Published online Aug 10, 2014. doi: 10.5306/wjco.v5.i3.348
Revised: May 14, 2014
Accepted: June 10, 2014
Published online: August 10, 2014
Processing time: 129 Days and 14.2 Hours
This review examines the biological properties of coumarins, widely distributed at the highest levels in the fruit, followed by the roots, stems and leaves, by considering their beneficial effects in the prevention of some diseases and as anti-cancer agents. These compounds are well known photosensitizing drugs which have been used as pharmaceuticals for a broad number of therapeutic applications requiring cell division inhibitors. Despite this, even in the absence of ultraviolet rays they are active. The current paper mainly focuses on the effects of psoralens on human breast cancer as they are able to influence many aspects of cell behavior, such as cell growth, survival and apoptosis. In addition, analytical and pharmacological data have demonstrated that psoralens antagonize some metabolizing enzymes, affect estrogen receptor stability and counteract cell invasiveness as well as cancer drug resistance. The scientific findings summarized highlight the pleiotropic functions of phytochemical drugs, given that recently their target signals and how these are modified in the cells have been identified. The encouraging results in this field suggest that multiple modulating strategies based on coumarin drugs in combination with canonical chemotherapeutic agents or radiotherapy could be a useful approach to address the treatment of many types of cancer.
Core tip: This review examines the biological properties of coumarins by considering their beneficial effects in the prevention of some diseases and as anti-cancer agents. The attention is mainly focused on the effects of psoralens on human breast cancer as they are able to influence many aspects of cell behavior. More recently, it has been reported that these drugs in breast cancer cells are capable of antagonizing some metabolizing enzymes, to affect estrogen receptor stability and to counteract cell invasiveness as well as cancer drug resistance.
- Citation: Panno ML, Giordano F. Effects of psoralens as anti-tumoral agents in breast cancer cells. World J Clin Oncol 2014; 5(3): 348-358
- URL: https://www.wjgnet.com/2218-4333/full/v5/i3/348.htm
- DOI: https://dx.doi.org/10.5306/wjco.v5.i3.348
Coumarins are classified as members of the benzopyrone family of compounds, all which consist of a benzene ring joined to a pyrone ring[1]. There are four main coumarin sub-types: the simple coumarins (e.g., coumarin, 7-hydroxycoumarin and 6,7-dihydroxycoumarin); the furanocoumarins (e.g., psoralen, angelicin) that consist of a five-membered furan ring attached to the coumarin nucleus, divided into linear or angular types with substituents at one or both of the remaining benzoid positions; the pyranocoumarins that are analogous to the furanocoumarins but contain a six-membered ring (e.g., seselin, xanthyletin); and the coumarins substituted in the pyrone ring, often at 3-C or 4-C positions (e.g., warfarin)[2].
Coumarins comprise a very large class of compounds found throughout the plant kingdom[3-5]. They are present in fruit, roots, stems and leaves, but some essential oils, particularly cinnamon bark, lavender oil and cassia leaf oil, are also a good source. Coumarin members have also been isolated from microorganisms. For example, coumarin group antibiotics, such as novobiocin, coumermycin A1 and clorobiocin, come from various Streptomyces species. These antibiotics are potent inhibitors of DNA gyrase[6,7]. Aflatoxins, isolated from the Aspergillus species, are a group of highly toxic fungal metabolites.
In the 1970s, furanocoumarins attracted scientific attention when they were introduced in clinical practice[8]. Two of the most important and well known furanocoumarins are psoralen and angelicin, which have been demonstrated to influence cell division and differentiation. In addition, anticancer[9,10], immunomodulatory[11], antibacterial[12], antioxidant[13] and neuroprotective functions[14] have also been shown.
Psoralens, extremely toxic to a wide variety of prokaryotic and eukaryotic organisms, are mainly extracted from the ripe fruit of Psoralea corylifolia (Leguminosae), an erect annual herb widely used in Ayurvedic medicine and traditional Chinese medicine. 8-methoxypsoralen (8-MOP) and 5-methoxypsoralen (5-MOP), or bergapten, are the psoralen compounds that occur in nature but several analogues have also been described[15-20].
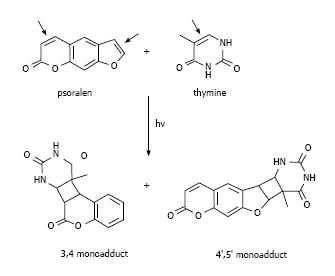
These molecules cause cell damage by covalent binding to DNA after ultraviolet radiation A (UVA) irradiation. They have a planar tricyclic structure with two photoreactive sites (3,4-pyrone and 4,5-furan double bonds). The initial intercalation and interaction with double-stranded DNA occur after absorption of a photon of UVA and afterwards a pyrimidine residue of the DNA covalently binds to the first photoreactive site with a 5,6-double bond. The psoralen monoadducts formed in the DNA can further react photochemically with a pyrimidine base on the complementary strand of the DNA. In fact, it is precisely the planar structure of psoralens which allows them to intercalate between DNA bases[21], thus preventing cell mitosis (Figure 1). This feature is clinically relevant and the combination of psoralen and UVA irradiation (PUVA therapy) has been employed in autoimmune or hyper-proliferative skin diseases, including psoriasis and vitiligo[8]. However, extensive studies have pointed to other biological and clinical applications.
Diets rich in fruit and vegetables are often a predominant source of phytochemical compounds with defensive health effects. Initially, natural products were used as concentrated herbal extracts. However, the identification of the biological activity of each single component became a very complex matter due to a mixture of other constituents. In fact, herbal drugs or extracts contain a combination of active constituents, which are well known in popular medicine for the treatment of various kinds of disorders, such as asthma, coughs, nephritis, vitiligo, calvities and gastroenteric diseases. In Asian countries such as Mongolia, when people were infected with Helicobacter pylori, an alternative non-antibiotic method based on green tea catechins was found to strongly inhibit H. pylori urease activity in vitro and to suppress the bacterial-induced gastritis[22]. A few years later, in a cohort study of gastric carcinomas after screening a total of 25 food phytochemicals, bergamottin was designed as the most promising agent[12]. Grapefruit and grapefruit-based products are rich in flavonoids, coumarins and carotenoids, which in the long run have been shown to have anti-inflammatory[23], anticarcinogenic[9,10,24], antibacterial[12] activities and significant protective effects on cardiovascular diseases[9,13]. Furthermore, these bioactive compounds possess antioxidant properties because they act as free radical scavengers, therefore protecting cellular structures and functions in many stressful conditions.
