Published online Dec 10, 2014. doi: 10.5306/wjco.v5.i5.1048
Revised: January 23, 2014
Accepted: June 18, 2014
Published online: December 10, 2014
Processing time: 351 Days and 4.1 Hours
Postoperative recurrence occurs in approximately half of patients with non-small cell lung cancer (NSCLC), even after complete resection. Disease recurrence after surgical resection reduces the patient’s life expectancy sharply. The prognosis after postoperative recurrence is considered to largely depend on both the mode of first recurrence (distant, locoregional or combined) and the treatment modality: (1) The majority of cases of postoperative recurrence involve distant metastasis with or without locoregional recurrence. Platinum-based systemic chemotherapy is practically accepted as the treatment for these diseases on the basis of evidence for original stage IV disease. The advent of both pemetrexed and molecular-targeted drugs has improved the survival of nonsquamous NSCLC and changed the chemotherapeutic algorithm for NSCLC; (2) Among patients with distant metastatic recurrence without locoregional recurrence at the primary tumor site, the metastasis is often limited in both organ and number. Such metastases are referred to as oligometastases. Local therapy, such as surgical resection and radiotherapy, has been suggested to be the first-line treatment of choice for oligometastatic recurrence; and (3) While locoregional recurrence is likely to cause troublesome symptoms, it is a potentially limited disease. Therefore, providing local control is important, and radiation is usually beneficial for treating local recurrence. In order to obtain better control of the disease and provide treatment with curative intent in patients with limited disease, the administration of concurrent platinum-based chemoradiotherapy is recommended according to the results of originally nonresectable stage IIIA and IIIB disease.
Core tip: The postrecurrence survival in non-small cell lung cancer (NSCLC) is considered to largely depend on both the mode of first recurrence (distant, locoregional or combined) and the treatment modality. Therefore, the therapeutic strategy for treating postoperative recurrence in patients with NSCLC should be considered according to the mode of first recurrence. In this way, proper treatment specific to the mode of recurrence will be developed and improvements of the postrecurrence survival can be obtained.
- Citation: Yano T, Okamoto T, Fukuyama S, Maehara Y. Therapeutic strategy for postoperative recurrence in patients with non-small cell lung cancer. World J Clin Oncol 2014; 5(5): 1048-1054
- URL: https://www.wjgnet.com/2218-4333/full/v5/i5/1048.htm
- DOI: https://dx.doi.org/10.5306/wjco.v5.i5.1048
Primary lung cancer is currently the leading cause of cancer-related mortality worldwide. Despite progress in both chemotherapy regimens and radiotherapy, surgical resection still remains the first choice of treatment for locally limited non-small cell lung cancer (NSCLC). Although the results of surgery for NSCLC have improved, this is mostly attributed to improvements in diagnostic techniques and early detection of the disease. In fact, the outcomes of surgical resection for locally advanced stages (II or IIIA) of disease are not acceptable, when a complete resection can be performed. According to the international database of the IASLC Lung Cancer Staging Project in 2007, the five-year-survival rate after complete resection is 73% for pathologic stage IA disease, 58% for IB disease, 46% for IIA disease, 36% for IIB disease, and 24% for IIIA disease[1].
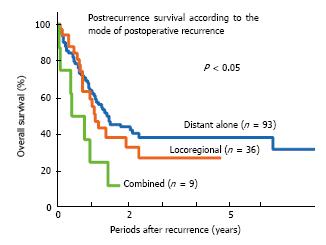
Postoperative recurrence occurs in approximately 45% of patients, even after complete resection of NSCLC[2]. Disease recurrence after curative surgery reduces the patient’s life expectancy sharply. The median postrecurrent survival time ranges from 8.1 to 17.7 mo in the literature[3-5]. The postrecurrence survival is considered to largely depend on both the mode of recurrence (Figure 1) and treatment modality[6,7]. To date, however, there have been few studies regarding the treatment of postoperative recurrence according to the mode of recurrence.
