INTRODUCTION
Breast cancer is the most commonly diagnosed malignancy and is the second leading cause of cancer-related death in the western world[1]. The incidence of breast cancer has continued to rise in the last several decades. In the United States alone, there will be an estimated 230000 new cases of invasive breast cancer diagnosed in 2013[2]. Fortunately, the prognosis of breast cancer has improved following advances in pharmacologic and surgical techniques in controlling regional disease. As a consequence, the prevalence of patients with a history of breast cancer is increasing as the rate of survival improves, making breast cancer in many aspects a chronic condition. According to recent statistics from the American Cancer Society, over 2.9 million United States women with a history of breast cancer are alive, highlighting the large and increasing population at risk for long-term complications of breast cancer[3].
While medical advances have prolonged survival for patients with breast cancer, metastatic progression involving distal sites such as bone, lung, liver, and brain remains common[4]. The bone is the most common site of metastasis, with osseous metastases developing in 8% of all patients with breast cancer and 69% of patients with advanced disease[5]. Consequences of bony metastases include pathologic fracture, spinal cord compression, anemia, and hypercalcemia[6]. Breast cancer has a particular affinity for the spine, accounting for approximately two-thirds of the osseous metastases discovered[7]. Of these lesions to the spine about one-third become symptomatic, causing intractable pain, neurological deficits, mechanical instability, and ultimately disability and a severe deterioration in quality of life[8,9]. Breast cancer metastases constitute the most common cause of symptomatic spine metastases, accounting for 9%-40% of reported clinical series of spinal epidural metastases in the literature[10].
The management of patients with symptomatic spinal metastases from breast cancer is often complex and requires a multidisciplinary approach[11]. The optimum treatment algorithm has not been definitively defined and varies per patient, with available options including pharmacologic management, radiotherapy, and surgery. Early management of metastatic spinal tumors traditionally emphasized treatment with radiotherapy over surgical decompression. Still, surgery continues to play a critical role in the treatment of metastatic spinal tumors. Advances in surgical techniques and instrumentation have allowed more effective decompression and stabilization of the spine, and with the support of recent evidence the trend has shifted towards using more advanced surgical options in appropriately selected patients[11]. However, while management strategies are continually evolving and physicians now have the capability to treat more aggressively, all therapies for spinal metastases unfortunately remain palliative. This review will detail the presentation, diagnosis, and surgical management options for patients with symptomatic breast cancer metastatic to the spine.
TUMOR CHARACTERISTICS
Multiple malignancies such as breast, prostate, kidney, and lung show a remarkable affinity to metastasize to bone[4]. Metastatic lesions can spread to the bone via several mechanisms, but the method of dissemination most likely responsible for breast cancer involves hematogenous seeding via venous routes[12]. Spread may be accomplished through the Batson plexus, a network of veins that connects the vertebral veins with other beds of venous drainage. This importantly includes the azygos vein, which receives blood draining from the breast via the intercostal veins. The venous plexus of Batson lacks valves to control the flow of blood, so changes in pressure within the body can lead to variable flow through the plexus, allowing retrograde or antegrade seeding of tumor cells[13]. The exact mechanism of metastatic seeding of the bone is unclear. There exists a prominent disparity between the abundance of circulating tumor cells and the relative rarity of metastatic seeding, suggesting a complex environmental barrier to metastasis[14]. Nevertheless, when metastatic events occur in the spine they most commonly occur within the vertebral body with or without extension into the posterior elements[13].
The majority of bone lesions caused by breast cancer is generally accepted to be osteolytic[6]. In reality, breast cancer metastases can cause osteolytic, osteoblastic, or most commonly mixed osteoblastic and osteolytic lesions in the bone[6]. Studies show that resident metastatic breast cancer cells secrete a multitude of osteolytic factors that directly and indirectly activate osteoclasts[15]. Indirect stimulation is mediated by up-regulation of RANK-RANKL signaling, either by osteoblast-mediated osteoclastogenesis or via stimulation of host immune cells by factors such as PTHrP[16]. In addition, there is evidence to suggest that bone breakdown releases previously deposited growth factors and cytokines within the matrix. This has a proliferative effect on the metastatic cells, thus creating a vicious cycle of bone resorption[17].