A rich source of coumarins and coumarin containing compounds are the Psoralea corylifolia L. seeds, well known in traditional Chinese medicine as “Buguzhi”. The plant has long been used for its magical effects to cure various skin diseases but, over the years, many other properties have been discovered[3,10]. The first historical notes on the biological effects of furanocoumarins are related to their photoactivation ability. As previously mentioned, PUVA has been suggested as a potential therapeutic to treat psoriatic lesions and other dermatological conditions[25,26]. Studies reproduced in the 1980-90s on PUVA therapy for psoriasis have reported the comparison of oral and bathwater delivery of 8-MOP[27]. Compared with systemic administration, selectively bathing the epidermis with concentrated psoralen leads to a more complete reversal of the pathological epidermal alterations[11].
Psoriatic keratinocytes inappropriately synthesize a number of immune-related molecules and express a higher amount of epidermal growth factor receptors and insulin-like growth factor receptors that can well support the cellular hyperplasia of the psoriatic lesions. In fact, one of the first studies on PUVA demonstrated how this therapy strongly suppressed the mitogenic stimuli on keratinocytes[11].
A number of conditions with an autoimmune basis other than psoriasis, such as vitiligo, cutaneous T-cell lymphoma, pemphigus vulgaris, systemic sclerosis and rheumatoid arthritis, have benefited from the above treatment[28-30].
The compounds best known and widely used for these applications are 4,5’,8-trimethylpsoralen (TMP), 8-MOP and 5-MOP. All these assessed in human cell line cultures in vitro as well as in in vivo studies showed anti-proliferative activity and apoptotic effects. Along with other derivatives, two angular furanocoumarins angelicin and 4,6,4’-trimethyl angelin (TMA) in human keratinocytes photoinduce cellular death and cell cycle arrest in G1 phase. The molecular responses involve up-regulation of p21 waf/Cip and p53 activation, with mitochondrial-induced cytochrome c release and the consequent apoptotic reaction[31].
In addition to these uses, coumarins display anticancer activities. Interest in this field stemmed from reports by Thornes who evidenced the immunomodulatory activity of coumarin and its utility in malignant melanoma[32]. The photoactivated coumarins are effective in preventing proliferation of bladder[33] and mucoepidermoid carcinoma[34], mammary cancer cell[35] and human melanoma cell line[36], with potential for their use in clinical treatments. Despite their photoactivity even in the absence of UV radiation, they have biological properties.
In fact, the native coumarins have been shown to affect adhesion and motility of neoplastic cells. This aspect was well elucidated in the highly invasive murine melanoma cell line B16-F10 by Velasco-Velaquez MA (2003).
In the latter cell type, compared to the non-malignant fibroblastic cells, the authors reported that 4-hydroxycoumarin (4-HC) was able to affect the assembly of actin filaments, thus decreasing the cellular adhesion to extracellular matrix proteins and motility only in the tumoral cell type.
Since adhesion of tumor cells to extracellular matrix is required during the metastatic process, 4-HC might be useful to prevent metastasis and could be used as an adjuvant therapy for melanoma[37].
The chemopreventive role of coumarin 5-MOP, in the absence of photoactivation, was investigated in human hepatocellular carcinoma (HCC) cell line by studying apoptotic and cytotoxic responses[38].
This study suggested that the suppressive effect of 5-MOP includes at least three modes of action: (1) it first kills cells directly; (2) induces apoptosis by arresting cells at the G2/M phase of cell cycle; and (3) induces apoptosis through an independent pathway with cell-cycle arrest. The authors concluded that the inhibition of cyclin B1 by 5-MOP may play an important role in mitotic arrest and provide an additional way to prevent cells from entering the M phase and undergoing apoptosis.
Antitumoral activity of the methanolic seed extract of P corylifolia L was evaluated in two human cancer cell lines: oral carcinoma cell line and erythroleukemia cells and their corresponding multidrug-resistant cell lines. Both psoralen and isopsoralen constituents were able to inhibit the growth of these cells in a dose-dependent manner. They can also inhibit the growth of normal human primary cells but the IC50 values were higher than those of tumoral cells, suggesting that these two active components had potential selective cytotoxicity[10].
The resistance aspect of cancer cells to chemotherapeutic agents is one of the major obstacles in achieving an effective treatment for cancer. This results from a variety of factors, including individual variations in patients and somatic genetic differences in tumors. The most common reason for cancer drug resistance involves over-expression of membrane drug efflux pumps, such as P-glycoprotein, but other mechanisms might be implicated. Various Chinese herbal drugs have been evaluated for their specific actions against multi drug resistant (MDR) cancer cells. In an experimental study by Wu JYC of the University of Hong Kong[39], a bioassay-guided fractionation of extracts from Radix Peucedani (also known as “Baihua Qianhu” in Chinese medicine) led to the isolation of the pyranocoumarin compounds, (±)-3′-angeloyl-4′-acetoxy-cis-khellactone, a good candidate as a MDR reversing agent for tumoral cells. Strong synergistic interactions were demonstrated when pyranocoumarins were combined with common anti-tumor drugs, including doxorubicin, paclitaxel, puromycin or vincristine, in multidrug resistant human oral epidermoid carcinoma cell line (MDR KB-V1) compared to its drug-sensitive cell line, KB-3-1. Pyranocoumarins increased doxorubicin accumulation in KB-V1 cells and the same treatment down-regulated the expression of P-glycoprotein.
Estrogenic hormones are essential for mammary gland development but the same microenvironment plays a pivotal role in the initiation and progression of breast tumorigenesis. In addition, the weight of genetic factors that contribute to the development of breast cancer must be also taken into account.
