2) | 0.01 |
BSG remains a therapeutic dilemma because of the location and heterogeneous biological behavior of these tumors, as seen in our cohort, comprised mainly of children and adolescents (81.6%). A median survival time of 1.5 years, one and two year OS rates of 51.1% and 41.9%, and clinical prognostic factors in our cohort were found to be in agreement with previously reported data[15,16]. We found no significant difference between children, adolescent and adult BSG in clinical presentation, MRI characteristics and treatment course, but there was a significant difference in median survival times (1.8 years in children/adolescents vs 1.2 years in adults) which is similar to other previous pediatric studies, although clearly shorter than reported by previous studies in adult BSG. The possible explanation for shorter median survival rates in adults could be high grade histology in our cohort. Similar findings were reported by Kaplan et al[15] and Reithmeier et al[16].
In our cohort, the most common subgroup was DIPG, of whom 27.3% underwent biopsy and subtotal resection, which is far from routine practice. The majority of biopsy proven DIPG had high grade astrocytoma on histology, which reflects the poor prognosis and shorter median survival in DIPG cases without histopathological confirmation. In addition, DIPG were also found to be more responsive to radiotherapy with concurrent chemotherapy in our cohort, suggesting that OS rates differ with different treatment strategies. However, trials of dose escalation (> 54Gy), hyperfractionated radiation therapy and incorporation of novel chemotherapeutic agents have failed to produce any meaningful change in the outcomes[11-14,16]. These findings are confirmatory for the heterogeneous nature of DIPG[17].
The subgroup of focal gliomas was the second most predominant in our cohort and these tumors were clearly found to be different from DIPG; however, the clinical picture was similar to DIPG. Complete removal in the majority of cases in our cohort reflected the improvement in median survival for such cases. Focal tectal gliomas constituted a small subgroup and these cases required CSF shunts for raised intracranial pressure, as reported by other studies[18]. However, adjuvant radiotherapy can be criticized in children as such patients have been managed with a CSF shunt or observation alone for long periods[19].
The third most common subgroup of dorsally exophytic gliomas in our cohort were managed successfully with complete resection in the majority of patients. However, in contradiction to the literature, our patients had a shorter median survival. A possible explanation could be high grade histology and no adjuvant radiotherapy[20,21]. A similar explanation for shorter median survival was also justified in our cohort of cervicomedullary glioma. Non-specific BSG, including medullary astroblastoma and pontomedullary BSG, had a similar clinical behavior and treatment outcome to DIPG[22].
In conclusion, brain stem gliomas have heterogeneous biological behavior. The DIPG subgroup had a dismal prognosis, needing more aggressive neurosurgical, radiation and chemotherapy techniques, while focal and tectal tumors were found to have a better prognosis.
BSG remains a therapeutic dilemma because of the location and heterogeneous biological behavior of these tumors and treatment is mainly through a multidisciplinary approach.
The present study focused on a Saudi population and found that the DIPG subgroup had a dismal prognosis, requiring more aggressive neurosurgical, radiation and chemotherapy techniques.
The present study revealed that stereotactic biopsy is feasible in DIPG and radiation therapy is associated with improvement of survival in patients with DIPG.
This study provides a treatment algorithm in brainstem glioma.
The article addressed an important disease with a poor prognosis.