In this review article, the authors would like to propose a perspective treatment strategy according to the mode of postoperative recurrence, which is expected to prolong postrecurrence survival in patients with a good performance status.
Information regarding the first recurrence site after surgery is useful for patient management. The mode of postoperative recurrence is usually classified into distant recurrence, locoregional recurrence and combined recurrence. From the point of view of the selection of treatment modality, local recurrence is defined as recurrent disease within the ipsilateral hemithorax and mediastinum, excluding pulmonary lesions. Postoperative recurrence in the ipsilateral lung or even the contralateral lung was previously classified as intrathoracic local failure. However, in view of the pathophysiological analysis and selection of treatment modalities, pulmonary lesions appearing after surgery should be differentiated into hematogenous distant metastases, locoregional recurrent tissues at the surgical margin or second primary cancer tumors. Therefore, ipsilateral pulmonary lesions including the surgical margin should be diagnosed as lesions of local recurrence.
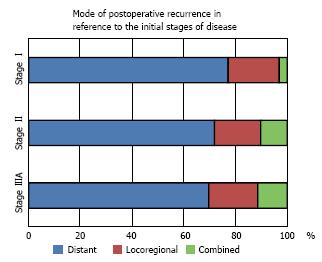
It was first reported in 1994 that the mode of recurrence does not differ with respect to the pathological stage at the time of surgery (Figure 2) and that the first site of recurrence is distant organs in 73.4% of cases, locoregional sites in 19.0% of cases, and combined sites in 7.6% of cases[2]. Common sites of distant metastasis include the brain, bone and lungs[8]. Recently, both whole-body 18fluorine deoxy-2fluoro-D-glucose positron emission tomography (FDG-PET) and brain magnetic resonance imaging have become commonly included for meticulous preoperative screening. Consequently, the incidence of distant metastasis after surgery has decreased due to better preoperative staging and improved selection of surgical patients. Hence, the incidence of distant metastases as the first site of recurrence site has decreased substantially[6,9].
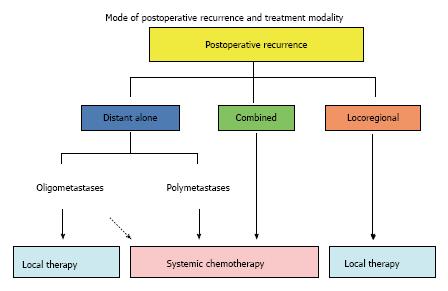
The majority of recurrent NSCLC patients after surgery involve distant metastasis with or without locoregional recurrence[2,6,8,10]. Although there are no definitive therapeutic guidelines for the treatment of recurrent disease after complete resection, the administration of systemic chemotherapy is practically accepted as the treatment for these diseases on the basis of evidence for original stage IV disease (Figure 3).
As to the first-line treatment for distantly metastatic stage IV disease, platinum-based chemotherapy is known to prolong survival, compared with the administration of best supportive care alone[11]. The median overall survival (OS) of NSCLC patients with clinical stage IV disease has been reported to be about one year with a median progression-free survival (PFS) of about five months in those treated with platinum-doublets chemotherapy consisting of platinum and third-generation cytotoxic drugs including paclitaxel, docetaxel, gemcitabine, vinorelbine and CPT-11[12,13]. The advent of both pemetrexed and molecular-targeted drugs has improved the survival of patients with nonsquamous NSCLC and drastically changed the chemotherapeutic treatment algorithm for NSCLC (Figure 4). Since pemetrexed has been identified to be more effective for nonsquamous NSCLC than squamous cell carcinoma[14], regimens of platinum plus pemetrexed are now standard first-line treatments for nonsquamous NSCLC. Furthermore, it is evident that both the PFS and OS are prolonged by treatment with platinum plus pemetrexed followed by continuous maintenance chemotherapy with pemetrexed alone[15]. In addition, the combined use of bevacizumab, anti-VEGF (vascular endothelial growth factor) antibodies prolongs the PFS in both the induction phase of platinum-doublet regimens and the continuous maintenance phase in the setting of nonsquamous NSCLC[16-19].