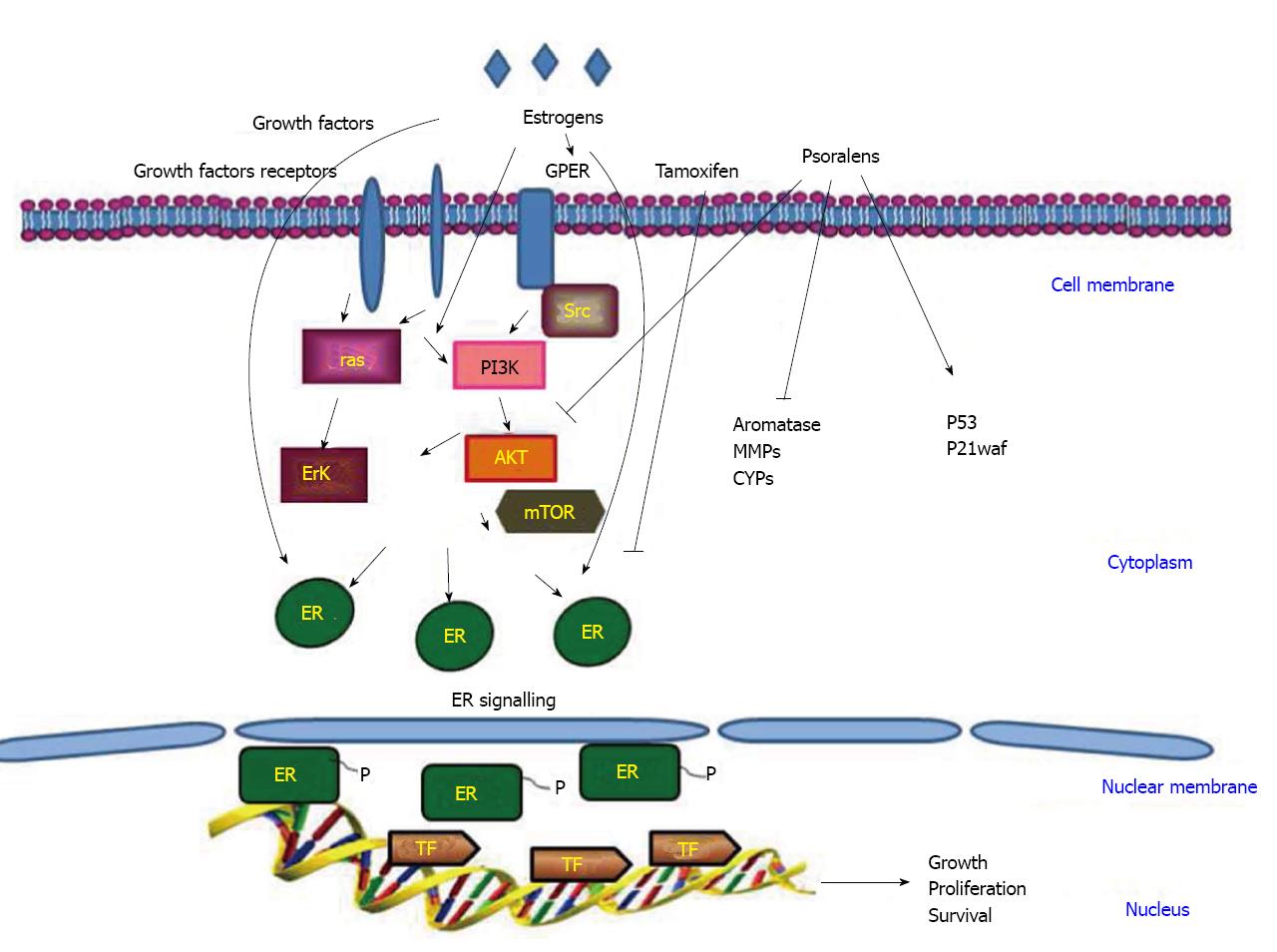
Estrogen signaling pathways are mediated by two nuclear estrogen receptor (ER) proteins, ER-alpha and ER-beta, with different roles. ER-alpha transduces proliferative responses, thus determining cellular growth and tumor progression, while ER-beta has inhibitory actions[40-43].
Besides these nuclear receptors, the GPR30/GPER, a member of the seven-transmembrane G protein-coupled receptor family, has been implicated in mediating the effects of estrogens in various normal and cancer cells. In particular, GPER is able to trigger gene expression and proliferative responses induced by estrogens and even ER antagonists in hormone-sensitive tumor cells[44,45].
Indeed, a whole series of intracellular events, such as the rapid phosphorylation of mitogen-activated protein kinases (MAPK) ERK1/2, the activation of PI3-kinase (PI3K) and phospholipase C (PLC), the increase in cAMP concentrations and intracellular calcium mobilization, was shown to follow GPER activation by both estrogens and anti-estrogens[45].
Approximately 70% of breast cancers are ER-alpha positive and estrogen-dependent. However, the majority of these tumors will transit from an estrogen-dependent to an estrogen independent state that is usually associated with an aggressive form of the disease. During the initial stages of the disease, depletion of ER-alpha from breast cancer cells is a potent approach to prevent estrogen-dependent growth. For this reason, an anti-estrogen such as tamoxifen, acting as a competitive inhibitor of the receptor, has been implemented in the therapeutical protocols for breast cancer. However, the anti-hormonal long-term use of tamoxifen for most treated women leads to the development of drug resistance and an increase of endometrial cell proliferation with risk of endometrial carcinogenesis[46-48].
Therefore, the goal of minimizing the negative side effects of estrogens on breast tumor has stimulated the search for a new molecule able to block the agonistic effects of both estrogen and tamoxifen. The pure antiestrogen, Fulvestrant (ICI 182780), which competes with estrogen for binding to ER with a higher affinity, fulfils these properties very well. This drug is particularly effective as a second-line treatment when tumor cells develop resistance to tamoxifen[49].
In addition to blocking ER activity, scientific research has recently focused attention on the possible use of the aromatase inhibitors (AIs) since breast cancer cell growth is closely supported by estrogen production. Steroidal (exemestane) and non-steroidal (anastrozole, letrozole) aromatase inhibitors are an additional strategy to counteract estrogen function and signaling. These compounds either bind and inactivate aromatase or compete with endogenous substrates to reduce estrogen synthesis. They are approved for use in endocrine treatment of post-menopausal breast cancer and have demonstrated efficacy in patients that develop resistance to anti-hormonal therapy[50-52] .
Several models have been proposed to explain the transition of breast tumor from an estrogen-dependent to an estrogen-independent status, including expression of variant or mutated ER-alpha, altered expression of co-factors or downstream estrogen target genes, post-receptor and pharmacological alterations[53] as well as ligand-independent activation of ER-alpha by other signaling pathways.
Accumulated evidence has indicated that a constitutive expression of growth factor or growth factor receptors in breast cancer cells plays an important role in pharmacological resistance. In many cases, an over-production of polypeptide growth factor with an increased activation of the corresponding signaling pathways can bypass the requirement of mitogenic estrogen signaling during progression of breast cancer[54].
Intricate interactions between ER-alpha and polypeptide growth factors, such as IGF-I, epidermal growth factor (EGF), transforming growth factor (TGF)-alpha and TGF-beta, are involved in the maintenance of proliferation and survival signals. In ER positive breast cancer cells, estrogens increase the mitogenic potential of IGF-I, sensitizing the cells to IGF-I action through the amplification of IGF-I signal[55-59].
The mechanisms of ER-alpha/IGF-I crosstalk is bidirectional and includes the ligand-independent activation of ER-alpha by IGF-I as well as the regulation of the IGF-I system by ER-alpha[60,61]. Other than this type of interaction, the crosstalk between ER-alpha and members of the EGFR family is well known. This receptor was found to be amplified in a human breast cancer cell line and named human epidermal growth factor receptor 2 (HER2)[62].
Amplification of HER2 in human mammary epithelial cells induces proliferative advantages, transformed characteristics, tumorigenic growth and in 3D models induces proliferative and anti-apoptotic changes that mimic early stages of epithelial cell transformation[63,64]. Overexpression of the HER2 protein, either through gene amplification or transcriptional deregulation, is seen in approximately 25%-30% of breast and ovarian cancers and confers worse biological behavior[65].
Patients with these characteristics have lower ER levels and are modestly less responsive to anti-estrogens; therefore, they develop the hormone-resistant phenotype. Moreover, high levels of HER-2/neu expression constitutively activate survival signals involving PI3K/Akt, which is closely related to MAPK hyperactivity[62,66].