Thus, the overall local reaction caused by metastatic breast cells is predominantly increased osteolysis. On the other hand, there is often an osteoblastic component due to three mechanisms. The first is tumor cells may influence other cells in its microenvironment such as stromal cells, which can differentiate into osteoblasts[16]. Alternatively, metastatic cells may simply secrete factors which directly stimulate osteoblast proliferation and bone formation[15]. Finally, there is commonly a local bone response to increased bone lysis as a natural response to injury[6]. Thus the resultant lesion may be quite variable, with these factors on a molecular level dictating the degree of overall bone distortion by the infiltrating metastatic tumor.
CLINICAL MANIFESTATIONS
Pain is the most common symptom and is the presenting complaint in nearly 90% of patients with spinal metastases from breast cancer[18]. Pain symptoms vary in intensity but may be vague and nonspecific, and patients with metastatic spinal cord compression have been found to have a delay in diagnosis of about 2 mo from first presenting to a physician to the time of diagnosis[19]. As the neurological status of the patient at time of diagnosis correlates strongly to the patient’s prognosis, a diagnosis before the onset of neurological compromise is essential[11,20]. Accordingly, any patient with a known history of malignancy who presents with new-onset back or neck pain should be promptly and thoroughly evaluated with a high suspicion for metastatic disease involving the spine. Common degenerative disorders less commonly affect the thoracic spine than the cervical or lumbar spine, hence pain in the thoracic spine warrants a high clinical suspicion for metastatic disease[11]. Likewise, patients with persistent nonmechanical pain should have a low threshold for evaluation of a neoplastic etiology[21].
Eliciting the type of spinal pain is important as one may receive clues to the etiology, location, and severity of the tumor infiltration. The pain may be biological, radicular, or mechanical pain. Biological, or local, pain is commonly described as a persistent deep “aching” unrelated to activity that is worse at night[22]. The mechanism is thought to be caused from local periosteal stretching from either tumor growth or tumor-induced inflammatory process[11]. Percussion over the spinous process may elicit local tenderness[13]. This type of pain usually responds to corticosteroids, anti-inflammatory medications, and tumoricidal treatments[22]. Radicular pain results from tumor infiltration causing compression or irritation of individual nerve roots. This pain classically presents in a dermatomal distribution as a band-like, burning, or shooting pain. Effective treatments for radicular pain include surgical nerve root decompression, conventional or stereotactic radiotherapy, or even neuroleptic medications such as gabapentin and pregabalin[22]. Finally, mechanical back pain results from spinal instability caused by the predominantly osteolytic metastatic breast lesion. This pain is aggravated with movement, activity, or activities that require axial loading of the spine, such as sitting or standing[13]. Mechanical pain is important to recognize as patients generally cannot find relief with pharmacologic or radiation therapy, and often require bracing or surgical stabilization[13].
Neurologic dysfunction is a feared consequence and is the second most common presenting symptom of metastatic cancer to the spine[22]. Symptoms are caused by direct tumor growth or pathological fracture that leads to compression of the spinal cord, individual nerve roots, or the cauda equina. First, epidural spinal cord compression may lead to varying degrees of motor weakness, gait instability, autonomic dysfunction, and diminished sensation below the level of injury[22]. Bladder dysfunction is the most common autonomic finding and commonly correlates well with the degree of motor impairment[11]. Manifestations of myelopathy may be noted on detailed physical exam, such as hyperreflexia, clonus, or positive Hoffman or Babinski signs. Second, compression of individual nerve roots can cause radicular pain, as mentioned above, as well as weakness or paresthesia in the associated muscle groups[22]. Thirdly, compression of the cauda equina may manifest as the characteristic constellation of symptoms including back pain, saddle anesthesia, bladder and bowel dysfunction, and lower extremity weakness. Importantly, patients who present with motor weakness may have a variable rate of neurologic deterioration, but in the absence of intervention usually progress to complete paralysis in the absence of any treatment[11].
IMAGING MODALITIES
The available imaging modalities to evaluate for suspected breast cancer metastatic to the spine include plain radiographs, skeletal scintigraphy (SS) (bone scan), computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography CT (PET-CT), and single photon emission CT (SPECT). These modalities carry varying sensitivities, degrees of information, and costs in evaluating for spinal metastases. Given the ability of metastatic breast cancer to present as osteoblastic, osteolytic, or mixed lesions in the bone, each imaging technique may play a valuable role in the evaluation of the at-risk patient depending on the clinical situation.
