Published online Oct 10, 2014. doi: 10.5306/wjco.v5.i4.568
Revised: March 28, 2014
Accepted: April 25, 2014
Published online: October 10, 2014
Processing time: 216 Days and 13.5 Hours
Protons interact with human tissue differently than do photons and these differences can be exploited in an attempt to improve the care of lung cancer patients. This review examines proton beam therapy (PBT) as a component of a combined modality program for locally advanced lung cancers. It was specifically written for the non-radiation oncologist who desires greater understanding of this newer treatment modality. This review describes and compares photon (X-ray) radiotherapy (XRT) to PBT. The physical differences of these beams are described and the clinical literature is reviewed. Protons can be used to create treatment plans delivering significantly lower doses of radiation to the adjacent organs at risk (lungs, esophagus, and bone marrow) than photons. Clinically, PBT combined with chemotherapy has resulted in low rates of toxicity compared to XRT. Early results suggest a possible improvement in survival. The clinical results of proton therapy in lung cancer patients reveal relatively low rates of toxicity and possible survival benefits. One randomized study is being performed and another is planned to clarify the clinical differences in patient outcome for PBT compared to XRT. Along with the development of better systemic therapy, newer forms of radiotherapy such as PBT should positively impact the care of lung cancer patients. This review provides the reader with the current status of this new technology in treating locally advanced lung cancer.
Core tip: This review was written for the non-radiation oncologist who wishes to understand the use of proton beam therapy (PBT) for locally advanced lung cancer. PBT can be used to create treatment plans delivering significantly lower doses of radiation to the adjacent organs at risk (lungs, heart, esophagus, and bone marrow) than photon (X-ray) radiotherapy (XRT). PBT combined with chemotherapy has resulted in relatively low toxicity and favorable survival. One randomized study is being performed and another is planned to clarify the differences in outcome for PBT compared to XRT. Newer forms of radiotherapy such as PBT should positively impact lung cancer patients.
- Citation: Schild SE, Rule WG, Ashman JB, Vora SA, Keole S, Anand A, Liu W, Bues M. Proton beam therapy for locally advanced lung cancer: A review. World J Clin Oncol 2014; 5(4): 568-575
- URL: https://www.wjgnet.com/2218-4333/full/v5/i4/568.htm
- DOI: https://dx.doi.org/10.5306/wjco.v5.i4.568
Lung cancer is the leading cancer killer in the United States and worldwide. It has been estimated that lung cancer has taken the lives of 159480 Americans in 2013[1]. Lung cancer has been historically divided into 2 major histologic types: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC).
Thoracic radiotherapy using photon (X-ray) radiotherapy (XRT) has been standard therapy for lung cancer since the 1960’s when the Veterans Administration Hospital System performed a phase III trial in patients with unresectable lung cancer (including both SCLC and NSCLC)[2]. Patients with localized but clinically inoperable tumors were assigned either XRT or a placebo. The median survival of patients given XRT was 142 d compared to 112 d (P = 0.05) for those who received a placebo. This study established the role of XRT in the treatment of unresectable lung cancer. XRT improved survival modestly in spite of the fact that many of these patients likely had distant metastatic disease that wasn’t recognized because this trial was performed prior to the advent of modern imaging (computed tomography, magnetic resonance imaging, or positron emission tomography).
Standard therapy for locally advanced SCLC (stages II-III) and NSCLC (stage III) includes concurrent chemotherapy plus XRT)[3]. SCLC patients who complete chemotherapy plus RT and have achieved stable disease or a better response should also receive prophylactic cranial irradiation (PCI)[3,4].
In spite of many years of investigation, the outcome for lung cancer patients remains poor due to the cancer’s propensity to persist locally and metastasize widely[5]. Local control rates have been poor when defined using post-chemo-RT tumor persistence on biopsy rather than radiologic imaging criteria[6]. The overall 5-year survival rates for locally advanced NSCLC and SCLC range from approximately 15% to 25%[3,4]. Concurrent chemotherapy plus XRT results in better survival than XRT alone or sequential therapy but with higher rates of severe toxicity[7,8]. For example, The North Central Cancer Treatment Group (NCCTG) performed a phase III trial to determine whether chemotherapy (cis-platin and etoposide) plus either twice daily (BID) XRT or daily (QD) XRT achieved a better outcome for patients with stage III NSCLC. Severe, grade ≥ 3 (3+) and grade 4+ toxicity was very common, occurring in 87% and 67% of patients, respectively[9]. Grade 3+ and 4+ hematologic toxicity occurred in 80% and 62%. Grade 3+ and 4+ esophageal toxicity occurred in 18% and 1%. Grade 3+ and 4+ pneumonitis occurred in 12% and 2%. The 5-year survival was 17%. These results form a reasonable standard for chemotherapy plus XRT to which other treatment programs can be compared. It is clear that more research is needed to develop therapies that result in better survival and less toxicity.