Tyrosine kinase associated receptors control most of the fundamental cellular processes, including cell proliferation, differentiation, metabolism, migration and survival. The over-expression of these signals facilitates the emergence of anti-hormone resistance in breast cancer. In such cases, potential interventions with anti-growth factor agents, either alone or in combination with anti-estrogen agents, have been reported and have shown promising results[67].
Targeting multiple pathways simultaneously may help to kill cancer cells and onset of slow drug resistance. In addition, the association of chemotherapy or radiotherapy with pure or synthetic analogs of phytochemical drugs may take advantage of the synergic effects of the combined protocols, resulting in the possibility of lower doses, consequently reducing toxicity. An overall view of the main signal transductions active in breast cancer is shown in Figure 2.
A large number of epidemiological studies suggest that a daily intake of phytochemicals can reduce the incidence of several types of cancers, including breast tumors[68-71]. Moreover, genetic variation in pathways affecting absorption, metabolism and distribution of these natural substances can influence exposure at the tissue level, thus modifying disease risk in individuals[72,73].
The increasing research on this has revealed that the antiproliferative action of psoralens in many tumoral cells, as well as in breast carcinoma, is not only due to their photoactivation, but that these molecules exert their responses even in the absence of radiation. The biological activities in target tissues have been related to the binding of psoralens with specific receptor proteins identified in cytoplasmic and membrane fractions of responsive cells. Binding of psoralens to these proteins is of high affinity and reversible[74]. Coumarins and coumarin-related compounds have been reported to possess significant growth inhibitory activities in in vivo models and against a panel of breast cancer cell lines, in which the structure-activity relationships has also been evaluated[75-77].
Until a few years ago, the transductional pathways activated by psoralens in target cells were not well known; however, the growing interest on this aspect led to the identification of main signals by which the anti-tumoral action is exerted. Moreover, in our first study[78] it was demonstrated that bergapten “per se” without photoactivation was able to influence transductional pathways mainly involved in the regulation of cell survival in two hormone-dependent and hormone-independent human mammary tumoral cell lines, expression of the two biological variants of breast cancer respectively, MCF-7 and SKBR-3. The psoralen induced growth inhibition and apoptosis through the up-regulation of the cyclin inhibitor p21 waf and p53 mRNA and proteins. The molecular study addressed how bergapten transactivated p53 gene promoter through the involvement of the NF-Y nuclear transcriptional factor and the p38 MAPK activation.
Besides this, in hormone-dependent MCF-7 cells, the psoralen counteracted the stimulatory effects of two important mitogenic factors, estradiol and IGF-I on the PI3 kinase/Akt survival pathway.
Similarly, in the presence of photoactivation, bergapten preferentially addressed apoptosis at lower doses than those reported in the previous paper, as revealed by the increase of p53, caspase activation and DNA ladder, while in the absence of UV, the psoralen significantly reduced the p-Akt survival signal[79]. In estrogen-receptor positive breast cancer cells, as previously mentioned, estradiol exerts its main role supporting the proliferation and growth of mammary tissue.
Aromatase is the enzyme responsible for the conversion of androgens into estrogens and synthetic aromatase inhibitors, such as letrozole, anastrozole and exemestane, have proven to be effective in endocrine regimens for ER-positive breast cancer. Together with these molecules, several flavones have also been demonstrated to be effective inhibitors of aromatase and NADPH-quinone reductase 1 and 2 both play an important role on breast carcinogenesis. Among the coumarin-derived compounds, the 4-benzyl-3-(4’-chlorophenyl)-7-methoxycoumarin has been shown to be a potent aromatase inhibitor. The structure-activity studies have evidenced that the three functional groups of the coumarin [the 3-(4’-chlorophenyl), 4-benzyl, and 7-methoxyl groups] are important for its ability to inhibit aromatase[72,80] and not only this (Figure 2). The realization that, in addition to the formation of estrogens by the aromatase pathway, steroids with estrogenic properties could also be formed via a sulfatase route has stimulated the interest of other authors in developing potent steroid sulfatase (STS) inhibitors. The furanocoumarins have proved to be successful steroid sulfatase inhibitors once tested in breast cancer cells and for this they may be useful in suppressing estrogen-dependent breast tumors[72,81,82]. The development and testing of both aromatase and sulfatase inhibitors are in progress and should resolve the question as to whether inhibition of only aromatase or sulfatase is superior to inhibition of only aromatase or STS activity when used for the treatment of hormone-dependent breast cancer.
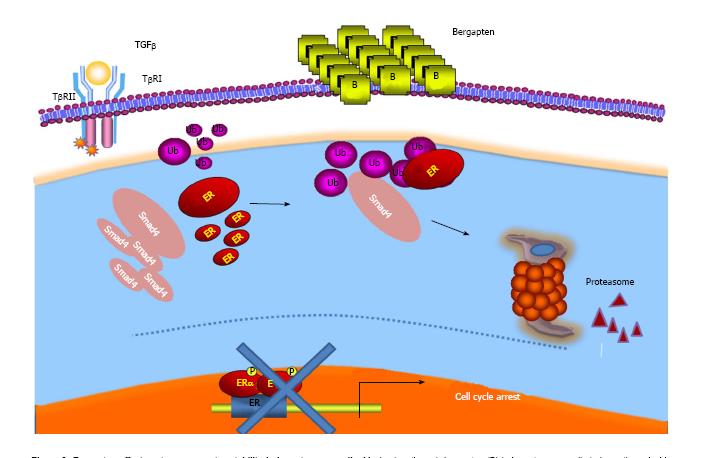
Several observations have also documented the interplay between E2/ER and growth factor signals such as the TGF-beta-dependent pathway. Indeed, it has been evidenced that ER-alpha is able to physically interact with components of the latter pathway, SMAD2, SMAD3 and SMAD4, and to abrogate TGF-beta signaling cascade[83,84]. On the other hand, while TGF-beta signaling has been demonstrated to stimulate ER-alpha transcriptional activity, the complex of SMAD3 and SMAD4 inhibits its activity[85,86]. Analogously, treatment of breast cancer-sensitive and tamoxifen-resistant cells with bergapten induced a depletion of ER protein through a degradative process that sees the involvement of the SMAD4 protein[87] (Figure 3).
This study once again draws attention to the anti-tumoral properties of psoralen and highlights a new molecular mechanism through which bergapten may prevent the crosstalk between the receptor and growth factor mitogenic signaling by affecting ER-alpha stability in breast cancer tamoxifen-sensitive and resistant cells. However, in a recent paper[88], it was demonstrated well that psoralen can also affect the Erb2 receptor tyrosine kinase whose over-expression, as previously reported, characterizes the most aggressive forms of breast cancer.