Plain radiographs are commonly the initial imaging study ordered in evaluating a patient presenting with back pain or neurological symptoms. Given its low cost and widespread availability, plain radiographs are a useful screening test to assess for lytic or sclerotic metastatic lesions, large masses, and pathological fractures. This imaging modality may detect the rare osteoblastic breast metastasis, but this would be more fruitful in a more osteoblastic lesion such as prostate cancer. Regarding osteolytic lesions, which is the predominant radiographic presentation of metastatic breast cancer, bone metastases only become visible on plain radiographs after 30%-50% of bone mineral loss has occurred[23]. Thus, plain radiographs are fairly insensitive and does not provide a definitive diagnosis.
Skeletal scintigraphy (bone scan) is highly sensitive in the detection of osseous metastases, provides images of the entire skeleton, and has been suggested as the first imaging study in asymptomatic patients[24]. This technique detects regions of remodeling within the skeletal system. Osteolytic lesions are accompanied by secondary formation of bone, which allows osteolytic bone metastases to be detected with skeletal scintigraphy several months before they appear on plain radiographs[6]. However, remodeling may also be a result of inflammation, infection, or fractures[22]. Thus, SS has limited specificity and findings need to be correlated by further imaging studies.
CThas become the preferred imaging modality for evaluation of the osseous structures of the spine. The ability of CT scanners to distinguish among materials of different densities allows it to show superior skeletal detail, including bone marrow[25]. Using the bone window setting, CT can be useful in determining the extent of tumor extension, osteoblastic vs osteolytic lesions, and assessing spinal stability[22]. CT is particularly better than plain radiographs and SS for evaluation of lesions in the spine, as these studies do not visualize the spine in sufficient anatomic detail[25]. CT also plays an essential role in preoperative planning and postoperative monitoring. CT myelography is a useful tool in imaging patients with prior spinal instrumentation, as the instrumentation artifact makes visualization with MRI difficult[22].
MRI is the gold-standard diagnostic modality in the imaging of metastatic spinal tumors[13]. This modality has superior sensitivity compared to standard radiographs, CT, and bone scans due to its superior resolution of soft-tissue structures of the spine. MRI is able to define important preoperative parameters such as the extent of epidural extension, degree of spinal cord compression, surrounding edema, and spinal root impingement. Further, evaluation of neighboring structures such as the ligaments, paraspinal muscles, and joints are able to be evaluated. Gadolinium contrast provides additional definition of soft-tissue infiltration and information regarding its vascularity[26]. T1 and T2 weighted studies and fat suppression studies are frequently ordered.
PET is a nuclear medicine technique that detects cellular metabolism of a glucose analog, most commonly fluorodeoxyglucose[24]. PET scans have limited resolutions, but fusion with CT (PET-CT) allows more precise localization of radiotracer uptake. While this modality is more sensitive and specific than SS, its role is controversial due to its increased radiation dose and high cost[27]. Currently it is only recommended if plain radiography, CT, SS, and MRI do not provide adequate information for diagnosis or treatment planning[24].
SPECT is similar to SS but acquires images in a cross-sectional fashion instead of a planar fashion[24]. SPECT shows a greater sensitivity and specificity for detecting spinal metastases[28]. Additionally, SPECT is able to differentiate tumors from inflammatory and infectious lesions, unlike SS[22]. Modern scanners now allow SPECT, diagnostic-quality CT, and fused SPECT-CT images to be done within 1 h on the same machine, which further improves the diagnostic accuracy of the modality[29].
Metastatic involvement of the spine can lead to emergent situations, such as spinal cord compression or pathologic fracture. In these patients, diagnosis in a timely manner is critical as the fragility of the spinal cord and nerve roots mandates urgent intervention. Failure to do so may result in vascular damage and spinal cord “stroke”, leading to irreversible neurological defect. Plain radiographs may be an initial study of choice to evaluate for compression fractures and overall spinal stability. An emergent MRI, which carries excellent soft-tissue contrast, is indicated in order to evaluate for the extent of tumor progression or retropulsed fracture fragments. A T1-weighted, fat saturated sequence after IV gadolinium contrast is optimum for imaging of spinal metastases as intramedullary and osseous lesions are best seen with this sequence[24]. CT (using bone windows) can be helpful for preoperative assessment of extent of bone destruction and mechanical instability.