One potential method to improve patient outcome is to optimize the radiotherapy. Historically, we have improved patient outcomes each time newer methods of radiation delivery were invented. First, radiotherapy was delivered with radio-isotopes placed directly into the tumor. This was problematic when treating lung cancer since the act of placing potential sources within a lung tumor would require physical damage to the lung. Low energy X-ray devices were then developed which delivered kilo-voltage X-rays. Unfortunately, these beams penetrated the tissues poorly and delivered the maximum dose on the skin with only a small fraction of the dose to a deep seated tumor. Kilovoltage X-ray beams were used in the VA study noted above. The isotope cobalt 60 was then used and produced a beam composed of gamma rays with two distinct energies of 1.17 and 1.33 million volts (MV) resulting in better skin sparing and improved depth-dose penetration. Linear accelerators were then developed which produced still higher energy MV X-rays or photons. These photon beams penetrated better but still delivered the maximal dose between 1.5 and 3.5 cm beneath the surface with the dose gradually decreasing as the beam penetrated straight through without stopping until exiting the body. The dose-distribution of X-rays within the body is due to their unique characteristics of having almost no mass and no charge.
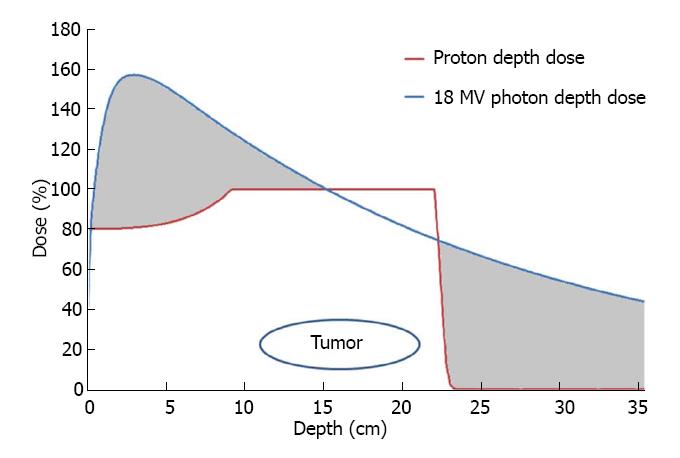
Protons, in contrast, have mass (approximately 1800x that of an electron) and hold a positive elementary charge. These characteristics create a much different distribution of dose deposition within the body. First, the accelerated protons enter the body with a high momentum which carries them to a specific depth dependent on the initial kinetic energy imparted upon them by the accelerating device (generally a cyclotron or synchrotron). As the proton beam travels to that depth, there is a relatively small amount of energy transmitted to the tissues. As the protons slow down, more and more energy is transferred to the surrounding tissues. The energy lost per unit path length is almost inversely proportional to the square of the speed of the proton. Shortly before the entire energy of the proton is lost, the energy loss rate reaches a sharp peak. Once the kinetic energy of the proton is entirely dissipated into the tissue, the proton comes to rest within the body. The energy of the proton is dissipated in collisions with the electrons of the neighboring atoms in the surrounding tissues causing ionizations which produce radiation damage. The region in the body where the maximum energy loss and final stopping of protons occurs is narrow and is located at a specific depth depending on the initial energy of the proton beam. This sharp, well defined peak of maximal dose from charged particles is referred to as the “Bragg peak”. Beyond this point, there is no radiation energy imparted upon surrounding tissues as the protons have stopped. So far we have only discussed mono-energetic proton beams, i.e., proton beams in which all protons have the same initial energy. The peak of a mono-energetic proton beam is so narrow that in order to generate a clinically useful proton beam one has to spread it out by giving repeated mono-energetic beams of protons with successively lower energies to cover a mass or tumor within the high dose region otherwise known as the “spread-out Bragg Peak” (Figure 1).
Figure 1 reveals depth-dose comparisons of a single photon (X-ray) beam and a proton beam. Each beam enters the body at 0 cm depth on the left side of the graph and travels into the body traveling to the right. The depth within the patient is shown on the X-axis and the percent of the prescribed dose delivered on the Y-axis. The 18 MV photon beam delivers the greatest dose at 3.5 cm depth within the patient and then decreases in dose delivered as it enters and exits the tumor at depths of 11 and 22 cm, respectively. The photon beam then continues through the body until it exits. In contrast, the proton beam administers the maximum dose in the tumor stopping just beyond the deepest portion of the tumor. There is less dose delivered as the proton beam travels to the tumor and no exit dose beyond the tumor. The grey areas correlate to the excess dose delivered by photons to normal healthy tissue that would not be irradiated by the proton beam.