Independently of interstrand DNA crosslinks, the photo-activated 8-MOP interacts with the Erb2 catalytic autokinase domain, blocking its activity, and furthermore, it can reverse therapeutic resistance by triggering tumor cell apoptosis.
A further negative aspect of estrogens is to promote the migration and motility of breast cancer cells, as demonstrated in an “in vitro” assay, and in fact, they increase the closure of wounded confluent culture. This phenomenon is dependent on the expression of ER, because antiestrogens completely abolish the migratory potency of estrogens[89]. A number of proteolytic enzymes participate in the degradation of environmental barriers, such as the cell-extracellular matrix (ECM) and basement membrane. Matrix metalloproteinases (MMPs), a family of zinc-dependent endopeptidases, play an important role in the proteolysis of ECM components, thus supporting metastatic and angiogenetic processes[90-92].
Agents able to suppress MMP signaling are useful to target cancer metastasis. Some natural products have been demonstrated to play a remarkable role in inhibiting MMP enzymes in breast and other type of cancers[93-97].
The antimetastatic activity of furanocoumarin bergamottin has been described in human fibrosarcoma HT-1080 cells and in MCF-7 breast cancer cells where the compound suppresses MMP-9 gene expression by repressing the transcriptional activation in the MMP-9 promoter. The results obtained in this study evidenced that the furanocoumarin inhibited PMA or TNF-alpha-induced activation of MMP-9 by suppressing NFκB activation in tumoral cells[98].
Considering that psoralen influences bone metabolism and that breast cancer frequently metastasizes to the skeleton, one study[99] investigated whether it can affect this process in vivo. Histological, molecular, biological and imaging analyses revealed that psoralen inhibits bone metastasis; in fact, it regulated the function of osteoblasts and osteoclasts in tumor-bearing mice. Accordingly, the authors suggest the possible therapeutic role of the drug for metastatic breast cancer.
In addition to the above mentioned effects, the furanocoumarin bergamottin has also been described to inhibit members of the family of CYP enzymes (cytochrome P450s) involved in the metabolism of many xenobiotics and drugs. It is well established that tumorigenesis is closely linked to the metabolism of pro-carcinogenic substances, which when subjected to biotransformation into the cells become dangerous for the body. Certain linear furanocoumarins (e.g., bergamottin, imperatorin, isopimpinellin) and a simple coumarin (osthruthin) were found to inhibit cytochrome P450 and to reduce the formation of DNA adducts induced by benzo[a]pyrene and 7,12-dimethylbenz[a] anthracene[24,100-103]. This evidence has encouraged more studies in which five new tetracyclic benzofurocoumarins were synthesized and then tested in three different human tumor cell lines: MDA MB 231 (breast adenocarcinoma), HeLa (cervix adenocarcinoma) and TCC-SUP (bladder transitional cell carcinoma). All of them significantly inhibited cell proliferation and this was mainly linked with the inhibition of CYP2A6 enzyme, belonging to the family of CYPs. The effectiveness of the drugs is related to the chelation of the oxygen from the furan ring with the iron from the heme, possibly resulting in the inactivation of the enzyme, and this might be one of the main causes that preclude tumor cell proliferation[104].
Currently, there is a great scientific interest towards natural anticancer drugs due to their multiple target activities on tumoral cells. As reported here, from the early studies on the activity of psoralens, much has now been documented, especially with regards to their mechanism of action at the molecular level.
The anti-tumoral activity of these molecules against breast cancer has been the main point reported in this review. Many intracellular signals that maintain high survival of breast cancer cells are selectively affected by these drugs. Starting from the latest experimental investigations, it appears that even the most aggressive and resistant cell phenotypes are responsive to psoralens since they antagonize metabolic pathways, protease enzymes, cell cycle progression and even interfere in the crosstalk between receptors and growth factor mitogenic signaling. The combination of natural products with the traditional chemotherapeutic agents, with the purpose of using low doses, can be well addressed and may be a new opportunity for the treatment of breast tumors, thereby decreasing the side effects at the systemic level.
We thank Dr. Tavolaro P for technical assistance and Dr. Sturino D for the English revision, Professor of English, University of Calabria, Cosenza.
P- Reviewer: Freitas VM, Gu GL, Matos MJ S- Editor: Ji FF L- Editor: Roemmele A E- Editor: Lu YJ
| 1. | Murray RD. Coumarins. Nat Prod Rep. 1989;6:591-624. [PubMed] |
| 2. | Keating G, O’Kennedy R. The Chemistry and Occurrence of Coumarins. Coumarins: Biology, Applications and Mode of Action. Chichester: John Wiley & Sons 1997; 23-66. |
| 3. | Egan D, O’Kennedy R, Moran E, Cox D, Prosser E, Thornes RD. The pharmacology, metabolism, analysis, and applications of coumarin and coumarin-related compounds. Drug Metab Rev. 1990;22:503-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 363] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | Egan DA, O’Kennedy R. Rapid and sensitive determination of coumarin and 7-hydroxycoumarin and its glucuronide conjugate in urine and plasma by high-performance liquid chromatography. J Chromatogr. 1992;582:137-143. [PubMed] |
| 5. | Finn GJ, Kenealy E, Creaven BS, Egan DA. In vitro cytotoxic potential and mechanism of action of selected coumarins, using human renal cell lines. Cancer Lett. 2002;183:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Cooke D, Fitzpatrick B, O’Kennedy R, McCormack T, Egan D. Coumarins-Multifaceted Molecules with Many Analytical and Other Applications. Coumarins: Biology, Applications and Mode of Action. Chichester: John Wiley & Sons 1997; 303-332. |
| 7. | Hoult JR, Payá M. Pharmacological and biochemical actions of simple coumarins: natural products with therapeutic potential. Gen Pharmacol. 1996;27:713-722. [PubMed] |
| 8. | Parrish JA, Fitzpatrick TB, Tanenbaum L, Pathak MA. Photochemotherapy of psoriasis with oral methoxsalen and longwave ultraviolet light. N Engl J Med. 1974;291:1207-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1085] [Cited by in RCA: 919] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 9. | Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc. 1996;96:1027-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1437] [Cited by in RCA: 1200] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 10. | Wang Y, Hong C, Zhou C, Xu D, Qu HB. Screening Antitumor Compounds Psoralen and Isopsoralen from Psoralea corylifolia L. Seeds. Evid Based Complement Alternat Med. 2011;2011:363052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Vallat VP, Gilleaudeau P, Battat L, Wolfe J, Nabeya R, Heftler N, Hodak E, Gottlieb AB, Krueger JG. PUVA bath therapy strongly suppresses immunological and epidermal activation in psoriasis: a possible cellular basis for remittive therapy. J Exp Med. 1994;180:283-296. [PubMed] |