MANAGEMENT
The management of patients with metastatic breast cancer to the spine is complex and frequently requires a multidisciplinary approach, involving numerous medical specialties (oncology, radiation oncology, pain management, rehabilitation medicine), surgery subspecialties (neurosurgery, orthopedics, surgical oncology), as well as radiologists and interventional radiologists. Advances in the past few decades have improved the treatment of both systemic disease as well as localized tumor burden to the spine. Cytotoxic agents remain the mainstay of treatment of patients with breast cancer. Hormone therapies such as selective estrogen receptor modulators and aromatase inhibitors have been shown to be effective against breast cancer, and human epidermal growth factor receptor 2 (HER2) targeting agents have also been effective in treating metastatic patients[30,31]. Moreover, bisphosphonates given with vitamin D and calcium, which inhibit tumor-related osteoclast activity, and corticosteroids, which may have oncolytic effects on breast cancer and decrease peritumor edema, give physicians an ever-growing array of tools to combat this disease. However, the treatment for symptomatic spinal metastases remains palliative and is not intended to prolong survival. The goals of treatment include restoration of and preservation of neurological function, maintaining spinal stability, and pain relief in an effort to achieve a better quality of life.
Treatment modality and tumor evaluation
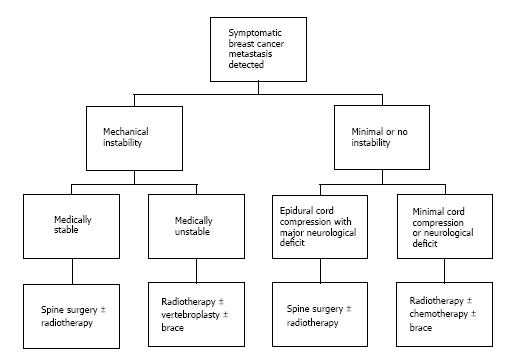
A proposed algorithm for the treatment of patients with symptomatic spinal metastasis from breast cancer is shown in Figure 1. Treatment decision-making can be further aided by considering neurologic, oncologic, mechanical, and systemic parameters (NOMS)[32,33]. Neurologic evaluation incorporates the degree of epidural tumor extension, as the presence of neurological deficits usually correlate with high-grade tumor extension. The Weinstein-Boriani-Biagini staging system was developed for primary spinal tumors in order to describe tumor involvement of vertebral body and adjacent tissues[13]. Briefly, the vertebral body in the axial plane is divided into 12 sectors and 5 tissue layers, which allows a reliable inter-physician description of tumor involvement. More recently, the Spine Oncology Study Group (SOSG) validated a 6-point scale devised specifically for metastatic spinal tumors[34]. Metastatic tumors are graded on a 3 point scale depending on tumor infiltration, with grade 0 representing no epidural extension and grade 3 representing spinal cord compression without CSF fluid around the cord. Grade 2 and 3 tumors are highly likely to result in neurologic deficits and generally require surgical decompression.
Figure 1 Proposed algorithm for treatment of patients with symptomatic spinal metastases from breast cancer.
The oncologic parameter takes into account specific features of the primary tumor histology. When compared to other metastatic spinal tumors, patients with breast cancer have a relatively long life expectancy[10,35]. Patients with breast cancer are also at higher risk for vertebral compression fractures because of age, osteoporosis, and the osteolytic nature of the tumor[36]. Patients with breast cancer are at increased risk of losing ambulation as compared with patients with other primary histologies[37,38]. For these reasons, patients with symptomatic spinal metastases from breast cancer may especially benefit from aggressive surgical intervention, in an attempt to achieve long-lasting symptom relief. Further, radiation response varies by tumor type and breast cancer metastases are generally more radiosensitive than other types of solid tumors[22,35]. Thus, radiation therapy can be useful treatment consideration in this patient cohort, in addition to or independent of surgery.
Spinal instability in the setting of metastatic disease is caused by tumor invasion and distortion of the vertebral body and posterior elements. This results in movement-related pain, symptomatic or progressive deformity, or neural compromise under physiologic loads. The SOSG recently devised the first classification system to aid in predicting spine stability of neoplastic lesions, called the Spine Instability Neoplastic Score (SINS)[39]. Briefly, the scoring system is a 6-point scale that takes into account location, pain, bone lesion type (lytic, blastic, or mixed), spinal alignment, extent of vertebral body collapse, and posterior element involvement. The SINS classification displays near-perfect intraobserver reliability and can be used to guide treatment decision-making, as unstable pathologies are likely to require surgical stabilization given that systemic or radiotherapy cannot restore spinal stability[40].
Surgical management and patient selection
Patients with spinal metastases may be candidates for a wide range of surgical interventions, ranging from limited posterior decompression to radical tumor excision and reconstruction. Surgical advances in the last few decades have allowed improved and more aggressive spinal cord decompression and tumor resection with acceptably low morbidity. Although surgery may be palliative from an oncologic perspective, patients may benefit significantly given the appropriate surgical indications. In 2005, Patchell et al[41] reported the results from the first prospective and randomized controlled trial of direct decompressive surgery plus radiation compared to radiation therapy only. The patients in the surgical arm of the study demonstrated significantly superior postoperative functional improvement (ambulation, neurologic function, muscle strength, continence) and decreased analgesic requirements. Patients with spinal metastases from multiple primary lesions were enrolled, with breast cancer accounting for 11% of the patients in both arms of the study. However, postoperative results were not stratified by primary tumor type. Possible mechanisms underlying improvements following surgical resection and stabilization may include direct alleviation of neural compression by tumor-related spinal pathology, and reduction of mechanical pain caused by tumor-induced instability[10].
The exact indications for surgery in patients with metastatic breast cancer to the spine are controversial, and evidence-based guidelines are not available due to the paucity of literature on this topic[10]. Currently, it is generally agreed that aggressive surgical resection may be appropriate for patients with at least 3 mo expected survival who present with progressive neurological deficit, vertebral column instability, tumors that progress despite maximal radiotherapy, and medically intractable pain[13,42,43]. The majority of the literature has studied metastatic spinal cord tumors as one large cohort, and there has been a recent effort to study histologic-specific spinal metastases. Surgical patients with various types of primary cancer have very different clinical characteristics, which can dictate a patient’s surgical approach, prognosis, and treatment options.
In 2007, Shehadi et al[10] reported the largest retrospective cohort of breast cancer patients treated with aggressive resection of metastatic spinal disease. Eighty-seven patients were treated with aggressive decompression and instrumentation. Patients generally did well neurologically, with 53% of patients who presented with neurologic deficits improving and 85% of all patients maintaining or improving their neurologic function at 1 year. Further, postoperative pain levels were significantly reduced from a preoperative visual analog scale (VAS) of 6 to a median VAS of 2. In 2011, Tancioni et al[35] reported on 23 breast cancer patients treated with more conservative decompressive surgery followed by radiotherapy. This retrospective study remarkably resulted in 96% complete remission of pain and 100% recovery of neurological defect with no major morbidity, which lasted until death or progression of disease at another site. Similarly, Chung et al[44] reported on 15 breast cancer patients who underwent aggressive spinal cord decompression, with 56% of patients who presented with neurologic deficits improving and all patients maintaining or improving their neurological status after surgery.
Several scoring systems have been proposed to determine which patients with metastatic spinal disease would benefit most from decompressive surgery[45]. Tokuhashi et al[46] was the first to propose a scoring system designed to estimate patient prognosis, which guides excisional surgery (> 6 mo survival) vs nonoperative treatment or limited intralesional curettage (< 6 mo survival). Tomita et al[47] also proposed a prognostic scoring system, recommending limited palliative decompression or supportive care vs total en bloc spondylectomy for long-term control. Both the Tomita and Tokuhashi scoring systems take into account the histology of the primary tumor as an important prognostic value, with breast cancer carrying the most favorable prognosis. However, both scores are based on a wide variety of tumor histopathologies with a limited number of breast cancer patients, multiple primary cancers are compiled into the same prognostic group, and medically intractable pain is not an indication for surgery. Therefore, these scores may not be entirely applicable for patients with metastatic breast cancer. Recent histopathologic-specific studies have reported potential prognostic variables, with estrogen receptor positivity, location of tumor, presence of other metastasis, and adjuvant radiotherapy potentially being important variables for patient risk stratification[20,44,45]. However no same prognostic variable has been consistently reported and the studies are all retrospective with small sample sizes.
Surgical techniques
The surgical approach to metastatic tumors of the spine depend on the tumor location, extent of infiltration, and type of reconstruction needed. The approach can be anterior, posterior, lateral or a combination of the above depending on the tumor location. Lesions may also involve multiple levels and require multiple-level decompression and excisions if the osteolytic lesions are extensive[21]. Because the vertebral body is most commonly involved in metastatic disease, an anterior approach often represents the most direct route to the tumor[22]. In the craniocervical region, access may be achieved with a transoral or transmandibular approach although these are associated with significant morbidity and are rarely used in the setting of metastatic disease. Recently, transnasal and transcervical approaches have been developed for improved access. The upper thoracic region (T1-T4) is difficult to access anteriorly due to obstruction by the great vessels and mediastinal organs[48] and a transpedicular posterior or posterolateral approach is generally preferred[13]. However, when necessary a manubriotomy, sternotomy, or trap-door approach may be used[22].
The remaining thoracic region (T5-T11) may be accessed ventrally via a thoracotomy and L2-L5 may be accessed through a retroperitoneal approach[49]. Care should be taken to screen patients who may have had previous radiation or surgery to the neck, thorax, or abdomen, as tissue planes may be disrupted and complicate ventral access. On the other hand, most spine surgeons have a greater familiarity with posterior approaches and thus this represents the most commonly used route for decompression and stabilization. Fortunately, T3-T12 nerve routs may generally be sacrificed without significant morbidity[22]. Posterior stabilization involve multilevel pedicle instrumentation using titanium polyaxial screw-rod systems, which are indicated for resections at high-stress areas and multilevel vertebrectomies[13,22]. Anterior reconstruction following a vertebrectomy is achieved using titanium or polyetherketone (PEEK) cages or with polymethyl-methacrylate (PMMA) cement and anterolateral plating. Newer surgical techniques such as minimally invasive surgery have shown efficacy in achieving neurological improvement and alleviating pain, while decreasing blood loss, operative time, and complication rates[50].
Vertebroplasty and kyphoplasty
Less invasive operative techniques such as percutaneous vertebroplasty and kyphoplasty are cement formulations that can provide additional reinforcement to the vertebral body. Vertebroplasty, which involves PMMA injection into the vertebral body under fluoroscopic or CT guidance, stabilizes the anterior column and prevents further compression fractures. Kyphoplasty is a similar procedure in which an inflatable balloon is first inserted in order to provide a void to inject cement under low pressure, which restores vertebral body height and corrects kyphotic deformity[22,51]. The analgesic properties are thought to be primarily secondary to mechanical stabilization, with potential contributions from thermoablation of nociceptive nerve endings and cytotoxic antitumor effects[52]. Preliminary retrospective reports have demonstrated effective pain relief for metastatic breast cancer to the spine. For example, in 2008 Lee et al[53] reported the results of vertebroplasty for patients with solitary metastases, 8 of whom had breast cancer. Following treatment, all breast cancer patients experienced immediate pain improvement and reduction in analgesic requirement. Treatment strategies have also been proposed that combine percutaneous techniques with surgical resection as well as stereotactic radiosurgery[54].
CONCLUSION
The prevalence of patients with a history of breast cancer is increasing as the rate of survival improves, highlighting the large population of patients at risk for symptomatic spinal metastases. The management of patients with metastatic breast cancer to the spine is often complex and requires a multidisciplinary approach. Precise diagnosis with history, physical, and imaging are imperative to initiate the appropriate treatment in a timely manner. The treatment for symptomatic spinal metastases remains palliative and is not intended to prolong survival. Surgical advances in the last few decades have allowed improved spinal cord decompression and tumor resection and continue to evolve. The goals of treatment include restoration of and preservation of neurological function, maintaining spinal stability, and pain relief in an effort to achieve a better quality of life. Further research should focus on pathology-specific whenever possible, given its implications for treatment selection and prognosis.
P- Reviewer: Garfield DH, Sidiropoulou Z S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