Protons are also different from photon irradiation in terms of killing power. The relative biological effectiveness (RBE) for proton RT is generally estimated at 1.1 X that for photons. Thus, one gets 10% more cancer kill power for each gray (Gy) of proton RT than photon RT. To simplify discussions, photon doses are described in Gy and proton doses described in Gy equivalence (GyE) to describe 2 beams with similar killing properties. One Gy is the addition of one joule of energy per kilogram of tissue. The energy absorbed results in free radical formation. The free radicals ionize and fracture DNA molecules within the cells’ nuclei. These DNA fractures result in cell death especially in rapidly reproducing cells which are the most sensitive to radiotherapy.
The primary benefits of PBT compared to XRT are based on the above mentioned interactions with matter when traveling into a patient to treat a tumor. The fact that the proton beam stops results in no radiation exposure beyond the tumor allows for the sparing of distally placed tissues. In contrast, photons don’t stop and travel through the entire body from the entrance to the exit point.
If one has a tumor in the anterior portion of the chest, it would be desirable to treat from the anterior perspective with PBT. After the proton beam treats the tumor, it stops. This results in substantially less dose than X-rays to deeper structures including the spine (spinal cord and marrow), esophagus, lung, and heart. Each of these normal dose-limiting organs is sensitive to radiation and can be injured during treatment. As discussed earlier, the standard therapy of concurrent chemotherapy plus XRT to 60 Gy in 30 daily fractions results in severe (grade 3+) toxicity in the vast majority of patients[9]. Thus, maximal sparing of these critical organs is important in potentially improving patient outcomes (survival, quality of life, and toxicity).
Detailed studies comparing the XRT plans using both 3-D planning and intensity modulated photon RT (IMRT) technology to proton TRT plans have been performed. Nichols et al[10] examined the dose distributions (dosimetry) from 8 consecutive stage III NSCLC patients. In all patients, 3-D XRT, IMRT, and proton therapy plans achieved the dose goals for the tumor volumes. Compared with 3-D XRT plans, proton plans offered a median 29% reduction in normal lung V20 (total lung volume receiving > 20 Gy), a median 33% reduction in mean lung dose (MLD), and a median 30% reduction in the volume of bone marrow receiving a dose of > 10 Gy. The V20 and MLD have been established to correlate well with the risk of radiation pneumonitis[11-14]. The 10 Gy dose to the bone marrow would be sufficient to suppress myelopoiesis within the irradiated marrow. Compared with the IMRT plans, the proton plans offered a median 26% reduction in normal lung V20, a median 31% reduction in MLD, and a median 27% reduction in the volume of bone marrow receiving a dose of > 10 Gy. They concluded that by reducing the volumes of normal structures irradiated, protons can potentially improve the therapeutic index for stage III NSCLC compared to with either 3-D XRT or IMRT. Similar results were found by Chang et al[15] when they compared the RT plans that delivered high dose RT with either protons or photons. PRT reduced the dose to normal tissues significantly, even with dose escalation, compared with standard-dose photon therapy, either 3-D XRT or IMRT.
The dosimetric studies highlight significant differences in the dose distributions when comparing protons to photons in the treatment of lung cancer. Because the proton beam can stop just beyond the far end of the target, normal tissues beyond the tumor can be spared from receiving radiation when compared to XRT. These differences can be used to produce potentially safer RT plans.