Recently, on the other hand, various types of molecular-targeted drugs have been developed in addition to conventional cytotoxic agents. It is now well-known that the response to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), such as erlotinib and gefitinib, is largely limited to specific mutations of the EGFR gene (EGFR) at exons 18 through 21[20-22] and that EGFR-TKIs thereby achieve a longer PFS (9.2-13.7 mo) than can be obtained with standard platinum-based chemotherapy (4.6-5.4 mo) for EGFR mutational NSCLC[23-26]. In patients with EGFR mutations, EGFR-TKIs are now preferentially administered as first-line treatment (Figure 4). In the subgroup analysis of a phase II study of first-line erlotinib, the MST of the patients with postoperative recurrence who exhibited EGFR mutations was 18.2 mo[27].
Following the identification of the EGFR mutation, the echinoderm microtubule-associated protein-like 4 (EML4)-anaplastic lymphoma kinase (ALK) fusion gene was discovered to be a driver oncogene for nonsquamous NSCLC in 2007[28]. Crizotinib, an ALK inhibitor, has been identified to be effective for EML4-ALK-positive NSCLC, with both a response rate of 60.8% and a PFS of 9.7 mo[29,30].
Since novel driver oncogenes have been extensively explored, it is essential to properly preserve surgical specimens for the future evaluation of biomarkers of molecular-targeted therapy.
Among patients with distant metastatic recurrence without locoregional recurrence at the primary tumor site, the metastasis is often limited in both organ and number. Such limited metastases are referred to as oligometastases. Local therapy, such as surgery and radiotherapy, has been applied successfully in appropriately selected patients, especially for patients with either brain metastasis alone or those with adrenal metastasis alone[31-34]. Recently, Yano et al. reported a retrospective study reviewing their therapeutic experience with postoperatively recurrent NSCLC patients and demonstrated that a histology of adenocarcinoma, a longer disease-free interval (≥ 1 year) and the use of local therapy are significantly preferable prognostic factors for the postrecurrence OS of patients with distant metastasis alone[6]. It has been suggested that local control of the metastatic tumor prolongs both the PFS and OS when distant metastases are limited in organ and number without local relapse at the primary site. These investigators subsequently reported findings of a prospective observational study that showed that 54.8% of postoperatively recurrent patients with distant metastasis alone exhibit oligometastatic metastasis without primary site recurrence and that the administration of local therapy, such as surgical resection or radiotherapy, results in a relatively long PFS of the patients with oligometastasis[35]. In that study, patients with only brain metastasis were excluded from the survival analysis since stereotactic radiotherapy is already practically accepted as the standard treatment for these limited brain metastases. In the oligometastatic patients who received local treatment, the median PFS was 20 mo. In that series, patients with metastasis to the lungs or bone were present among the long-term progression-free survivors.
Prior to application of local treatment for postoperative oligometastatic recurrence, it is essential to rule out both locoregional recurrence at primary site (in the locoregional lymph nodes) and other systemic metastasis. Therefore, for an accurate clinical diagnosis of oligometastases, FDG-PET examinations should be performed at the time of postoperative recurrence, as this modality has a high ability to detect asymptomatic recurrence[36].
While locoregional recurrence is likely to cause troublesome symptoms, it is a potentially limited disease. Therefore, providing local control is important, and the administration of local treatment, such as radiotherapy, is usually beneficial for local recurrence after complete resection in patients without pleural dissemination or effusion (Figure 3). In a study by Yano et al[2], half of the locoregionally recurrent patients who received radiation treatment exhibited a good local response, resulting in a prolonged survival, with a median survival time (MST) of 27 mo. On the other hand, the MST of the patients with uncontrolled disease was only six months. The administration of modern three-dimensional conformal radiotherapy with a curative dose of 60-66 Gy has been reported to achieve approximately 90% response rate (65% complete response and 24% partial response) for postoperative thoracic lymph node recurrence[37]. As a result, the five-year PFS and OS rates are 22.2% and 36.1%, respectively,
Postoperative locoregional recurrence is considered to be pathophysiologically the same as originally nonresectable stage IIIA and IIIB diseases, although the MST after treatment of a curative dose of radiation is longer for patients with postoperative locoregional recurrence (ranging from 14 mo to 19 mo[38-40]) than for nonresectable stage IIIA and IIIB diseases (ranging from 8.5 to 14.1 mo[41]). The therapeutic outcomes of the nonresectable stage IIIA and IIIB disease have been improved with recent developments in chemoradiotherapy, particularly platinum-based regimens, compared with that achieved with radiation alone[41]. In patients with a good performance status, the administration of concurrent chemoradiotherapy improves survival compared with the use of sequential chemoradiotherapy. Therefore, postoperative locoregional recurrence should be treated with concurrent chemoradiotherapy in order to obtain better control of the disease and provide curative treatment in patients with limited disease.
The potential of radiotherapy to control localized lesions is clearly best with small-volume disease[37]. Furthermore, in patients with small-volume disease which potentially remains localized without any hematogenous distant metastasis, curative radiotherapy is considered to be the treatment of choice. When deemed feasible, surgical resection is also another potential treatment of choice. However, early small-volume locoregional recurrence, especially in the hilar and mediastinal lymph nodes, is rarely detected on chest X-rays. Therefore, in addition to periodically obtaining chest X-rays, chest computed tomography should be performed annually for the first two years during the postoperative period[42].
In conclusion, the therapeutic strategy for treating postoperative recurrence in patients with NSCLC should be considered according to the mode of first recurrence. In this way, proper treatment specific to the mode of recurrence will be developed and improvements of the postrecurrence survival can be obtained. Based on the treatment algorithm shown in Figures 3 and 4, a multi-institutional prospective cohort study on treatment modalities for postoperative recurrence is currently proceeded by the Kyushu University Lung Surgery Study Group.
P- Reviewer: Bueno V, Freixinet JL, Hida T S- Editor: Gou SX L- Editor: A E- Editor: Lu YJ
| 1. | Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2389] [Cited by in RCA: 2581] [Article Influence: 143.4] [Reference Citation Analysis (0)] |
| 2. | Yano T, Hara N, Ichinose Y, Asoh H, Yokoyama H, Ohta M, Hata K. Local recurrence after complete resection for non-small-cell carcinoma of the lung. Significance of local control by radiation treatment. J Thorac Cardiovasc Surg. 1994;107:8-12. [PubMed] |
| 3. | Ichinose Y, Yano T, Yokoyama H, Inoue T, Asoh H, Tayama K, Takanashi N. Postrecurrent survival of patients with non-small-cell lung cancer undergoing a complete resection. J Thorac Cardiovasc Surg. 1994;108:158-161. [PubMed] |