| 12. | Sekiguchi H, Washida K, Murakami A. Suppressive Effects of Selected Food Phytochemicals on CD74 Expression in NCI-N87 Gastric Carcinoma Cells. J Clin Biochem Nutr. 2008;43:109-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Bubols GB, Vianna Dda R, Medina-Remon A, von Poser G, Lamuela-Raventos RM, Eifler-Lima VL, Garcia SC. The antioxidant activity of coumarins and flavonoids. Mini Rev Med Chem. 2013;13:318-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Lee MH, Kim JY, Ryu JH. Prenylflavones from Psoralea corylifolia inhibit nitric oxide synthase expression through the inhibition of I-kappaB-alpha degradation in activated microglial cells. Biol Pharm Bull. 2005;28:2253-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Lake BG. Coumarin metabolism, toxicity and carcinogenicity: relevance for human risk assessment. Food Chem Toxicol. 1999;37:423-453. [PubMed] |
| 16. | Stolk LM, Siddiqui AH. Biopharmaceutics, pharmacokinetics and pharmacology of psoralens. Gen Pharmacol. 1988;19:649-653. [PubMed] |
| 17. | Dalla Via L, Gia O, Marciani Magno S, Santana L, Teijeira M, Uriarte E. New tetracyclic analogues of photochemotherapeutic drugs 5-MOP and 8-MOP: synthesis, DNA interaction, and antiproliferative activity. J Med Chem. 1999;42:4405-4413. [PubMed] |
| 18. | Gia O, Anselmo A, Conconi MT, Antonello C, Uriarte E, Caffieri S. 4’-Methyl derivatives of 5-MOP and 5-MOA: synthesis, photoreactivity, and photobiological activity. J Med Chem. 1996;39:4489-4496. [PubMed] |
| 19. | Chilin A, Marzano C, Guiotto A, Manzini P, Baccichetti F, Carlassare F, Bordin F. Synthesis and biological activity of (hydroxymethyl)- and (diethylaminomethyl)benzopsoralens. J Med Chem. 1999;42:2936-2945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Dalla Via L, Mammi S, Uriarte E, Santana L, Lampronti I, Gambari R, Gia O. New furan side tetracyclic allopsoralen derivatives: synthesis and photobiological evaluation. J Med Chem. 2006;49:4317-4326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Zarebska Z, Jarzabek-Chorzelska M, Rzesa G, Gliński W, Pawińska M, Chorzelski T, Jablońska S. Detection of DNA-psoralen photoadducts in situ. Photochem Photobiol. 1984;39:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Matsubara S, Shibata H, Ishikawa F, Yokokura T, Takahashi M, Sugimura T, Wakabayashi K. Suppression of Helicobacter pylori-induced gastritis by green tea extract in Mongolian gerbils. Biochem Biophys Res Commun. 2003;310:715-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 23. | Murakami A, Gao G, Kim OK, Omura M, Yano M, Ito C, Furukawa H, Jiwajinda S, Koshimizu K, Ohigashi H. Identification of coumarins from the fruit of Citrus hystrix DC as inhibitors of nitric oxide generation in mouse macrophage RAW 264.7 cells. J Agric Food Chem. 1999;47:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Kleiner HE, Reed MJ, DiGiovanni J. Naturally occurring coumarins inhibit human cytochromes P450 and block benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene DNA adduct formation in MCF-7 cells. Chem Res Toxicol. 2003;16:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18:1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2162] [Cited by in RCA: 1756] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 26. | Wolff K, Hönigsmann H. Safety and therapeutic effectiveness of selected psoralens in psoriasis. Natl Cancer Inst Monogr. 1984;66:159-164. [PubMed] |
| 27. | Lowe NJ, Weingarten D, Bourget T, Moy LS. PUVA therapy for psoriasis: comparison of oral and bath-water delivery of 8-methoxypsoralen. J Am Acad Dermatol. 1986;14:754-760. [PubMed] |
| 28. | Gasparro FP. The role of PUVA in the treatment of psoriasis. Photobiology issues related to skin cancer incidence. Am J Clin Dermatol. 2000;1:337-348. [PubMed] |
| 29. | Dalla Via L, Marciani Magno S. Photochemotherapy in the treatment of cancer. Curr Med Chem. 2001;8:1405-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Oliven A, Shechter Y. Extracorporeal photopheresis: a review. Blood Rev. 2001;15:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Viola G, Fortunato E, Cecconet L, Del Giudice L, Dall’Acqua F, Basso G. Central role of mitochondria and p53 in PUVA-induced apoptosis in human keratinocytes cell line NCTC-2544. Toxicol Appl Pharmacol. 2008;227:84-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Thornes RD, Lynch G, Sheehan MV. Cimetidine and coumarin therapy of melanoma. Lancet. 1982;2:328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Keane TE, Petros JA, Velimirovich B, Yue KT, Graham SD. Methoxypsoralen phototherapy of transitional cell carcinoma. Urology. 1994;44:842-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Wu JZ, Situ ZQ, Wang W, Chen JY, Liu B. Antitumor activity of psoralen on mucoepidermoid carcinoma cell line MEC-1. Chin Med J (Engl). 1992;105:913-917. [PubMed] |
| 35. | Wu S, Zhang Z, Zhao J. [An experimental study on antitumor activity of psoralen on mammary cancer cell line EMT6 in vitro and in vivo]. Zhongguo Zhongyao Zazhi. 1998;23:303-35, inside back cover. [PubMed] |
| 36. | Carneiro Leite V, Ferreira Santos R, Chen Chen L, Andreu Guillo L. Psoralen derivatives and longwave ultraviolet irradiation are active in vitro against human melanoma cell line. J Photochem Photobiol B. 2004;76:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Velasco-Velázquez MA, Agramonte-Hevia J, Barrera D, Jiménez-Orozco A, García-Mondragón MJ, Mendoza-Patiño N, Landa A, Mandoki J. 4-Hydroxycoumarin disorganizes the actin cytoskeleton in B16-F10 melanoma cells but not in B82 fibroblasts, decreasing their adhesion to extracellular matrix proteins and motility. Cancer Lett. 2003;198:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Lee YM, Wu TH, Chen SF, Chung JG. Effect of 5-methoxypsoralen (5-MOP) on cell apoptosis and cell cycle in human hepatocellular carcinoma cell line. Toxicol In Vitro. 2003;17:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Wu JY, Fong WF, Zhang JX, Leung CH, Kwong HL, Yang MS, Li D, Cheung HY. Reversal of multidrug resistance in cancer cells by pyranocoumarins isolated from Radix Peucedani. Eur J Pharmacol. 2003;473:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 158] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 40. | Kumar V, Green S, Staub A, Chambon P. Localisation of the oestradiol-binding and putative DNA-binding domains of the human oestrogen receptor. EMBO J. 1986;5:2231-2236. [PubMed] |
| 41. | Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925-5930. [PubMed] |
| 42. | Pike AC, Brzozowski AM, Hubbard RE, Bonn T, Thorsell AG, Engström O, Ljunggren J, Gustafsson JA, Carlquist M. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J. 1999;18:4608-4618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 680] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 43. | Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1186] [Cited by in RCA: 1305] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 44. | Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1078] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 45. | Prossnitz ER, Maggiolini M. Mechanisms of estrogen signaling and gene expression via GPR30. Mol Cell Endocrinol. 2009;308:32-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 287] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 46. | Katzenellenbogen BS. Antiestrogen resistance: mechanisms by which breast cancer cells undermine the effectiveness of endocrine therapy. J Natl Cancer Inst. 1991;83:1434-1435. [PubMed] |
