Published online Aug 10, 2013. doi: 10.5306/wjco.v4.i3.70
Revised: April 23, 2013
Accepted: May 7, 2013
Published online: August 10, 2013
Processing time: 123 Days and 13.2 Hours
Gastrointestinal stromal tumors (GISTs) represent a malignant gastrointestinal tumor of neurofibromatosis type 1 (NF1) Von Recklinghausen disease. In the current case, we report a 27-year-old woman with NF1, who presented with a lower abdominal mass, symptomatic anaemia, and significant weight loss. We employed multiple approaches to assess the tumor behavior, including computed tomography (CT) scan, surgical tumor resection, histological and immunohistochemical analysis and gene sequencing. Additionally, the patient was given Imatinib mesylate (Gleevec) as adjuvant therapy. CT scan delineated a large thick wall cavity lesion connecting to the small bowel segment. Resection of the tumor yielded a mass of 17 cm × 13 cm with achievement of safety margins. The diagnosis was GIST, confirmed by immunohistochemical expression of CD117, CD34, and Bcl-2. Sequencing revealed no mutations in either KIT or platelet-derived growth factor receptor-alpha, genes which are mutated in over 85% of sporadic GIST cases. Further, there was no evidence of recurrence, metastasis or metachronous GIST for over three years in our patient. From our analyses, we believe selective genotyping is advisable for high risk patients to predict potential tumor behavior.
Core tip: There are several differences between gastrointestinal stromal tumors (GISTs) in a neurofibromatosis type 1 (NF1) patient and sporadic GISTs with regard to the tumor site, tumor behavior, targeted therapeutic approach, survival, prognosis, and recurrence based on genomic mutations. Wild type GISTs, those that do not have mutations in KIT or platelet-derived growth factor receptor-alpha (PDGFR-α), are typically resistant to imatanib therapy. Mutational analysis is critical in predicting tumor behavior and might individualize targeted therapy. We show that sequencing for KIT and PDGFR-α provide a useful tool in predicting tumor behavior of GIST in a NF1 patient.
- Citation: Sawalhi S, Al-Harbi K, Raghib Z, Abdelrahman AI, Al-Hujaily A. Behavior of advanced gastrointestinal stromal tumor in a patient with von-Recklinghausen disease: Case report. World J Clin Oncol 2013; 4(3): 70-74
- URL: https://www.wjgnet.com/2218-4333/full/v4/i3/70.htm
- DOI: https://dx.doi.org/10.5306/wjco.v4.i3.70
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract[1] and originate from specific primitive cells, the interstitial cells of Cajal (ICCs). Most sporadic GISTs contain mutations in the KIT gene (80%-85%)[1]. Some GISTs have mutations in platelet-derived growth factor receptor-alpha (PDGFR-α) as an alternate oncogenic mechanism[1]. The 10%-15% of GISTs without activating mutations in either KIT or PDGFR-α are referred to as “Wild type” (WT) which are resistant to imatinib (Gleevec, Novartis, Basel, Switzerland) therapy[2].
Neurofibromatosis type 1 (NF1), also called Von Recklinghausen disease, is an autosomal dominant disorder with a genetic abnormality at chromosome 17q11.2, affecting 1/3000 individuals worldwide[3]. GISTs occur in approximately 5%-25% of NF1 patients[4]. WT GISTs in NF1 patients tend to be multiple and are located predominantly within the small intestine[5]. Mutation of KIT and PDGFR-α genes do not occur in GISTs from NF1 patients[4]. The underlying molecular mechanism for oncogenesis in these tumors has not been elucidated. Recently, Yantiss et al[4] discovered the same KIT point mutation (V559G) in exon 11 occurred in 3 separate GISTs from 1 NF1 patient, whereas 2 patients showed WT KIT and PDGFR-α sequences. However, the possibility of a constitutional mutation was not definitively ruled out. From the case reviewed here, we shed light not only regarding clinical and pathological parameters, but also assess the mutation status of KIT and PDGFR-α genes that predict the prognosis, survival, relapse and the behavior of the GIST tumor in a patient with NF1.
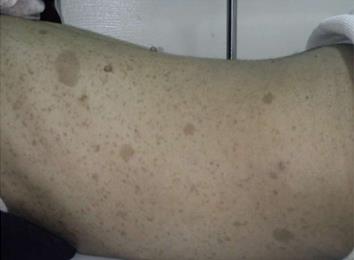
A 27-year-old female patient with known NF1 was admitted to our hospital complaining of lower abdominal dragging pain, symptomatic anemia, with significant weight loss during the previous two years. Our patient appeared cachectic and pale. Physical examination revealed multiple cutaneous and subcutaneous nodules and Café’-au-lait pigmentation all over the body (Figure 1). There was a large solitary lower abdominal mass (17 cm × 13 cm) that was not tender, but was firm in consistency, had active bowel sound, and had no hepatosplenomegally or ascites. Laboratory tests including the tumor marker, serum metanephrine, were normal; however, low hemoglobin levels were detected.