P- Reviewer: Ho I S- Editor: Ji FF L- Editor: Roemmele A E- Editor: Lu YJ
| 1. | Laigle-Donadey F, Doz F, Delattre JY. Brainstem gliomas in children and adults. Curr Opin Oncol. 2008;20:662-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Kesari S, Kim RS, Markos V, Drappatz J, Wen PY, Pruitt AA. Prognostic factors in adult brainstem gliomas: a multicenter, retrospective analysis of 101 cases. J Neurooncol. 2008;88:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Ueoka DI, Nogueira J, Campos JC, Maranhão Filho P, Ferman S, Lima MA. Brainstem gliomas--retrospective analysis of 86 patients. J Neurol Sci. 2009;281:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Bauman G, Pahapill P, Macdonald D, Fisher B, Leighton C, Cairncross G. Low grade glioma: a measuring radiographic response to radiotherapy. Can J Neurol Sci. 1999;26:18-22. [PubMed] |
| 5. | Wagner S, Warmuth-Metz M, Emser A, Gnekow AK, Sträter R, Rutkowski S, Jorch N, Schmid HJ, Berthold F, Graf N. Treatment options in childhood pontine gliomas. J Neurooncol. 2006;79:281-287. [PubMed] |
| 6. | Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7:241-248. [PubMed] |
| 7. | Frazier JL, Lee J, Thomale UW, Noggle JC, Cohen KJ, Jallo GI. Treatment of diffuse intrinsic brainstem gliomas: failed approaches and future strategies. J Neurosurg Pediatr. 2009;3:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Schumacher M, Schulte-Mönting J, Stoeter P, Warmuth-Metz M, Solymosi L. Magnetic resonance imaging compared with biopsy in the diagnosis of brainstem diseases of childhood: a multicenter review. J Neurosurg. 2007;106:111-119. [PubMed] |
| 9. | Kebudi R, Cakir FB. Management of diffuse pontine gliomas in children: recent developments. Paediatr Drugs. 2013;15:351-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Kretschmar CS, Tarbell NJ, Barnes PD, Krischer JP, Burger PC, Kun L. Pre-irradiation chemotherapy and hyperfractionated radiation therapy 66 Gy for children with brain stem tumors. A phase II study of the Pediatric Oncology Group, Protocol 8833. Cancer. 1993;72:1404-1413. [PubMed] |
| 11. | Doz F, Neuenschwander S, Bouffet E, Gentet JC, Schneider P, Kalifa C, Mechinaud F, Chastagner P, De Lumley L, Sariban E. Carboplatin before and during radiation therapy for the treatment of malignant brain stem tumours: a study by the Société Française d’Oncologie Pédiatrique. Eur J Cancer. 2002;38:815-819. [PubMed] |
| 12. | Jennings MT, Sposto R, Boyett JM, Vezina LG, Holmes E, Berger MS, Bruggers CS, Bruner JM, Chan KW, Dusenbery KE. Preradiation chemotherapy in primary high-risk brainstem tumors: phase II study CCG-9941 of the Children’s Cancer Group. J Clin Oncol. 2002;20:3431-3437. [PubMed] |
| 13. | Drabko K, Wiśniewska-Slusarz H, Wójcik B, Choma M, Zaucha-Prazmo A, Kowalczyk JR. [Megachemotherapy followed by autologous haematopoietic stem cell rescue in children with high risk CNS tumours]. Med Wieku Rozwoj. 2005;9:439-447. [PubMed] |
| 14. | Jalali R, Raut N, Arora B, Gupta T, Dutta D, Munshi A, Sarin R, Kurkure P. Prospective evaluation of radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. Int J Radiat Oncol Biol Phys. 2010;77:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Kaplan AM, Albright AL, Zimmerman RA, Rorke LB, Li H, Boyett JM, Finlay JL, Wara WM, Packer RJ. Brainstem gliomas in children. A Children’s Cancer Group review of 119 cases. Pediatr Neurosurg. 1996;24:185-192. [PubMed] |
| 16. | Reithmeier T, Kuzeawu A, Hentschel B, Loeffler M, Trippel M, Nikkhah G. Retrospective analysis of 104 histologically proven adult brainstem gliomas: clinical symptoms, therapeutic approaches and prognostic factors. BMC Cancer. 2014;14:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Warren KE. Diffuse intrinsic pontine glioma: poised for progress. Front Oncol. 2012;2:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 18. | Gomez-Gosalvez FA, Menor F, Morant A, Clemente F, Escriva P, Carbonell J, Mulas F. Tectal tumours in paediatrics. A review of eight patients. Rev Neurol. 2001;33:605-611. |
| 19. | Dağlioğlu E, Cataltepe O, Akalan N. Tectal gliomas in children: the implications for natural history and management strategy. Pediatr Neurosurg. 2003;38:223-231. [PubMed] |
| 20. | Sridhar K, Sridhar R, Venkatprasanna G. Management of posterior fossa gliomas in children. J Pediatr Neurosci. 2011;6:S72-S77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Oka H, Utsuki S, Tanizaki Y, Hagiwara H, Miyajima Y, Sato K, Kusumi M, Kijima C, Fujii K. Clinicopathological features of human brainstem gliomas. Brain Tumor Pathol. 2013;30:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Notarianni C, Akin M, Fowler M, Nanda A. Brainstem astroblastoma: a case report and review of the literature. Surg Neurol. 2008;69:201-205. [PubMed] |