| 47. | Wiebe VJ, Osborne CK, Fuqua SA, DeGregorio MW. Tamoxifen resistance in breast cancer. Crit Rev Oncol Hematol. 1993;14:173-188. [PubMed] |
| 48. | Herynk MH, Fuqua SA. Estrogen receptors in resistance to hormone therapy. Adv Exp Med Biol. 2007;608:130-143. [PubMed] |
| 49. | Osborne CK, Coronado-Heinsohn EB, Hilsenbeck SG, McCue BL, Wakeling AE, McClelland RA, Manning DL, Nicholson RI. Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. J Natl Cancer Inst. 1995;87:746-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 238] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 50. | Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, Sahmoud T. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1484] [Cited by in RCA: 1420] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 51. | Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, Jones SE, Alvarez I, Bertelli G, Ortmann O. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 1278] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 52. | Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1392] [Cited by in RCA: 1306] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 53. | Murphy LC. Mechanisms of hormone independence in human breast cancer. In Vivo. 1998;12:95-106. [PubMed] |
| 54. | Nicholson RI, Staka C, Boyns F, Hutcheson IR, Gee JM. Growth factor-driven mechanisms associated with resistance to estrogen deprivation in breast cancer: new opportunities for therapy. Endocr Relat Cancer. 2004;11:623-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Lanzino M, Morelli C, Garofalo C, Panno ML, Mauro L, Andò S, Sisci D. Interaction between estrogen receptor alpha and insulin/IGF signaling in breast cancer. Curr Cancer Drug Targets. 2008;8:597-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Lee AV, Jackson JG, Gooch JL, Hilsenbeck SG, Coronado-Heinsohn E, Osborne CK, Yee D. Enhancement of insulin-like growth factor signaling in human breast cancer: estrogen regulation of insulin receptor substrate-1 expression in vitro and in vivo. Mol Endocrinol. 1999;13:787-796. [PubMed] |
| 57. | Mauro L, Salerno M, Panno ML, Bellizzi D, Sisci D, Miglietta A, Surmacz E, Andò S. Estradiol increases IRS-1 gene expression and insulin signaling in breast cancer cells. Biochem Biophys Res Commun. 2001;288:685-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Stewart AJ, Johnson MD, May FE, Westley BR. Role of insulin-like growth factors and the type I insulin-like growth factor receptor in the estrogen-stimulated proliferation of human breast cancer cells. J Biol Chem. 1990;265:21172-21178. [PubMed] |
| 59. | Yang XF, Beamer WG, Huynh H, Pollak M. Reduced growth of human breast cancer xenografts in hosts homozygous for the lit mutation. Cancer Res. 1996;56:1509-1511. [PubMed] |
| 60. | Lannigan DA. Estrogen receptor phosphorylation. Steroids. 2003;68:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 343] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 61. | Kahlert S, Nuedling S, van Eickels M, Vetter H, Meyer R, Grohe C. Estrogen receptor alpha rapidly activates the IGF-1 receptor pathway. J Biol Chem. 2000;275:18447-18453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 338] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 62. | Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469-6487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 906] [Cited by in RCA: 839] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 63. | Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 443] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 64. | Woods Ignatoski KM, Grewal NK, Markwart S, Livant DL, Ethier SP. p38MAPK induces cell surface alpha4 integrin downregulation to facilitate erbB-2-mediated invasion. Neoplasia. 2003;5:128-134. [PubMed] |
| 65. | Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4972] [Cited by in RCA: 5040] [Article Influence: 140.0] [Reference Citation Analysis (0)] |
| 66. | Faridi J, Wang L, Endemann G, Roth RA. Expression of constitutively active Akt-3 in MCF-7 breast cancer cells reverses the estrogen and tamoxifen responsivity of these cells in vivo. Clin Cancer Res. 2003;9:2933-2939. [PubMed] |
| 67. | Schiff R, Massarweh SA, Shou J, Bharwani L, Mohsin SK, Osborne CK. Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res. 2004;10:331S-336S. [PubMed] |
| 68. | Sporn MB, Suh N. Chemoprevention: an essential approach to controlling cancer. Nat Rev Cancer. 2002;2:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 243] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 69. | Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2044] [Cited by in RCA: 1899] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 70. | Russo M, Spagnuolo C, Tedesco I, Russo GL. Phytochemicals in cancer prevention and therapy: truth or dare? Toxins (Basel). 2010;2:517-551. [PubMed] |
| 71. | D’Incalci M, Steward WP, Gescher AJ. Use of cancer chemopreventive phytochemicals as antineoplastic agents. Lancet Oncol. 2005;6:899-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 72. | Manach C, Hubert J, Llorach R, Scalbert A. The complex links between dietary phytochemicals and human health deciphered by metabolomics. Mol Nutr Food Res. 2009;53:1303-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 73. | Ross SA. Nutritional genomic approaches to cancer prevention research. Exp Oncol. 2007;29:250-256. [PubMed] |
| 74. | Laskin JD. Cellular and molecular mechanisms in photochemical sensitization: studies on the mechanism of action of psoralens. Food Chem Toxicol. 1994;32:119-127. [PubMed] |
| 75. | Musa MA, Cooperwood JS, Khan MO. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr Med Chem. 2008;15:2664-2679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 376] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 76. | Musa MA, Khan MO, Cooperwood JS. Synthesis and antiproliferative activity of coumarin-estrogen conjugates against breast cancer cell lines. Lett Drug Des Discov. 2009;6:133-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Lacy A, O’Kennedy R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr Pharm Des. 2004;10:3797-3811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 415] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 78. | Panno ML, Giordano F, Palma MG, Bartella V, Rago V, Maggiolini M, Sisci D, Lanzino M, De Amicis F, Andò S. Evidence that bergapten, independently of its photoactivation, enhances p53 gene expression and induces apoptosis in human breast cancer cells. Curr Cancer Drug Targets. 