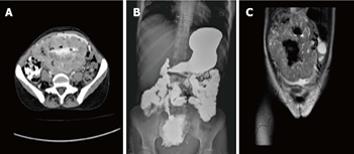
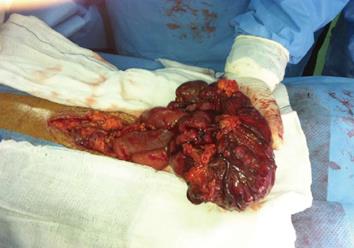
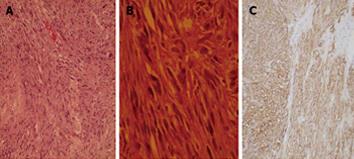
Computed tomography (CT) scan with oral and intravenous contrast revealed a large thick wall cavity lesion with multiple surrounding tortuous vessels, making a percutaneous biopsy high-risk (Figure 2A). Barium follow-through revealed a large cavity connected to a small bowel segment located at the mid-pelvic region which gradually filled with contrast on subsequent films (Figure 2B). Pelvic magnetic resonance imaging was requested to confirm that the lesion did not involve the ovaries (Figure 2C). Informed consent for exploratory laparatomy was obtained from the patient. From this procedure, we found an exophytic mass originating from the jejunal wall and covered by hypervascular omentum with dilated and engorged vessels (Figure 3). There was no evidence of obstruction, or liver and peritoneal metastasis. Resection of the mass was performed with adequate safety margins. Analysis of the gross specimen showed a small intestinal segment measuring 15 cm in length with an attached mass measuring 17 cm × 11 cm × 7 cm. The mass showed congestion and hemorrhage on the external surface. Microscopically, the mass possessed an invasive tumor comprised of spindle shaped cells arranged in bundles and fascicles along with a large area of necrosis. The individual tumor cells were spindle in shape with oval to elongated hyperchromatic nuclei (Figure 4A). Many abnormal nuclei and multinucleated tumor giant cells were noted, with mitotic figures measured at 4/50 (high power field) (Figure 4B).The proximal and distal margins were free of tumor.
An immunohistochemical analysis revealed that the tumor cells expressed KIT, CD34 (Figure 4C), Bcl-2 and focally expressed S-100 protein. The tumor was negative for desmin and smooth muscle-α actin. Genomic DNA analysis of the GIST sample was prepared from formalin-fixed tumor tissue (Qiagen). Direct sequencing of exon and intron-exon boundaries of the c-KIT and PDGF-α genes was performed (Applied Biosystems 3130 XL Genetic analyzer) (Table 1). There were no mutations in exons 9, 11, 13 or 17 in the c-KIT gene or in exons 12, 14, or 18 in the PDGFR-α gene.
| Exon | Primers sequencing |
| c-kit gene | |
| 9F | GTA GTG CGA TGG CCA GTATGCCACATCCCAAGTGTTT |
| 9R | CAG TGT GCA GCG ATG ACTGACATGGTCAATGTTGGAA |
| 11F | GTA GTG CGA TGG CCA GTTTTGTTCTCTCTCCAGAGTGCT |
| 11R | CAG TGT GCA GCG ATG ACACCCAAAAAGGTGACATGGA |
| 13F | GTA GTG CGA TGG CCA GTCATGCGCTTGACATCAGTTT |
| 13R | CAG TGT GCA GCG ATG ACCAATAAAAGGCAGCTTGGACA |
| 17F | GTA GTG CGA TGG CCA GTGTTTTCTTTTCTCCTCCAACC |
| 17R | CAG TGT GCA GCG ATG ACGGACTGTCAAGCAGAG |
| PDGFA gene | |
| 12F | GTA GTG CGA TGG CCA GTTCCAGTCACTGTGCTGCTTC |
| 12R | CAG TGT GCA GCG ATG ACGCAAGGGAAAAGGGAGTCTT |
| 14F | GTA GTG CGA TGG CCA GTTGGTAGCTCAGCTGGACTGAT |
| 14R | CAG TGT GCA GCG ATG ACGGGATGGAGAGTGGAGGATT |
| 18F | GTA GTG CGA TGG CCA GTCTTGCAGGGGTGATGCTATT |
| 18R | CAG TGT GCA GCG ATG ACTGAAGGAGGATGAGCCTGAC |
The post-operative course was uneventful and the patient underwent a routine follow-up with CT scan and positron emission tomography (PET) scan every six months for three years. Imantinib mesylate (Gleevec; 400 mg) was given daily as adjuvant treatment throughout this time period. There was no evidence of recurrence, metastasis or metachronous GIST during the three year follow-up.
GISTs are typically classified into two subtypes: with or without mutations in KIT and PDGFR-α with the former being more malignant[6]. There are several differences between GISTs in a NF1 patient and sporadic GISTs with regard to the tumor site, tumor behavior, targeted therapeutic approach, survival, prognosis and recurrence based on genomic mutations. Many reports have demonstrated that NF1-GISTs are multicentric, and mainly found in the small intestine, a scenario which is rarely observed in sporadic GISTs[5], whereas our patient had a solitary tumor. Moreover, sporadic GISTs usually express a mutation of the KIT gene in exons 9, 11, 13 or 17; while NF1-GISTs lack KIT and PDGFR-α mutations[4]. There is evidence supporting the hypothesis that tumor mutational status may be a prognostic factor for GIST[6]. Patients with mutation-positive GISTs exhibit more frequent recurrence and higher mortality rates than patients without KIT mutations. Lau et al[7] found the 5-year relapse-free survival rate in patients with KIT mutations was 21% compared with 60% in patients without a KIT mutation.
Neurofibromin is a member of GTPase-activating protein family of ras regulatory proteins, and acts as a tumor suppressor[8]. Therefore, inactivation of neurofibromin in NF1-related GISTs is the mechanism leading to tumor formation[8]. In general, NF1 patients develop GISTs at a younger age compared to individuals with sporadic GISTs, as was the case with our patient. Definitive diagnosis of GISTs before surgery is not mandatory, especially because small biopsy samples can be inconclusive. GISTs are fragile and bleed easily, therefore if a suspected GIST is considered to be resectable, biopsy prior to surgery is not recommended because of the high risk of tumor dissemination. Surgery is the first-line therapy for patients with primary resectable GISTs. The object of surgical resection of a primary GIST is complete gross resection without rupturing the tumor psuedocapsule. Resection of adjacent organs in cases of adherent GISTs should be performed with the goal of obtaining negative margins. Lymphadenectomy is usually not warranted, with the exception of evident nodal involvement.
A multimodality approach for treatment combining surgery with systemic therapy is ideal. Marrari et al[9] recommended starting dose of imatinib (400 mg once daily) for patients with advanced GIST carrying mutations in KIT exon 11, PDGFR-α, or those with a WT genotype, with the option of dose escalation upon progression. Response to imatinib and dose escalation have been shown to correlate with the mutational status of KIT and PDGFR-α in GISTs[2]. Patients with KIT exon 9 mutations exhibit noticeably prolonged tumor-free survival when they start imatinib at 800 mg daily[10], but we decided to give our patient imatinib with a dose of 400 mg daily for three years, because she was considered a high risk patient. The risk classification stratifies tumors into very low, low, intermediate, and high-risk levels. Large tumors with high mitotic rates have the highest risk. However, a tumor greater than 10 cm with any mitotic rate, as in our case, or a tumor with more than 10 mitoses per 50 high powered fields, regardless of size, is considered to be high risk[11]. It is unclear if adjuvant therapy in high risk WT-GISTs yields benefit, and the effect of adjuvant imatinib on WT-GISTs may be variable. Regardless, adjuvant treatment with imatinib for three years was associated with a relapse-free survival and overall survival advantage in a randomized trial compared to one year of therapy in high-risk patients[12]. Supporting this, our patient also did not show resistance to imatinib or have tumor relapse within three years. Continuous treatment is recommended because often treatment interruption is followed by rapid tumor progression[13].
Clinical decision making should be individualized based on the mutational analysis (where available) in order to exclude resistant genotypes (e.g., PDGFR-α D842V mutation) from imatinib therapy and permits the usage of an appropriate dose for KIT exon 9 mutations[14]. Marrari et al[9] demonstrated that patients with mutations in exon 11 of the KIT gene show more favourable clinical responses with imatinib treatment compared to those with exon 9 mutations or WT KIT. Surgery should not be delayed in those patients without mutations or who are imatinib resistant. Resistance to imatinib therapy can be primary (as with KIT exon 9 and PDGFR-α exon 18 mutations or WT GISTs[15]) or secondary (related to new kinase mutations). Sunitinib malate is approved for the treatment of advanced imatinib-refractory GIST and is associated with longer overall survival in patients with primary KIT exon 9 mutations and WT GIST compared with KIT exon 11 mutations in a retrospective study[16]. We believe that the clinical course of our patient may have also been attributed to the natural behavior of the tumor and not to imatinib. Tarn et al[17] recently published a study suggesting that WT GISTs are dependent on signalling through the insulin-like growth factor 1 receptor (IGF-IR), therefore monoclonal antibodies a against IGF-IR may be an option of targeted therapy in patients with WT GIST.
According to the European Society for Medical Oncology consensus recommendations, GIST tumors should be considered for molecular analysis for KIT or PDGFR-α mutations[14]. Experts recommend genotyping for all high-risk situations, such as our patient, primary imatinib resistance and metastatic GISTs[18]. Low-risk tumors and those fully resected do not require this type of testing. Furthermore, the optimal timing of mutational analysis to predict tumor behavior, at initial diagnosis or at time of surgery, remain unknown.
P- Reviewer Petros Z S- Editor Song XX L- Editor A E- Editor Lu YJ
| 1. | Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813-3825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 837] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 2. | Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342-4349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1817] [Cited by in RCA: 1642] [Article Influence: 74.6] [Reference Citation Analysis (1)] |
| 3. | Nemoto H, Tate G, Schirinzi A, Suzuki T, Sasaya S, Yoshizawa Y, Midorikawa T, Mitsuya T, Dallapiccola B, Sanada Y. Novel NF1 gene mutation in a Japanese patient with neurofibromatosis type 1 and a gastrointestinal stromal tumor. J Gastroenterol. 2006;41:378-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Yantiss RK, Rosenberg AE, Sarran L, Besmer P, Antonescu CR. Multiple gastrointestinal stromal tumors in type I neurofibromatosis: a pathologic and molecular study. Mod Pathol. 2005;18:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Andersson J, Sihto H, Meis-Kindblom JM, Joensuu H, Nupponen N, Kindblom LG. NF1-associated gastrointestinal stromal tumors have unique clinical, phenotypic, and genotypic characteristics. Am J Surg Pathol. 2005;29:1170-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Taniguchi M, Nishida T, Hirota S, Isozaki K, Ito T, Nomura T, Matsuda H, Kitamura Y. Effect of c-kit mutation on prognosis of gastrointestinal stromal tumors. Cancer Res. 1999;59:4297-4300. [PubMed] |
| 7. | Lau S, Tam KF, Kam CK, Lui CY, Siu CW, Lam HS, Mak KL. Imaging of gastrointestinal stromal tumour (GIST). Clin Radiol. 2004;59:487-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Maertens O, Prenen H, Debiec-Rychter M, Wozniak A, Sciot R, Pauwels P, De Wever I, Vermeesch JR, de Raedt T, De Paepe A. Molecular pathogenesis of multiple gastrointestinal stromal tumors in NF1 patients. Hum Mol Genet. 2006;15:1015-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Marrari A, Wagner AJ, Hornick JL. Predictors of response to targeted therapies for gastrointestinal stromal tumors. Arch Pathol Lab Med. 2012;136:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Duffaud F, Salas S, Huynh T. Recent advances in the management of gastrointestinal stromal tumors. F1000 Med Rep. 2010;2. [PubMed] |
| 11. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2147] [Article Influence: 93.3] [Reference Citation Analysis (1)] |
| 12. | Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster J, Al-Batran SE. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 680] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 13. | Le Cesne A, Ray-Coquard I, Bui BN, Adenis A, Rios M, Bertucci F, Duffaud F, Chevreau C, Cupissol D, Cioffi A. Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomised phase 3 trial. Lancet Oncol. 2010;11:942-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 198] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 14. | ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii49-vii55. [PubMed] |
| 15. | Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1-S41; quiz S42-S44. [PubMed] |
| 16. | Blay JY. New paradigms in gastrointestinal stromal tumour management. Ann Oncol. 2009;20 Suppl 1:i18-i24. [PubMed] |
| 17. | Tarn C, Rink L, Merkel E, Flieder D, Pathak H, Koumbi D, Testa JR, Eisenberg B, von Mehren M, Godwin AK. Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proc Natl Acad Sci USA. 2008;105:8387-8392. [PubMed] |
| 18. | Debiec-Rychter M, Dumez H, Judson I, Wasag B, Verweij J, Brown M, Dimitrijevic S, Sciot R, Stul M, Vranck H. Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2004;40:689-695. [PubMed] |