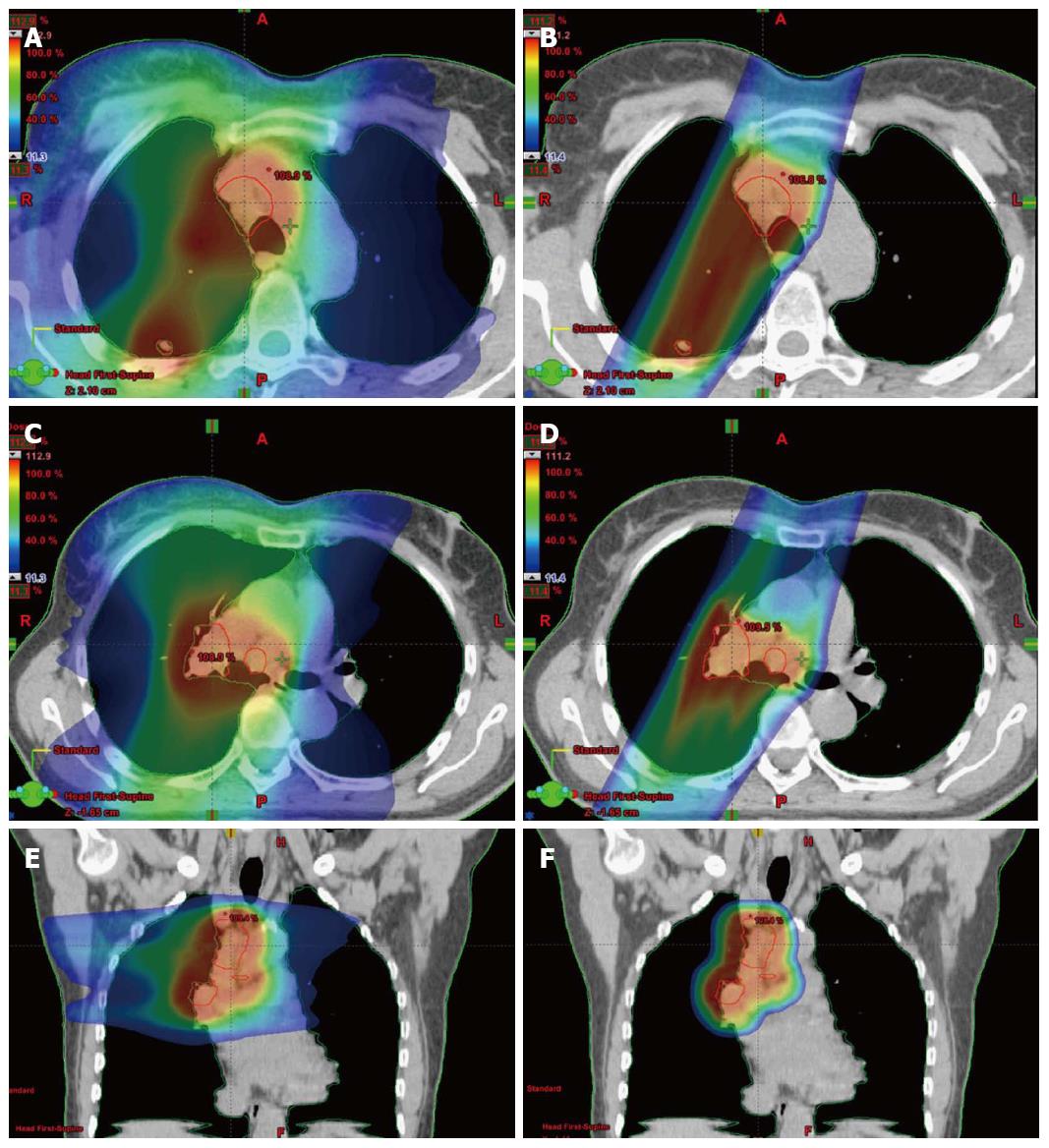
Clinical data is required to substantiate that the gains found dosimetrically translate into improvements in patient outcome. Newer proton facilities are employing scanned pencil beams instead of the broad passively scattered proton beams which are shaped with apertures and range compensators. Pencil beams are very narrow proton beams steered with magnetic fields which move in a raster pattern back and forth through the tumor volume. The intensity (or dose) can be modified as the beam moves allowing the delivery of intensity modulated proton beam therapy (IMPT). Zhang et al[16] was able to show that IMPT produced better dosimetric results than more conventional proton beam therapy. Figure 2 includes dosimetric comparisons between photon and proton plans for a patient with stage III lung cancer. The doses delivered shown as a color wash with lower doses denoted in blue and higher doses in red. On the left is a rapid arc intensity modulated photon RT (IMRT) plan which included 6 MV photons which delivered IMRT while the gantry rotated around the patient. On the right are shown the same patient treated with intensity modulated proton RT. It is clear from figures that the IMPT plan delivers the same dose to the target (red outlined region) with less dose to the surrounding normal tissues.
Proton beams are more sensitive to a variety of uncertainties such as respiratory motion, changes in patient positioning, and tumor shrinkage. This creates more technical challenges in planning and delivery of radiotherapy. The term, “robust” is used to describe treatment plans which can accommodate these uncertainties.
Colaco et al[17] reported the first known series of limited stage SCLC (L-SCLC) patients treated with PBT. All 6 patients also received chemotherapy and PCI. Five patients received 60-66 GyE in 30-34 daily fractions and one patient received 45 GyE in 30 BID fractions. With one year of median follow up, the one-year overall survival rates was 83%. Treatment was well tolerated; there were no cases of acute grade 3+ esophagitis or grade 2+ pneumonitis, and no other grade 3+ non-hematological toxicities were seen. Dosimetric comparison revealed better sparing of lung and esophagus with PBT than IMRT. They concluded that PBT merits further investigation as a method of reducing toxicity in L-SCLC.
Results of PBT in patients with stage III NSCLC
Clinical outcomes of phase I and II studies are available. Standard therapy for stage III NSCLC in fit patients is concurrent radiation and chemotherapy. As such, this summary focuses on the studies which delivered combined modality therapy. Chang et al[18] reported the early findings of a phase II trial of high-dose PBT that included 44 patients with stage III NSCLC treated with 74 GyE in 37 fractions with weekly carboplatin and paclitaxel. Protons were delivered as passively scattered beams and adaptive re-planning. The median overall survival time was 29.4 mo and no patients suffered grade 4 or 5 proton-related toxicity. The most common non-hematologic grade 3 toxicities were dermatitis (n = 5.11%), esophagitis (n = 5.11%), and pneumonitis (n = 1.2%). Nine (20.5%) patients experienced local disease recurrence, four (9.1%) patients had regional lymph node recurrence, and 19 (43.2%) patients developed distant metastasis. Of the 44 patients, 9 (20%) had their original plans adapted to changes in their tumor volume[19]. Changes in tumor volume due to response can sufficiently alter the proton stopping power of the tissues to require changing the plan in order to administer the prescription dose to the tumor and protect normal tissues. The authors concluded that concurrent high-dose PBT and chemotherapy were well tolerated and that the median survival was encouraging.
Hoppe et al[20] reported on 19 NSCLC patients (n = 18:stage III, n = 1:stage IIb) treated with carboplatin, paclitaxel, and PBT to 74 Gy/37 fractions. There were only 1 (5%) acute grade ≥ 3 non-hematologic toxicity and 2 (11%) chronic non-hematologic toxicity with a median follow up of 16 mo. Oshiro compiled a series of 57 patients with stage III NSCLC treated with a median dose of 74 Gy with PBT and no chemotherapy[21]. The median survival was 21.3 mo which was similar to many combined modality series. Toxicity was very modest with grade 3+ lung toxicity in 11% and no grade 3+ esophageal toxicity.
Clinical comparisons of patients treated with either photon or proton therapy for lung cancer are rare. This is in part due to the relatively few clinical experiences with proton therapy for lung cancer. This is in part due to the difficulty in getting lung cancer covered by insurance for PBT. Sejpal et al[22] reported a retrospective comparative analysis of the MD Anderson experience in patients with stage 3 NSCLC. Their rational for the use of proton beam therapy was the recognition that concurrent chemotherapy plus XRT, the standard of care for stage 3 NSCLC, causes severe toxicity in most patients. Photon based TRT cannot be given at doses associated with a high chance of cure without excessive toxicity. They hypothesized that PBT could permit higher tumor doses with less normal-tissue toxicity than XRT delivered as 3-D XRT or IMRT. They compared the outcome of PBT + chemotherapy (n = 62), 3D-XRT + chemotherapy (n = 74) or IMRT + chemotherapy (n = 66). RT was delivered to the gross tumor volume with weekly paclitaxel and carboplatin. The median total radiation dose was 74 GyE for the proton group and 63 Gy for the other groups. Severe (grade 3+) pneumonitis and esophagitis in the proton group (2% and 5%) were lower despite the higher radiation dose(3-D XRT, 30% and 18%; IMRT, 9% and 44%; P < 0.001 for all). The median survival times were 17.7 mo for the 3-D XRT group, 17.6 mo for the IMRT group, and 24.4 mo for the proton therapy group (P = 0.1). They found that higher doses of PBT could be delivered to lung tumors with lower rates of esophagitis and pneumonitis.
The above findings were provocative enough to justify a randomized trial of IMRT vs PBT. This trial is entitled, “A bayesian randomized trial of image-guided adaptive conformal photon vs proton therapy, with concurrent chemotherapy, for locally advanced NSCLC.” The primary objective is to compare the incidence of grade 3+ treatment related pneumonitis (TRP) or local failure. In addition, the Radiation Therapy and Oncology Group (RTOG) is planning a phase III trial (RTOG 1308) comparing chemotherapy plus either XRT or PBT in doses of 60 to 70 Gy. The primary endpoint is overall survival. Other trials exist and can be found at: http://clinicaltrials.gov and are summarized in Table 1.
| Non-small cell lung cancer: |
| 1 ClinicalTrials.gov identifier NCT00881712 (LU02: University of Florida): A phase II trial of 3-D proton radiotherapy with concomitant chemotherapy for patients with initially unresectable stage III non-small cell lung cancer: |
| (1) Arm 1: Concurrent chemotherapy and PBT (74 GyE/37 fractions) for unresectable stage 3 patients with nodes > 15 mm in diameter. |
| (2) Arm 2: Concurrent chemotherapy and PBT (60 GyE/30 fractions) for unresectable stage 3 patients with nodes < 15 mm in diameter. |
| (3) Arm 3: Resectable stage 3 diseases: preoperative PBT (50 GyE/25 fractions) and surgery. |
| 2 ClinicalTrials.gov identifier NCT01993810: (RTOG1308) phase III randomized trial comparing overall survival after photon vs proton chemoradiotherapy for inoperable stage II-III B NSCLC: |
| (1) Arm I: XRT (70 Gy/35 fractions) and either paclitaxel and carboplatin or etoposide and cisplatin. |
| (2) Arm II: PBT (70 GyE/35 fractions) and either paclitaxel and carboplatin or etoposide and cisplatin. |
| 3 ClinicalTrials.gov identifier NCT01770418 (Proton Collaborative Group): A Phase I/II study of hypofractionated proton therapy for stage II-III non-small cell lung cancer: |
| (1) Proton radiotherapy |
| Dose Level 1: 60 Gy (RBE) at 2.5 Gy (RBE) per fraction × 24 fractions |
| Dose Level 2: 60 Gy (RBE) at 3 Gy (RBE) per fraction × 20 fractions |
| Dose Level 3: 60.01 Gy (RBE) at 3.53 Gy (RBE) per fraction × 17 fractions |
| Dose Level 4: 60 Gy (RBE) at 4 Gy (RBE) per fraction × 15 fractions |
| (2) Paclitaxel and carboplatin or cisplatin and etoposide |
| 4 ClinicalTrials.gov identifier NCT01565772 (MGH): A phase I trial of hypofractionated PBT with cisplatin and etoposide followed by surgery in stage III non-small cell lung cancer: |
| Radiation (PBR): 45-55 Gy total, 1.8-2.2 Gy × 25 fractions with cisplatin and etoposide followed by resection |
| 5 Clinical trials.gov identifier NCT01165658 (MD Anderson) phase I study of Hypofractionated Proton Radiation Therapy in Thoracic Malignancies: The radiation prescription dose ranges from 45 Gy in 3 Gy fractions to 60 GyE in 4 Gy fractions. This is for patients ineligible for chemotherapy. |
| 6 Clinical trials.gov identifier NCT00614484 (Loma Linda University): phase I/II study of combined chemotherapy and high dose, accelerated proton radiation for the treatment of locally advanced non-small cell lung carcinoma. Carboplatin and taxol and 5 wk of daily proton therapy. |
| 7 Clinical trials.gov identifier NCT01629498 (MD Anderson): phase I/II trial of Image-guided, intensity-modulated photon or scanning beam proton therapy. Both with SIB dose escalation to the GTV with concurrent chemotherapy for stage II/III NSCLC |
| (1) Experimental. IMPT + SIB + chemotherapy phase I starting IMPT dose: 60 Gy (RBE) in 30 fractions; phase I starting SIB dose: (72-84) Gy (RBE). All patients receive standard concurrent chemotherapy. |
| (2) Experimental. IMRT + SIB + chemotherapy: phase I Starting IMRT dose: 60 Gy (RBE) in 30 fractions; phase I starting SIB Dose: (72-84) Gy (RBE). All patients receive standard concurrent chemotherapy |
| 8 Clinical trials.gov identifier NCT00915005 (MD Anderson): A bayesian randomized trial of image-guided adaptive conformal photon vs proton therapy, with concurrent chemotherapy, for locally advanced NSCLC (treatment related pneumonitis and locoregional recurrence) |
| (1) Experimental group 1 (photon therapy): 74 Gy/37 fractions + carboplatin and paclitaxel |
| (2) Experimental group 2 (proton therapy): 74 Gy/37 fractions + carboplatin and paclitaxel |
| (3) Experimental group 3 (proton therapy): 66 Gy/33 fractions + carboplatin and paclitaxel |
| 9 ClinicalTrials.gov identifier NCT01076231 (University of Pennsylvania) feasibility and phase I/II trial of preoperative PBR with concurrent chemotherapy for resectable stage IIIA or superior sulcus NSCLC: pbr over 5.5-7.5 wk plus concurrent chemotherapy comprising cisplatin and etoposide. Then, patients may undergo surgical resection or additional chemoradiotherapy |
| 10 ClinicalTrials.gov identifier NCT01108666 (University of Pennsylvania): phase I dose escalation trial of PBR with concurrent chemotherapy and nelfinavir for inoperable stage III NSCLC. Determine MTD of nelfinavir and MTD of PBR when given with chemotherapy for stage III NSCLC. |
| 11 ClinicalTrials.gov identifier NCT01808677 (MD Anderson): registry study of thoracic reirradiation for NSCLC utilizing PBT or intensity modulated radiation therapy. Primary objective to assess the prevalence of high-grade toxicity in patients being treated with thoracic re-irradiation with PBT or IMRT for NSCLC, with or without chemotherapy. |
| 12 ClinicalTrials.gov identifier NCT01386697 (University of Pennsylvania): A prospective radiation oncology planning study for lung, gastrointestinal and lymphomatous malignancies using proton radiotherapy as compared to 3D conformal and intensity-modulated X-ray therapy for dosimetric evaluation of tumor coverage and dose to organs-at-risk. The overall objective is to estimate the actual or potential benefit of DIBH treatment in the context of proton radiotherapy as compared to 3DCRT and IMXT. |
PBT offers stage III lung cancer patients potentially safer radiotherapy plans with significantly lower doses delivered to the lungs, esophagus, and bone marrow. This has resulted in less toxicity[22] in retrospective comparisons. Safe dose escalation with photon therapy was not possible in stage III NSCLC patients treated on RTOG 0617 which compared chemotherapy plus either 60 Gy or 74 Gy of photon irradiation. The higher dose arm resulted in poorer survival which appears to be secondary to increases in normal tissue (pulmonary and/or cardiac) doses[23]. PBT can better spare the normal surrounding organs at risk and, thus, offers investigators an opportunity to safely deliver higher tumor doses which may result in higher local control rates and improved survival.
One randomized study is being performed and another is being planned to further elucidate the potential clinical benefits of PBT compared to traditional XRT. Technology continues to improve and pencil beam PBT and IMPT may produce better clinical results than scattered PBT. Along with the development of better systemic therapy, improvements in radiotherapy technology should positively impact on the care of unresectable lung cancer. Protons may allow the safe escalation of dose to levels considered to be tumorcidal while sparing the critical normal tissues[24]. This was not possible using photons either with 3-D treatment planning or IMRT when tested in RTOG 0617[25].
More research is needed to optimize proton administration especially for the newer pencil beam systems. This requires greater physics understanding in order to create plans which are robust in the face of uncertainty. Comparative studies will elucidate the potential value of this newer radiotherapy modality in terms of both clinical outcomes (survival, toxicity, and QOL) and cost effectiveness.
P- Reviewer: Hattori N, Nosotti M S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9856] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 2. | Wolf J, Patno ME, Roswit B, D’Esopo N. Controlled study of survival of patients with clinically inoperable lung cancer treated with radiation therapy. Am J Med. 1966;40:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Jett JR, Schild SE, Keith RL, Kesler KA. Treatment of non-small cell lung cancer, stage IIIB: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132:266S-276S. [PubMed] |
| 4. | Jett JR, Schild SE, Kesler KA, Kalemkerian GP. Treatment of small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e400S-e419S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 249] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 5. | Le Chevalier T, Arriagada R, Tarayre M, Lacombe-Terrier MJ, Laplanche A, Quoix E, Ruffie P, Martin M, Douillard JY. Significant effect of adjuvant chemotherapy on survival in locally advanced non-small-cell lung carcinoma. J Natl Cancer Inst. 1992;84:58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 196] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Le Chevalier T, Arriagada R, Quoix E, Ruffie P, Martin M, Douillard JY, Tarayre M, Lacombe-Terrier MJ, Laplanche A. Radiotherapy alone versus combined chemotherapy and radiotherapy in unresectable non-small cell lung carcinoma. Lung Cancer. 1994;10 Suppl 1:S239-S244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 103] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Curran WJ, Scott CB, Langer CJ, Komaki R, Lee JS, Hauser S, Movsas B, Wasserman T, Sause WT, Cox JD. Long-term benefit is observed in a phase III comparison of sequential vs concurrent chemo-radiation for patients with unresected stage III nsclc: RTOG 9410 (abstract #2499). Proceedings of the 2003 annual meeting of ASCO. Proc Am Soc Clin Oncol. 2003;621. |
| 8. | Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, Katagami N, Ariyoshi Y. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692-2699. [PubMed] |
| 9. | Schild SE, Stella PJ, Geyer SM, Bonner JA, McGinnis WL, Mailliard JA, Brindle J, Jatoi A, Jett JR. The outcome of combined-modality therapy for stage III non-small-cell lung cancer in the elderly. J Clin Oncol. 2003;21:3201-3206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 174] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 10. | Nichols RC, Huh SN, Henderson RH, Mendenhall NP, Flampouri S, Li Z, D’Agostino HJ, Cury JD, Pham DC, Hoppe BS. Proton radiation therapy offers reduced normal lung and bone marrow exposure for patients receiving dose-escalated radiation therapy for unresectable stage iii non-small-cell lung cancer: a dosimetric study. Clin Lung Cancer. 2011;12:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Graham MV, Purdy JA, Emami B, Harms W, Bosch W, Lockett MA, Perez CA. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 1999;45:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 959] [Cited by in RCA: 915] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 12. | Jin H, Tucker SL, Liu HH, Wei X, Yom SS, Wang S, Komaki R, Chen Y, Martel MK, Mohan R. Dose-volume thresholds and smoking status for the risk of treatment-related pneumonitis in inoperable non-small cell lung cancer treated with definitive radiotherapy. Radiother Oncol. 2009;91:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Kwa SL, Lebesque JV, Theuws JC, Marks LB, Munley MT, Bentel G, Oetzel D, Spahn U, Graham MV, Drzymala RE. Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys. 1998;42:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 569] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 14. | Wang S, Liao Z, Wei X, Liu HH, Tucker SL, Hu CS, Mohan R, Cox JD, Komaki R. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys. 2006;66:1399-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 281] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 15. | Chang JY, Zhang X, Wang X, Kang Y, Riley B, Bilton S, Mohan R, Komaki R, Cox JD. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in Stage I or Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:1087-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 239] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 16. | Zhang X, Li Y, Pan X, Xiaoqiang L, Mohan R, Komaki R, Cox JD, Chang JY. Intensity-modulated proton therapy reduces the dose to normal tissue compared with intensity-modulated radiation therapy or passive scattering proton therapy and enables individualized radical radiotherapy for extensive stage IIIB non-small-cell lung cancer: a virtual clinical study. Int J Radiat Oncol Biol Phys. 2010;77:357-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 227] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 17. | Colaco RJ, Huh S, Nichols RC, Morris CG, D’Agostino H, Flampouri S, Li Z, Pham DC, Bajwa AA, Hoppe BS. Dosimetric rationale and early experience at UFPTI of thoracic proton therapy and chemotherapy in limited-stage small cell lung cancer. Acta Oncol. 2013;52:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Chang JY, Komaki R, Lu C, Wen HY, Allen PK, Tsao A, Gillin M, Mohan R, Cox JD. Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer. 2011;117:4707-4713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Koay EJ, Lege D, Mohan R, Komaki R, Cox JD, Chang JY. Adaptive/nonadaptive proton radiation planning and outcomes in a phase II trial for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;84:1093-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Hoppe BS, Flampouri S, Henderson RH, Pham D, Bajwa AA, D’Agostino H, Huh SN, Li Z, Mendenhall NP, Nichols RC. Proton therapy with concurrent chemotherapy for non-small-cell lung cancer: technique and early results. Clin Lung Cancer. 2012;13:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Oshiro Y, Mizumoto M, Okumura T, Hashimoto T, Fukumitsu N, Ohkawa A, Kanemoto A, Hashii H, Ohno T, Sakae T. Results of proton beam therapy without concurrent chemotherapy for patients with unresectable stage III non-small cell lung cancer. J Thorac Oncol. 2012;7:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Sejpal S, Komaki R, Tsao A, Chang JY, Liao Z, Wei X, Allen PK, Lu C, Gillin M, Cox JD. Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer. 2011;117:3004-3013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Cox JD. Are the results of RTOG 0617 mysterious? Int J Radiat Oncol Biol Phys. 2012;82:1042-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Fletcher GH, Shukovsky LJ. The interplay of radiocurability and tolerance in the irradiation of human cancers. J Radiol Electrol Med Nucl. 1975;56:383-400. [PubMed] |
| 25. | Bradley JD, Paulus R, Komaki R, Masters GA, Forster K, Schild SE. A randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy with or without cetuximab for stage III non-small cell lung cancer: Results on radiation dose in RTOG 0617. J Clin Oncol. 2013;31:abstr 7501. |