2009;9:469-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 79. | Panno ML, Giordano F, Mastroianni F, Palma MG, Bartella V, Carpino A, Aquila S, Andò S. Breast cancer cell survival signal is affected by bergapten combined with an ultraviolet irradiation. FEBS Lett. 2010;584:2321-2326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 80. | Chen S, Cho M, Karlsberg K, Zhou D, Yuan YC. Biochemical and biological characterization of a novel anti-aromatase coumarin derivative. J Biol Chem. 2004;279:48071-48078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 81. | Shields-Botella J, Bonnet P, Duc I, Duranti E, Meschi S, Cardinali S, Prouheze P, Chaigneau AM, Duranti V, Gribaudo S. In vitro and in vivo models for the evaluation of new inhibitors of human steroid sulfatase, devoid of residual estrogenic activity. J Steroid Biochem Mol Biol. 2003;84:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 82. | Purohit A, Woo LW, Chander SK, Newman SP, Ireson C, Ho Y, Grasso A, Leese MP, Potter BV, Reed MJ. Steroid sulphatase inhibitors for breast cancer therapy. J Steroid Biochem Mol Biol. 2003;86:423-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 83. | Matsuda T, Yamamoto T, Muraguchi A, Saatcioglu F. Cross-talk between transforming growth factor-beta and estrogen receptor signaling through Smad3. J Biol Chem. 2001;276:42908-42914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 202] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 84. | Ito I, Hanyu A, Wayama M, Goto N, Katsuno Y, Kawasaki S, Nakajima Y, Kajiro M, Komatsu Y, Fujimura A. Estrogen inhibits transforming growth factor beta signaling by promoting Smad2/3 degradation. J Biol Chem. 2010;285:14747-14755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 85. | Wu L, Wu Y, Gathings B, Wan M, Li X, Grizzle W, Liu Z, Lu C, Mao Z, Cao X. Smad4 as a transcription corepressor for estrogen receptor alpha. J Biol Chem. 2003;278:15192-15200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 86. | Ren Y, Wu L, Frost AR, Grizzle W, Cao X, Wan M. Dual effects of TGF-beta on ERalpha-mediated estrogenic transcriptional activity in breast cancer. Mol Cancer. 2009;8:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 87. | Panno ML, Giordano F, Rizza P, Pellegrino M, Zito D, Giordano C, Mauro L, Catalano S, Aquila S, Sisci D. Bergapten induces ER depletion in breast cancer cells through SMAD4-mediated ubiquitination. Breast Cancer Res Treat. 2012;136:443-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 88. | Xia W, Gooden D, Liu L, Zhao S, Soderblom EJ, Toone EJ, Beyer WF, Walder H, Spector NL. Photo-activated psoralen binds the ErbB2 catalytic kinase domain, blocking ErbB2 signaling and triggering tumor cell apoptosis. PLoS One. 2014;9:e88983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 89. | Malek D, Gust R, Kleuser B. 17-Beta-estradiol inhibits transforming-growth-factor-beta-induced MCF-7 cell migration by Smad3-repression. Eur J Pharmacol. 2006;534:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 90. | Westermarck J, Kähäri VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781-792. [PubMed] |
| 91. | Hadler-Olsen E, Winberg JO, Uhlin-Hansen L. Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumour Biol. 2013;34:2041-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 282] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 92. | Artacho-Cordón F, Ríos-Arrabal S, Lara PC, Artacho-Cordón A, Calvente I, Núñez MI. Matrix metalloproteinases: potential therapy to prevent the development of second malignancies after breast radiotherapy. Surg Oncol. 2012;21:e143-e151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 93. | Ngameni B, Touaibia M, Patnam R, Belkaid A, Sonna P, Ngadjui BT, Annabi B, Roy R. Inhibition of MMP-2 secretion from brain tumor cells suggests chemopreventive properties of a furanocoumarin glycoside and of chalcones isolated from the twigs of Dorstenia turbinata. Phytochemistry. 2006;67:2573-2579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 94. | Shen SC, Lin CW, Lee HM, Chien LL, Chen YC. Lipopolysaccharide plus 12-o-tetradecanoylphorbol 13-acetate induction of migration and invasion of glioma cells in vitro and in vivo: Differential inhibitory effects of flavonoids. Neuroscience. 2006;140:477-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 95. | Jang SY, Lee JK, Jang EH, Jeong SY, Kim JH. Shikonin blocks migration and invasion of human breast cancer cells through inhibition of matrix metalloproteinase-9 activation. Oncol Rep. 2014;31:2827-2833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 96. | Kim C, Kim D, Nam D, Chung WS, Ahn KS, Kim SH, Choi SH, Shim BS, Cho SK, Ahn KS. Anti-Metastatic Effect of Supercritical Extracts from the Citrus hassaku Pericarp via Inhibition of C-X-C Chemokine Receptor Type 4 (CXCR4) and Matrix Metalloproteinase-9 (MMP-9). Phytother Res. 2014;Mar 17; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 97. | Zheng L, Zhang YM, Zhan YZ, Liu CX. Momordica cochinchinensis seed extracts suppress migration and invasion of human breast cancer ZR-75-30 cells via down-regulating MMP-2 and MMP-9. Asian Pac J Cancer Prev. 2014;15:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Hwang YP, Yun HJ, Choi JH, Kang KW, Jeong HG. Suppression of phorbol-12-myristate-13-acetate-induced tumor cell invasion by bergamottin via the inhibition of protein kinase Cdelta/p38 mitogen-activated protein kinase and JNK/nuclear factor-kappaB-dependent matrix metalloproteinase-9 expression. Mol Nutr Food Res. 2010;54:977-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 99. | Wu C, Sun Z, Ye Y, Han X, Song X, Liu S. Psoralen inhibits bone metastasis of breast cancer in mice. Fitoterapia. 2013;91:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 100. | Cai Y, Kleiner H, Johnston D, Dubowski A, Bostic S, Ivie W, DiGiovanni J. Effect of naturally occurring coumarins on the formation of epidermal DNA adducts and skin tumors induced by benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene in SENCAR mice. Carcinogenesis. 1997;18:1521-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 101. | Kleiner HE, Vulimiri SV, Miller L, Johnson WH, Whitman CP, DiGiovanni J. Oral administration of naturally occurring coumarins leads to altered phase I and II enzyme activities and reduced DNA adduct formation by polycyclic aromatic hydrocarbons in various tissues of SENCAR mice. Carcinogenesis. 2001;22:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 102. | Kelly VP, Ellis EM, Manson MM, Chanas SA, Moffat GJ, McLeod R, Judah DJ, Neal GE, Hayes JD. Chemoprevention of aflatoxin B1 hepatocarcinogenesis by coumarin, a natural benzopyrone that is a potent inducer of aflatoxin B1-aldehyde reductase, the glutathione S-transferase A5 and P1 subunits, and NAD(P)H: quinone oxidoreductase in rat liver. Cancer Res. 2000;60:957-969. [PubMed] |
| 103. | Wattenberg LW, Lam LK, Fladmoe AV. Inhibition of chemical carcinogen-induced neoplasia by coumarins and alpha-angelicalactone. Cancer Res. 1979;39:1651-1654. [PubMed] |
| 104. | Francisco CS, Rodrigues LR, Cerqueira NM, Oliveira-Campos AM, Rodrigues LM. Synthesis of novel benzofurocoumarin analogues and their anti-proliferative effect on human cancer cell lines. Eur J Med Chem. 2012;47:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |