INTRODUCTION
Radical treatment of cancer cervix with radiotherapy is incomplete without the use of brachytherapy. The present standard of care for treatment of locally advanced cancer cervix is concurrent chemoradiation followed by brachytherapy. The probability of local control and development of normal tissue toxicity is related to the dose of radiation delivered. Since brachytherapy delivers a significant proportion of the dose, it is imperative to properly estimate the tumour extension and place the applicators optimally to decrease normal tissue toxicity and improve local controls.
The practice of external beam radiotherapy (EBRT) has seen radical changes with the advances in the field of imaging, radiation treatment planning and treatment delivery. Standard fields bounded by just “four lines” have given way to treatment portals encompassing the tumour in all its “four dimensions”. Thus individualized target delineation, dose computation and dose delivery dose has become the routine for any EBRT treatments.
However, practice of brachytherapy has not changed much and continues to be based on systems that were developed in early 20th century. Brachytherapy practices in cancer cervix had perhaps lesser scope for development, given the relatively fixed configuration of the applicators, poor visualisation of the standard applicators on computed tomography (CT) scans and the limited scope of dose optimisation with a single uterine tandem. With the advent of CT/magnetic resonance imaging (MRI) compatible applicators, availability of three-dimension (3D) cross sectional imaging and brachytherapy treatment planning software, it is time to explore the arena of cervical brachytherapy beyond the “point” based dose prescriptions.
This article summarises the evolution of brachytherapy in cancer cervix, focusing specifically on the intracavitary brachytherapy (ICBT), an integral component of radiotherapeutic management in cancer cervix. It analyses the problems inherent with the traditional point A based prescriptions and evaluates the present day scenario to explore the future approaches and possibilities that could provide a definitive dose prescription and reporting parameters on an individualized basis.
POINT A: THE TRADITIONAL BASIS OF INTRACAVITARY BRACHYTHERAPY
Use of radium sources in ICBT of cancer cervix started in 1903 and dose prescription at that time was largely empirical or subjective, on account of lack of data regarding biological effects of radiation on tumour and the surrounding normal tissues. To surmount this problem, dosimetric systems were formulated that provided guidelines regarding loading, arrangement and duration of treatment with a set of specific radioisotopes in a designated manner to deliver the desired dose. The three dosimetric systems in use were-Paris system, Stockholm system and the Manchester system. With ICBT prescriptions being specified in terms of mg-hours, only the amount of radium required and the time duration were specified; doses to the normal tissues were neither calculated nor quantified.
To overcome these issues, Tod et al[1,2] formulated the Manchester system in 1938 and they subsequently modified it in 1953. Within this system, they attempted to define the treatment in terms of dose to a point, which they believed to be representative of the target itself and was reproducible from patient to patient. To better define the actual dose that was delivered with specific “mg-hr systems”, Tod and Meredith calculated the dose (in Röntgen) at multiple points in the pelvis. They showed that the initial lesion of radiation necrosis was due to the high dose effects in the area at the medial edge of the broad ligament, within the pyramidal shaped area (paracervical triangle), where the uterine vessels cross the ureter. Keeping this triangle in mind as the dose limiting region, the authors defined point A, lying within the paracervical triangle that was 2 cm lateral to the center of the uterine canal and 2 cm superior to the mucosa of the lateral fornix, in the plane of the uterus. Additionally, applicator design, loading and arrangements were specified so as to deliver the same dose-rate at point A, regardless of the combination of applicators used for treatment.
The strong point of Manchester system point A prescription was the constancy of dose rate at the point A, irrespective of the combination of tandems and ovoids used. Point A prescription was easily taken up into clinical practice on account of its simplicity and was most suitable at that time with limited imaging modalities available (i.e., orthogonal radiographs). As the originally defined point A could not be visualised on a radiograph, it was modified and redefined in relation to the applicator itself that could be visualized on radiographs. As per the new definition, point A was defined as a point 2 cm above the external os of uterus and 2 cm lateral to the uterine tandem in the plane of the uterus[2].
HOW WELL DOES BRACHYTHERAPY POINT A REPRESENT THE ANATOMICAL POINT A?
The point A, defined in the paracervical triangle was based on the assumption that the region represented the tolerance limits due to crossing of the uterine artery and ureter. Wang et al[3] correlated the anatomical point A and the brachytherapy point A in 11 patients undergoing ICBT. The anatomical point As-both right and left, were marked with radio-opaque clips and their positions compared with the brachytherapy point A. During the 64 brachytherapy applications, it was observed that the mean distances between the brachytherapy point A and anatomical point A were 5.2 cm (SD: ± 1.0) on right and 5.4 cm (SD: ± 1.1) on left. Furthermore, the dose received to the anatomical point A on right and left were 35.2% and 30% of the doses prescribed to the right and left brachytherapy point as respectively. This, questions the basic assumption of point A (the so called crossing point of the uterine artery and ureter) as being the dose limiting point. Lewis et al[4] too, have demonstrated that the location of point A was far from the ureter in 93% of their observations and at a distance of 0.8 cm or more.
INTERNATIONAL COMMISSION ON RADIATION UNITS AND MEASUREMENTS REPORT 38 RECOMMENDATIONS AND ITS IMPLICATIONS
To provide an uniformity in the reporting of ICBT of cancer cervix practiced at different centres, International Commission on Radiation Units and Measurements (ICRU) proposed reporting guidelines in 1985 in its ICRU Report 38[5]. As per the report, the type of source, the applicator, total reference air kerma (TRAK), dimensions of the reference volume (60 Gy or other dose equivalent volume), description of the dose distribution, dose rate and/or treatment time, absorbed dose at reference points and regional structures and organs at risk (OAR) were to be reported when using the 2D method of treatment planning.
Pötter et al[6] reported their outcomes in 189 patients of cancer cervix stages Ia to IVb treated between 1993-1997 in Vienna using the ICRU Report 38. They used a combination of a box technique for EBRT and a high-dose-rate ICBT using ring-tandem applicator. Small tumours were treated with 50 Gy of EBRT (25 Gy in brachytherapy reference volume) and 5-6 fractions of 7 Gy at point A (isoeffective at 76-86 Gy at point A) while the larger tumours received 3-4 fractions of 7 Gy after 50 Gy EBRT with open fields, (isoeffective to 82-92 Gy at point A). The 60 Gy ICRU volumes for the irradiation of small tumors ranged from 240 to 407 cm3 (mean: 337 cm3) and for larger tumors from 452 to 785 cm3 (mean: 607 cm3). At a mean follow-up of 34 mo, depending on the disease stage, their actuarial pelvic control and disease-specific survival rates varied from 52.7% to 100% and 52.1% to 100% respectively. The actuarial late grades 3 and 4 complication rate (LENT/SOMA) was 2.9% for the bladder, 4.0% for the bowel, 6.1% for the rectum and 30.6% for the vagina (shortening and obliteration). The authors felt that, in future the outcomes could be further improved using image based ICBT for a highly individualized treatment planning based on the topography of the actual tumour and OARs.
DOSE PRESCRIPTIONS IN HDR ERA USING POINT A AND ICRU REPORT 38
Over the years, the practice of ICBT has changed from low-dose-rate (LDR) to high-dose-rate (HDR), taking into considerations several logistics and technical advantages of HDR over LDR. The use of HDR brachytherapy has increased substantially in the last decade all over the world. As per the recent Quality Research in Radiation Oncology (formerly Patterns of Care) survey (2007-2009), 62% facilities in United States were using HDR as compared to 13% in the 1996-1999 survey[7]. During a recent global survey, HDR was found to be practiced by 85% of the respondents[8].
Three randomized controlled trials have proved HDR brachytherapy to be comparable to LDR brachytherapy in terms of loco-regional control and complication rates[9-11]. A meta-analysis showed that there were no significant difference in terms of outcomes between LDR and HDR[12].
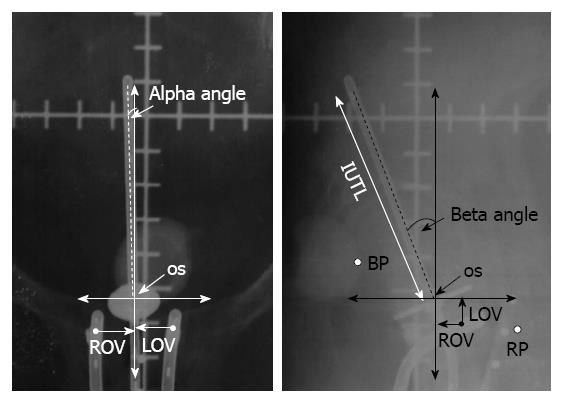
However, depending on the institutional protocols HDR ICBT requires multiple applications. This could lead to a variation in the applicator geometry and its spatial position in relation to the pelvic organs, pelvic bony anatomy and the organs at risk[13-17]. These have been reported in terms of changes in the uterine axis, uterine length, slippage of tandem, colpostat separation and vaginal packing, resulting in fluctuations in spatial location of the applicator in craniocaudal axis, lateral and antero-posterior rotation as well as variation in coronal, transverse and saggital planes (Figure 1)[18]. This has been attributed to mainly patient movement, vaginal packings and tumour regression during the interval between multiple fractions of HDR ICBT.
Figure 1 Schematic representation of antero-posterior and lateral radiographs with the applicator for estimation of various applicator components.
IUTL: Intrauterine length; VDL: Vertical displacement; ADL: Antero-posterior displacement; ROV: Right ovoid to os; LOV: Left ovoid to os LOV; BP: ICRU bladder point; RP: ICRU rectal point; ICRU: International Commission on Radiation Units and Measurements. Reproduced with permission[18].
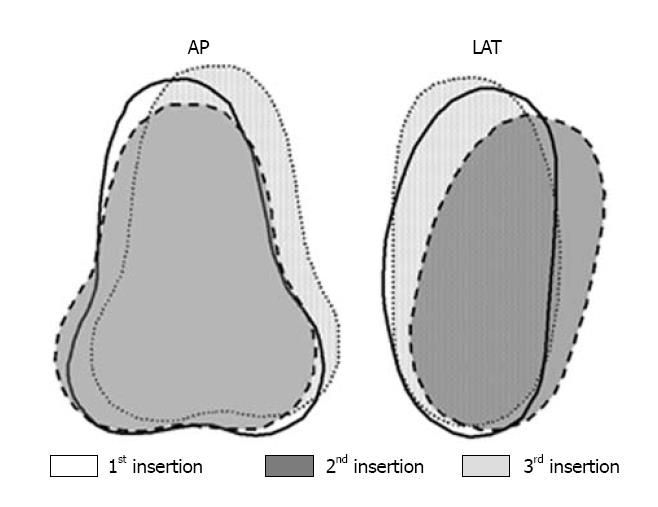
The variation in the applicator position in successive brachytherapy sessions, results in varying location of point A, as it is primarily defined in relation to the applicator itself[14,19,20]. Multiple HDR applications, thus result in different set of point As for both right and left sides, each set corresponding to each application. Thus, the resultant of multiple ICBT in HDR brachytherapy could lead to multiple point As on right and left side, each corresponding to a particular application. This eventually results in loss of the geometrical definition of a point (a dimensionless entity), as multiple points would result in a volume encompassed by these multiple points on both sides of the intrauterine tandem (Figure 2)[21]. Multiple point As, thus tend to change into “volume A” for multiple HDR applications in a single patient.
Figure 2 Fusion of standard Fletcher-Suit application positions of three insertions in a given patient with respect to the bony pelvis depicted in antero-posterior and lateral projections.
Reproduced with permission[21]. LAT: Lateral; AP: Antero-posterior.
As a consequence to the above, in a given patient, multiple HDR ICBT could result in multiple ICRU volumes, with different volumes of common intersections depending on the variability of the applicator positions during these multiple ICBT applications (Figure 3)[21]. All these could result in variation in the doses to various ICRU Report 38 reporting parameters-OARs, ICRU volumes, total reference air kerma in the same patient during the course of multiple HDR ICBT[14,18,19].
Figure 3 Fusion of three 6 Gy International Commission on Radiation Units and Measurements 38 dose distribution in a given patient with respect to the cervical os depicted in antero-posterior and lateral projections.
Reproduced with permission[21].
Continuing to report dose to point A or ICRU Report 38 parameters, is therefore fraught with uncertainty. Apart from the ease of defining these points based on orthogonal radiographs, it is quite imperative that the extent of tumour coverage within the prescribed dose would still remain ambiguous. Moreover, the outcomes in cancer cervix have not been found to correlate with the point A doses. Katz et al[22], evaluated the outcomes for tumour control and bladder and rectal morbidity in 808 applications in 396 patients with respect to the dose at point A and also the ICRU Report 38 rectal and bladder points. They reported a lack of correlation between the reference doses and outcomes[22]. Thus, point A as a panacea of reporting or dose prescription for ICBT is questionable.
Although ICRU Report 38 recommended reporting of the 60 Gy reference isodose dimensions and other parameters including the ICRU reference volumes and doses to OARs, a survey by Pötter et al[23] showed that these guidelines are usually not followed, nor are they reported by most centres in clinical practice or in the literature related to HDR ICBT. The variations in the ICRU Report 38 reference volumes with multiple HDR applications could have further added complexity to the reporting parameters and therefore might not have been favoured by most workers, although point A still continued to be the most common reporting parameter.
A NEED TOWARDS IMAGE GUIDED BRACHYTHERAPY: CT BASED INTRACAVITARY BRACHYTHERAPY
The limitations of orthogonal radiographs and dose prescriptions based on point A has mandated exploration of brachytherapy based on the actual position of the tumour and OARs in relation to the applicator. In the 90’s, changes in brachytherapy practice started owing to the availability of CT scans and applicators that were CT/MRI compatible[24]. Major changes in the design of the applicators were incorporated in keeping with the requirements of the tumour volume to be treated, viz a combined intracavitary/interstitial approach using Vienna ring applicator[25] or Utrecht ovoid applicator[26]. Shin et al[27] performed CT based intracavitary brachytherapy and compared them with conventional point A based treatment plans. For CT based plans, dose was prescribed to the outermost point that covered all CTVs. In 30 treatment plans with HDR ICBT, the mean target volume coverage index, conformal index, significantly improved with CT based treatment plans. However, the mean values of bladder and rectal point doses and volume fractions receiving 50%, 80% and 100% of the reference dose did not differ between the plans based on CT or point A.
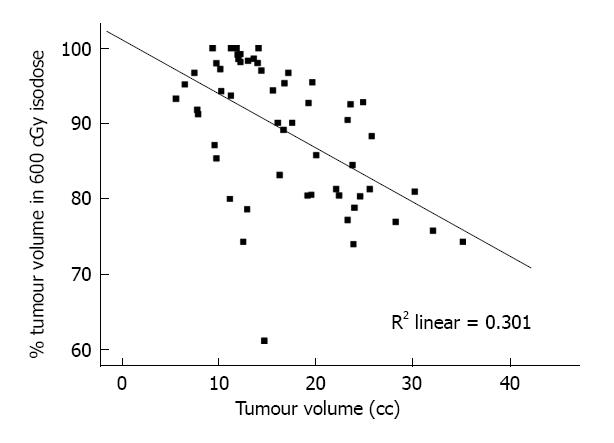
In another study, reported by Datta et al[28], patients underwent an ICRT application and thereafter underwent a CECT scan with the CT compatible applicator in situ. On the scans, the target was delineated by including the entire cervix mass along with any parametrial or intrauterine or vaginal extension. The plans were generated with doses prescribed at point A and the tumour coverage within the prescribed isodose was evaluated on the axial CT images. It was observed that in FIGO stages II and III, when prescribing doses at point A, the mean percentage tumour volume encompassed within the prescribed isodose (of 600 cGy) ranged from 60.8% to 100% with a mean ± SD) of 88.8% (± 9.2). The extent of target coverage was inversely correlated with the target volume delineated at the time of brachytherapy (Figures 4 and 5)[28]. Apart from this, the true maximal doses to bladder and rectum were underestimated when compared to the ICRU Report 38 reference points for these OARs and represented the 90th and 95th percentile of the maximum doses to these organs respectively.
Figure 4 Scatter-plot for tumour volume vs percentage of tumour enclosed within the 6 Gy isodose lines.
Reproduced with permission[28].
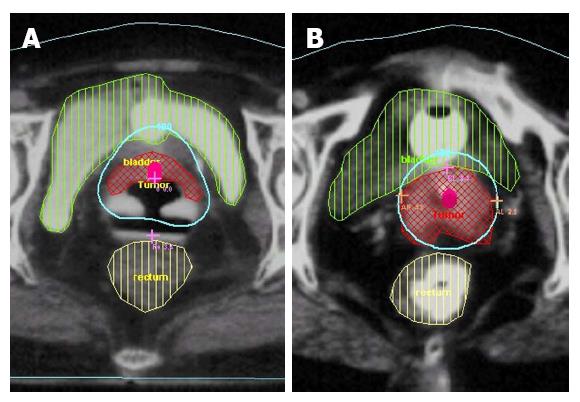
Figure 5 Tumour well covered within 6 Gy isodose volume (A) and tumour lying outside the 6 Gy isodose volume (B).
Reproduced with permission[28].
Impact of use of 3D image based (mainly CT based) brachytherapy on outcomes was reported by Charra-Brunaud et al[29]. For the radical radiotherapy treatment arm, the local relapse free survival at 24 mo was 73.9% and 78.5% while the grade 3-4 toxicity rate was 22.7% and 2.6% in 2D vs 3D plans, respectively. Clearly improved outcomes both in terms of local control rates and toxicity rates have been demonstrated with use of image based brachytherapy planning.
Soft tissue imaging is best done with MRI. However, CT scans are widely available in most of the radiation oncology departments and could be used more frequently for logistic reasons. It is has been shown that although CT scans are adequate for contouring the OARs, the CT based contours could significantly overestimate the tumour width[30]. The results of the CT contouring could be improved by contrast enhanced images for both bladder and rectum. Intravenous contrast could enhance the central areas of the cervix more than the peripheral areas and may also help to identify the uterine artery, thereby assisting the delineation of the upper border of cervix. The guidelines using CT scans for ICBT have been detailed using a comparative study with MRI[30].
CT GUIDED INTERSTITIAL BRACHYTHERAPY
Apart from intracavitary brachytherapy, availability of CT compatible interstitial needles, permits CT guided interstitial brachytherapy. This approach could facilitate a better assessment of the target volumes and its delineation and dose adaptation leading to improved outcomes. Wang et al[31] reported the use of CT guided HDR interstitial implantation in 20 patients and had achieved a median 90% dose of 45 Gy for high risk clinical target volume by brachytherapy alone. Together with external radiotherapy, the median 90% dose reached to 94 Gy. At a median follow-up of 15 mo, only 2 patients experienced local failure. In a similar report, by Lee et al[32], 68 patients with both primary and recurrent disease were treated with CT guided interstitial brachytherapy. A median cumulative equivalent dose of 78.4 Gy was delivered by interstitial brachytherapy. At a median follow-up of 17 mo, the actuarial local control reported was 86% with grade 3 late toxicities in nine patients.
MRI BASED IMAGE GUIDED BRACHYTHERAPY AND GUIDELINES
MRI with its superior soft tissue contrast and visualization is able to detect subtle abnormalities that may not be appreciated on CT. It has been found to accurately estimate tumour size to within ± 5 mm and correctly identify the parametrial invasion in 88% of cases[33]. In contrast to CT which is needed for EBRT treatment planning as it requires tissue electron density data for computation, brachytherapy calculations rely on the inverse square law. Thus, MRI based treatment planning represents the current state of the art in ICBT of cancer cervix.
The Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) published their detailed guidelines in 2005 and 2006 on the 3D image-based treatment planning in ICBT for cancer cervix[34,35]. Recently, the American Brachytherapy Society (ABS) has also framed their recommendations and adopted the GEC-ESTRO guidelines for contouring, image-based treatment planning, and dose reporting[24,36]. The guidelines provides clear recommendations for tumour delineation-gross target volume (GTV), high risk (HR) clinical target volume (CTV), intermediate risk (IR) CTV and OARs (rectum, bladder, sigmoid colon, and any adjacent bowel loops). The GTV, HR-CTV and IR-CTV represent a declining tumour cell density and thus are expected to have different radiotherapy dose requirements. As per the recommendations, parameters for reporting are based on 3D image based dosimetric evaluation of ICBT and these include the reference volume, TRAK, prescribed dose, point A dose, D90 CTV (dose in 90% of CTV), D100 CTV (minimum target dose), D100 GTV (minimum dose in GTV), V100 CTV (CTV volume receiving > 100% of the prescribed dose) and dose volume parameters for OAR’s. The recently published ABS guidelines provides dose limits for target and OARs for ICBT based on both radiographs and image based brachytherapy. The guidelines also suggests doses for template based interstitial HDR brachytherapy following 45-50.4 Gy of EBRT[24].
The transition from 2D to 3D CT to 3D MRI based brachytherapy has been rapid. Radiation oncologists need to get accustomed to correctly interpreting the MRI data before delineation of the GTV and the high risk CTV. There is a learning curve involved and interobserver variations with MRI have been found to be lesser as compared to CT scans[37,38]. GEC-ESTRO recommends to start with the standard method of prescription and then adjust the loading pattern and dwell times for optimisation. So, the starting point could either be point A prescription or the 60 Gy reference volume. The ABS recommends cautious use of optimisation based exclusively on dose volume histogram parameters as changes in spatial dose distribution may be significant and if not carefully analysed there may be unfavourable results[24,36]. Using the response adapted CTV defined at the time of brachytherapy, there is an option of using dose escalation and delivering much higher dose than is feasible with EBRT[39].
Apart from usual T2 images used for MRI based ICBT, diffusion weighted imaging (DWI) and the derived apparent diffusion coefficient (ADC) values, could also add additional biological information on tumour cell density. Haack et al[40] have demonstrated significant differences in ADC values for the three GEC-ESTRO target. The mean ADC values were lowest for the IR-CTV, followed by HR-CTV and highest for GTV of the GEC-ESTRO target volumes.
The GEC-ESTRO working group recently issued guidelines for the MRI for 3D image guided cervical cancer brachytherapy[41]. They recommended pelvic MRI scanning prior to radiotherapy and at the time of ICBT with one MR image. Multiplanar (transversal, sagittal, coronal and oblique image orientation) T2-weighted images with pelvic surface coils have been considered as the golden standard for delineating the topography of the tumour and the critical organs, while the use of complementary MRI sequences (e.g., contrast-enhanced T1-weighted or 3D isotropic MRI sequences) was considered as optional.
Beriwal et al[42] investigated the dosimetric consequences of brachytherapy planning using individualized MRI/CT based 3D-treatment plans for each ICBT application vs plans based on a single scan for all fractions. They observed that a single-plan procedure achieved acceptable dosimetry in most patients but individualized planning at each application improved dosimetry as it took into account the variation in the applicator geometry and position of critical organs during each HDR ICBT.
Nesvacil et al[43] evaluated the feasibility of adaptive 3D image based ICBT using a combination of MRI for the first BT application and planning the subsequent fractions on CT. They reported that such an approach is feasible, especially in small tumours for HR CTV coverage and OARs. However, for larger tumours, MRI based ICBT was preferred for all BT applications. This could be required in departments with limited access to MRI.
Tanderup et al[44] in 72 consecutive patients compared the point doses to 3D dose volume parameters for tumour and OARs. They reported that the HR CTV90 was highly variable in standard plans with point A dose prescriptions. Although for small tumours (< 31cc), HR-CTV were well covered by standard plans in 94% patients, the OAR constraints exceeded in 72% of the cases. This was improved by MRI based optimization. On the contrary, optimization resulted in full coverage of the HR-CTV90 in 72% of the patients as compared to 25% with standard plans. The authors, concluded that point A was a poor surrogate of HR-CTV doses and MRI based image guided adaptive brachytherapy could improve target coverage and OAR doses.
OUTCOMES WITH MRI BASED IMAGE GUIDED ADAPTIVE BRACHYTHERAPY
The practice of image guided adaptive brachytherapy (IGABT) is gradually gaining momentum and the number of centres opting for IGABT is increasing. In United Kingdom, 71% of the centres in 2011 had embarked on IGABT compared to just 26% in 2008[45]. In Canada, although point A is still the most common dose prescription point, but 73% of the centres have expressed their desire to change to 3D IGABT[46]. Most of these centres are either using 3D imaging and planning or are in transition towards 3D IGABT.
The data on outcomes with MR based treatment planning is emerging gradually[47-49]. Lindegaard et al[48] have demonstrated that a point A based non-optimised plan will result in discrepancy to the target doses ranging from 50% to 150%. The ICRU Report 38 bladder reference point underestimated the 2 cm3 bladder dose by 75%-300%, while it overestimated the rectal dose in 75% patients. 3D MRI based planning, improved the optimization to the various dose-volume parameters[48]. Pötter et al[50] have reported excellent local control rates of 95%-100% at 3 years in limited/favourable tumours (stage IB1/IIB proximal, less that 4-5 cm) and 85%-90% in larger tumours (stage IIB-IV) with acceptable treatment related morbidity (< 5%). Pelvic recurrences in this series had decreased by 65%-70%, as compared to historical series. They attributed this to the practice of MRI guided dose volume adaptation that enabled dose escalation in larger tumours (prescribed D90 > 85 Gy) often with interstitial needles as used in Vienna applicators.
In the Nordic study, Lindegaard et al[51] reported comparative results of outcome of cohort of patients treated at 2 different time periods with orthogonal radiograph based point A dose prescription (99 patients in NOCECA study, between 1994-2000) and those with MRI based IGABT (140 consecutive patients, 2005-2011). With IGABT, the actuarial local control reported was 91% at 3 years. MRI based IGABT significantly improved both overall and cancer specific survivals by 16% (P < 0.005) and 19% (P < 0.001) at three years, respectively. The improved survival was also seen in stage IIB to IV stages (P = 0.01). Additionally, significant reduction in moderate gastrointestinal and vaginal morbidity was reported with MRI based plans (P < 0.001). There were also few patients who reported severe morbidity and grade 3 urological and gastrointestinal morbidities were reduced by more than 50% with MRI based IGABT over point A dose prescription brachytherapy (P = 0.02). This study further highlights the improved outcomes both in terms of local control, survival and reduced toxicities with MRI based IGABT in comparison to point A based conventional brachytherapy.
Studies from Vienna explored feasibility of dose escalation with CT based vs MRI based treatment planning[52]. They found that compared to conventional radiograph based treatment planning, it was possible to escalate the dose to 95% of the target volume by a mean factor of 1.2 (range 1-1.7). The doses to the OARs, rectum and bladder could be maintained within the prescribed tolerance limits of 71% of the prescribed dose. Further, in a subgroup of 10 patients, MRI based ICBT permitted a dose escalation to 138% compared to 124% by CT based planning.
Using 3D IGABT with MRI, Tharavichitkul et al[53] reported their intermediate term results in 47 patients of locally advanced cancer cervix. Patients received a combination of 45-46 Gy EBRT followed by 6.5-7 Gy of 4 fractions of HDR IGABT. At 26 mo, the local control, disease free survival and overall survival were reported to be 97.9%, 85.1% and 95.6% respectively. The grade 3-4 bladder and rectum morbidity was 2.1% for each of these OARs.
Results of a retrospective analysis of 46 patients using MR based 3D IGABT along with chemoradiation was reported from University Medical Centre, Utrecht, The Netherlands[54]. At a median follow up of 41 mo, 3 year local control, progression free survival and overall survival was 93%, 71% and 65% respectively. Late grade 3-4 gastrointestinal and vaginal toxicities were observed in 4 patients (9.5%).
A multi-centric collaborative study, (intErnational study on MRI guided BRAchytherapy in locally advanced Cervical cancer) was launched in 2008 to validate these results prospectively[55]. The study attempts to use MRI based IGABT in locally advanced cervical cancers in a multi-centric setting to establish benchmark for local control, overall survival, morbidity and quality of life. It also would correlate local control with GEC-ESTRO dose volume parameters for GTV, HR CTV, and IR CTV as well as late morbidity and dose volume parameters for OARs. With an expected sample size of more than 600 patients and a proposed long term follow up of 3-6 years, the outcomes from this study are keenly awaited to reconfirm the utility and efficacy of MRI based IGABT.
POSITRON EMISSION TOMOGRAPHY-CT BASED IMAGE GUIDED ADAPTIVE BRACHYTHERAPY
Positron emission tomography-CT (PET-CT) has been used increasingly for initial tumour evaluation and also during follow up in cancer cervix. PET-CT has also been explored for brachytherapy planning in cervical cancers (Figure 6). PET-CT is superior to other modalities for ruling out any positive regional lymph node or distant metastasis. The additional information could also be used to assess the target volumes for image based brachytherapy[56,57]. Olsen et al[58] recently reported on a comparative evaluation of PET-CT and MRI. They compared the ADC maps on DWI MRI to evaluate the concordance of two functional imaging techniques and observed a good correlation of functional imaging between FDG-PET and DWI for cervical cancer. Tumor subvolumes with increased metabolic activity on FDG-PET also were found to have greater cell density by DWI. With the availability of PET-MRI in clinics, it could be expected that in near future the IGABT could be based not just on anatomic imaging as evident on MRI or CT, but on an anato-metabolic-imaging using PET- CT/MRI.
Figure 6 Positron emission tomography-computed tomography explore for brachytherapy planning in cervical cancers.
A and B: Positron emission tomography-computed tomography with the Vienna applicator; C and D: Depiction of the 6 Gy isodose volume. The tumour in relation to the applicator is visualized within the 6 Gy volume.
FUTURE CONSIDERATIONS AND PRACTICAL ISSUES FOR CLINICAL ADAPTION OF IGABT
Even though GEC-ESTRO recommends 3D MRI image-based treatment planning, point A continues to be used as a starting point of optimisation and dose to point A is still reported as a bridge between the 2D and 3D treatment planning. The superiority of MRI based treatment planning has been documented in several publications. Without contesting the superiority of MRI based planning at each brachytherapy application, there is much trepidation in accepting it in routine clinical practice worldwide, especially in resource constraint settings on account of the additional cost, extra manpower and infrastructural requirement. Recently several publications have explored alternative techniques to acquire 3D image data without escalating the cost of the treatment[59-62].
Trans-rectal ultrasound and MRI have been demonstrated by Schmid et al[59] as having high correlation in accurately measuring the target width and thickness at the time of brachytherapy, when the target itself was defined as the complete macroscopic tumour mass and the remaining cervix. Trans-abdominal ultrasound too has been used to delineate the uterus, cervix and the central disease and has been demonstrated to have a fairly strong correlation with MRI[60].
In absence of MRI, CT scans alone when used for brachytherapy planning can ensure OAR doses to be kept within acceptable limits. However, CT based target volumes have been overestimated as compared to MRI volumes[30,63]. In limited resource setting, MRI based pre-planning at the first brachytherapy application and consecutive CT/MRI data fusion has been demonstrated to be safe and feasible with acceptable inaccuracy of soft tissue registration by Dolezel et al[64].
Incorporation of successive clinical examination to CT based delineation has been explored by Hegazy et al[65]. The study concluded that target delineation accuracy can systematically improve through incorporation of additional information from comprehensive 3D documentation of repetitive gynaecological examinations and can improve accuracy of dose optimization in settings with limited imaging facilities. With availability of CT images alone, a minimum two-third uterine height may be a good surrogate for height of HR CTV.
Considering the practical logistic issues, that could arise during practice of IGABT, one may have to judiciously select the patient population who may benefit with MRI based IGBT vs CT based technique. For early and favourable disease cases, excellent local control rates have been reported with CT based planning[45]. For these cases, dose escalation may not be required and may not need MRI based IGABT. For locally advanced diseases, the standard point A based prescriptions can result in under dosage or geographic misses as has been documented on CT images[28].
The best way forward will be to perform a 3D cross sectional imaging prior to brachytherapy to correctly estimate the residual disease and thereafter proceed either with CT based or preferably MRI based planning[34,35,63,64]. An alternate in form of an ultrasound based planning as evident from some of the recent studies could also be explored[61,62,66]. It would still need some more time till we can integrate PET-CT/MRI into IGABT in cervical cancers.
CONCLUSION
Continuing to use point A dose prescriptions in ICBT at a time when the practice of EBRT has moved away from point prescriptions towards biological target based planning, amounts to an inequality in the fundamental approaches to planning for EBRT and ICBT. This is neither acceptable nor desirable. Point A prescription has for long served as the workhorse of intracavitary brachytherapy. Now, it may be time to honour it with a well-deserved place in the archives of ICBT of cancer cervix and move ahead and adapt image based intracavitary brachytherapy for an individualized and evidence based adaptive brachytherapy.