| 4. | Sugimura H, Nichols FC, Yang P, Allen MS, Cassivi SD, Deschamps C, Williams BA, Pairolero PC. Survival after recurrent nonsmall-cell lung cancer after complete pulmonary resection. Ann Thorac Surg. 2007;83:409-417; discussioin 417-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 233] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 5. | Saisho S, Yasuda K, Maeda A, Yukawa T, Okita R, Hirami Y, Shimizu K, Nakata M. Post-recurrence survival of patients with non-small-cell lung cancer after curative resection with or without induction/adjuvant chemotherapy. Interact Cardiovasc Thorac Surg. 2013;16:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Yano T, Haro A, Yoshida T, Morodomi Y, Ito K, Shikada Y, Shoji F, Maruyama R, Maehara Y. Prognostic impact of local treatment against postoperative oligometastases in non-small cell lung cancer. J Surg Oncol. 2010;102:852-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Nakagawa T, Okumura N, Ohata K, Igai H, Matsuoka T, Kameyama K. Postrecurrence survival in patients with stage I non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;34:499-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Yano T, Yokoyama H, Inoue T, Asoh H, Tayama K, Takai E, Ichinose Y. The first site of recurrence after complete resection in non-small-cell carcinoma of the lung. Comparison between pN0 disease and pN2 disease. J Thorac Cardiovasc Surg. 1994;108:680-683. [PubMed] |
| 9. | Williams BA, Sugimura H, Endo C, Nichols FC, Cassivi SD, Allen MS, Pairolero PC, Deschamps C, Yang P. Predicting postrecurrence survival among completely resected nonsmall-cell lung cancer patients. Ann Thorac Surg. 2006;81:1021-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Yano T, Yokoyama H, Inoue T, Asoh H, Tayama K, Ichinose Y. Surgical results and prognostic factors of pathologic N1 disease in non-small-cell carcinoma of the lung. Significance of N1 level: lobar or hilar nodes. J Thorac Cardiovasc Surg. 1994;107:1398-1402. [PubMed] |
| 11. | Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ. 1995;311:899-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2673] [Cited by in RCA: 2431] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 12. | Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3965] [Cited by in RCA: 4050] [Article Influence: 176.1] [Reference Citation Analysis (0)] |
| 13. | Ohe Y, Ohashi Y, Kubota K, Tamura T, Nakagawa K, Negoro S, Nishiwaki Y, Saijo N, Ariyoshi Y, Fukuoka M. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol. 2007;18:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 482] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 14. | Scagliotti GV, De Marinis F, Rinaldi M, Crinò L, Gridelli C, Ricci S, Matano E, Boni C, Marangolo M, Failla G. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002;20:4285-4291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 675] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 15. | Paz-Ares L, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 447] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 16. | Botrel TE, Clark O, Clark L, Paladini L, Faleiros E, Pegoretti B. Efficacy of bevacizumab (Bev) plus chemotherapy (CT) compared to CT alone in previously untreated locally advanced or metastatic non-small cell lung cancer (NSCLC): systematic review and meta-analysis. Lung Cancer. 2011;74:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Lima AB, Macedo LT, Sasse AD. Addition of bevacizumab to chemotherapy in advanced non-small cell lung cancer: a systematic review and meta-analysis. PLoS One. 2011;6:e22681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Barlesi F, Scherpereel A, Rittmeyer A, Pazzola A, Ferrer Tur N, Kim JH, Ahn MJ, Aerts JG, Gorbunova V, Vikström A. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089). J Clin Oncol. 2013;31:3004-3011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 19. | Patel JD, Socinski MA, Garon EB, Reynolds CH, Spigel DR, Olsen MR, Hermann RC, Jotte RM, Beck T, Richards DA. PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:4349-4357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 301] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 20. | Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8739] [Cited by in RCA: 8781] [Article Influence: 418.1] [Reference Citation Analysis (0)] |
| 21. | Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1687] [Cited by in RCA: 1823] [Article Influence: 113.9] [Reference Citation Analysis (0)] |
| 22. | Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64:8919-8923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 955] [Cited by in RCA: 1005] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 23. | Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2837] [Cited by in RCA: 3283] [Article Influence: 218.9] [Reference Citation Analysis (0)] |
| 24. | Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380-2388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3961] [Cited by in RCA: 4390] [Article Influence: 292.7] [Reference Citation Analysis (0)] |
| 25. | Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2700] [Cited by in RCA: 3261] [Article Influence: 232.9] [Reference Citation Analysis (0)] |
| 26. | Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4228] [Cited by in RCA: 4358] [Article Influence: 335.2] [Reference Citation Analysis (0)] |
| 27. | Goto K, Nishio M, Yamamoto N, Chikamori K, Hida T, Maemondo M, Katakami N, Kozuki T, Yoshioka H, Seto T. A prospective, phase II, open-label study (JO22903) of first-line erlotinib in Japanese patients with epidermal growth factor receptor (EGFR) mutation-positive advanced non-small-cell lung cancer (NSCLC). Lung Cancer. 2013;82:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3816] [Cited by in RCA: 4100] [Article Influence: 227.8] [Reference Citation Analysis (0)] |
| 29. | Koivunen JP, Mermel C, Zejnullahu K, Murphy C, Lifshits E, Holmes AJ, Choi HG, Kim J, Chiang D, Thomas R. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14:4275-4283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 817] [Cited by in RCA: 817] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 30. | Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693-1703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3613] [Cited by in RCA: 3543] [Article Influence: 236.2] [Reference Citation Analysis (0)] |
| 31. | Macdermed DM, Weichselbaum RR, Salama JK. A rationale for the targeted treatment of oligometastases with radiotherapy. J Surg Oncol. 2008;98:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 32. | Hishida T, Nagai K, Yoshida J, Nishimura M, Ishii G, Iwasaki M, Nishiwaki Y. Is surgical resection indicated for a solitary non-small cell lung cancer recurrence? J Thorac Cardiovasc Surg. 2006;131:838-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Mariya Y, Sekizawa G, Matsuoka Y, Seki H, Sugawara T. Outcome of stereotactic radiosurgery for patients with non-small cell lung cancer metastatic to the brain. J Radiat Res. 2010;51:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Tanvetyanon T, Robinson LA, Schell MJ, Strong VE, Kapoor R, Coit DG, Bepler G. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small-cell lung cancer: a systematic review and pooled analysis. J Clin Oncol. 2008;26:1142-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 236] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 35. | Yano T, Okamoto T, Haro A, Fukuyama S, Yoshida T, Kohno M, Maehara Y. Local treatment of oligometastatic recurrence in patients with resected non-small cell lung cancer. Lung Cancer. 2013;82:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Toba H, Sakiyama S, Otsuka H, Kawakami Y, Takizawa H, Kenzaki K, Kondo K, Tangoku A. 18F-fluorodeoxyglucose positron emission tomography/computed tomography is useful in postoperative follow-up of asymptomatic non-small-cell lung cancer patients. Interact Cardiovasc Thorac Surg. 2012;15:859-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Okami J, Nishiyama K, Fujiwara A, Konishi K, Kanou T, Tokunaga T, Teshima T, Higashiyama M. Radiotherapy for postoperative thoracic lymph node recurrence of non-small-cell lung cancer provides better outcomes if the disease is asymptomatic and a single-station involvement. J Thorac Oncol. 2013;8:1417-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Kagami Y, Nishio M, Narimatsu N, Mjoujin M, Sakurai T, Hareyama M, Saito A. Radiotherapy for locoregional recurrent tumors after resection of non-small cell lung cancer. Lung Cancer. 1998;20:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Jeremic B, Shibamoto Y, Milicic B, Milisavljevic S, Nikolic N, Dagovic A, Aleksandrovic J, Radosavljevic-Asic G. External beam radiation therapy alone for loco-regional recurrence of non-small-cell lung cancer after complete resection. Lung Cancer. 1999;23:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Tada T, Fukuda H, Nakagawa K, Matsui K, Hosono M, Takada Y, Inoue Y. Non-small cell lung cancer: radiation therapy for locoregional recurrence after complete resection. Int J Clin Oncol. 2005;10:425-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Okawara G, Mackay JA, Evans WK, Ung YC. Management of unresected stage III non-small cell lung cancer: a systematic review. J Thorac Oncol. 2006;1:377-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Yano T, Maehara Y. Lung carcinoma surveillance counterpoint: Japan. New York: Human Press 2013; 79-81. |