Published online Mar 10, 2012. doi: 10.5306/wjco.v3.i3.32
Revised: February 12, 2012
Accepted: March 5, 2012
Published online: March 10, 2012
Cutaneous malignant melanoma is the most aggressive form of skin cancer with an extremely poor survival rate for the patients diagnosed with locally invasive and metastatic disease states. Intensive research has led in last few years to an improvement of the early detection and curative treatment of primary cutaneous melanomas that are confined to the skin by tumor surgical resection. However, locally advanced and disseminated melanomas are generally resistant to conventional treatments, including ionizing radiation, systemic chemotherapy, immunotherapy and/or adjuvant stem cell-based therapies, and result in the death of patients. The rapid progression of primary melanomas to locally invasive and/or metastatic disease states remains a major obstacle for an early effective diagnosis and a curative therapeutic intervention for melanoma patients. Importantly, recent advances in the melanoma research have led to the identification of different gene products that are often implicated in the malignant transformation of melanocytic cells into melanoma cells, including melanoma stem/progenitor cells, during melanoma initiation and progression to locally advanced and metastatic disease states. The frequent deregulated genes products encompass the oncogenic B-RafV600E and N-RasQ61R mutants, different receptor tyrosine kinases and developmental pathways such as epidermal growth factor receptor (EGFR), stem cell-like factor (SCF) receptor KIT, hedgehog, Wnt/β-catenin, Notch, stromal cell-derived factor-1 (SDF-1)/CXC chemokine receptor-4 (CXCR4) and vascular endothelial growth factor (VEGF)/VEGFR receptor. These growth factors can cooperate to activate distinct tumorigenic downstream signaling elements and epithelial-mesenchymal transition (EMT)-associated molecules, including phosphatidylinositol 3’-kinase (PI3K)/Akt/ molecular target of rapamycin (mTOR), nuclear factor-kappaB (NF-κB), macrophage inhibitory cytokine-1 (MIC-1), vimentin, snail and twist. Of therapeutic relevance, these deregulated signal transduction components constitute new potential biomarkers and therapeutic targets of great clinical interest for improving the efficacy of current diagnostic and prognostic methods and management of patients diagnosed with locally advanced, metastatic and/or relapsed melanomas.
- Citation: Mimeault M, Batra SK. Novel biomarkers and therapeutic targets for optimizing the therapeutic management of melanomas. World J Clin Oncol 2012; 3(3): 32-42
- URL: https://www.wjgnet.com/2218-4333/full/v3/i3/32.htm
- DOI: https://dx.doi.org/10.5306/wjco.v3.i3.32
Cutaneous malignant melanoma represents the major cause of mortality among skin cancers and its incidence rate is increasing during last years[1-5]. Although the localized cutaneous melanomas diagnosed in the early stages are usually curable by surgical resection of malignant tumors, the rapid progression to invasive and metastatic disease states is generally associated with a poor median survival of 6 mo to 12 mo and a five year survival rate of less than 10%[1,2,6-8]. The therapeutic options for the patients with unresectable melanomas and metastases at distant organs such as lungs, liver and brain consisting to the radiation therapy and/or chemotherapy are only palliative, aiming to improve the quality of life of patients[8-10]. Especially, the standard treatment with alkylating agent, dacarbazine or its orally active analog temozolomide, alone or in combination with other cytotoxic agents, is ineffective in the most cases and culminate to the development of drug resistance, disease relapse and the death of melanoma patients[11-13].
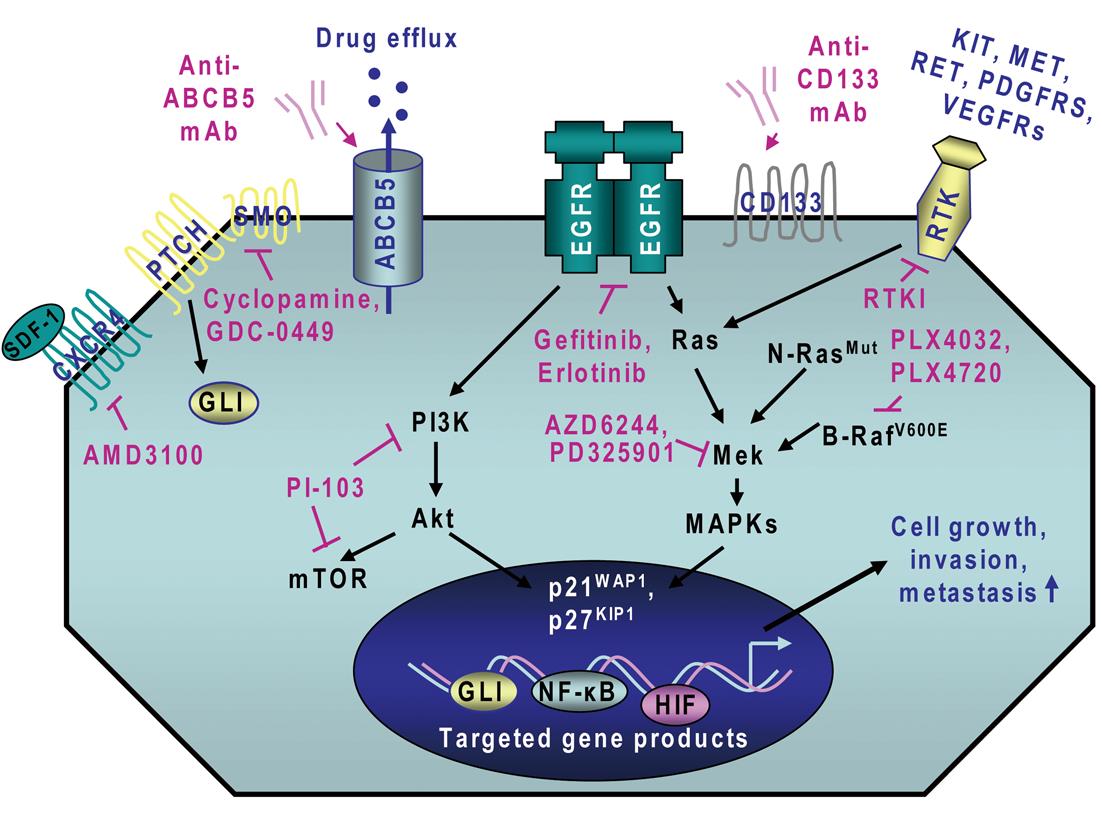
Importantly, recent advances in melanoma research have led to the establishment of the molecular oncogenic events that may contribute to melanoma initiation and progression and treatment resistance of melanoma cells. It has been observed that the persistent activation of different oncogenic signaling cascades initiated in an autocrine or a paracrine manner by distinct growth factors and cytokines through their cognate receptors is typically involved in the sustained proliferation, survival, invasion and metastases at near lymph nodes and distant sites of melanoma cells and angiogenic process[2,14-23]. These deregulated gene products include B-RafV600E, N-RasQ61R, epidermal growth factor receptor (EGFR), hepatocyte growth factor (HGF) receptor MET, platelet-derived growth factor receptors (PDGFRs), sonic hedgehog, Wnt/β-catenin, Notch, Nodal/Cripto, hyaluronan (HA)/CD44, stem cell-factor (SCF) receptor KIT, stromal cell-derived factor-1 (SDF-1)/CXC chemokine receptor-4 (CXCR4), and vascular endothelial growth factor (VEGF)/VEGFR receptor (Figure 1)[13,14,17,19,22,24-47]. These tumorigenic pathways can cooperate for the sustained activation of downstream signaling effectors such as mitogen-activated protein kinases (MAPKs), phosphatidylinositol 3’-kinase (PI3K)/Akt, nuclear factor-kappaB (NF-κB) and hypoxia-inducible factors (HIFs) for the acquisition of a more malignant behavior by melanoma cells during disease progression to locally advanced and metastatic states.
In addition, recent advances in skin stem/progenitor cell research have led to the identification of melanoma cells endowed with stem cell-like properties and which can provide critical functions for tumor growth, metastases at distant sites, treatment resistance and disease relapse[17,18,20,21,23,48,49]. More specifically, highly tumorigenic melanoma stem/progenitor cells have been identified in situ and isolated from primary and secondary melanoma tumors, circulating melanoma cells and established melanoma cell lines[20,21,50-64]. Melanoma stem/progenitor cells may express different stem cell-like markers such as CD133, nestin, aldehyde dehydrogenase (ALDHhigh), CD166, neural crest nerve growth factor receptor (CD271) and/or ATP-binding cassette (ABC) multidrug resistance transporters such as multidrug resistance-1 encoding P-glycoprotein (P-gp), ABCG2 and ABCB5. It has been shown that highly tumorigenic melanoma stem/progenitor cells can give arise to the total tumor cell mass in vivo with the phenotypic features resembling to original patient’s melanomas and metastasize at distant sites[50-58,60,61,63-65]. In this matter, we review the most recent advancements on the gene products that are often altered during melanoma initiation and progression to locally invasive and metastatic disease states and which may be exploited to develop novel multiplex biomarker detection methods for optimizing diagnosis and prognosis and multitargeted therapies for a more effective management of melanoma patients.
The clinical diagnosis of cutaneous malignant melanomas at early stages retains a big challenge for the experimented pathologists and is generally made only after they become visible on skin[66]. Moreover, a skin biopsy and different tumor imaging tests such as X-rays, computed tomography (CT) scan, magnetic resonance imaging (MRI) and positron emission tomography (PET) tests are often performed to establish the grades and stages of melanomas and screen for metastatic melanomas[66,67].
In addition, the immunohistochemical staining of tissue specimens with different antibodies directed against different melanocytic markers such as S-100 and melanoma-associated antigen recognized by T-cells (MART-1) also designated as melanocyte antigen (Melan-A), which is expressed by melanoma cells, is useful for improving the accuracy of the pathological diagnosis and prognosis of melanoma patients[68-70]. Moreover, monoclonal antibody gp100 corresponding to clone HMB-45, which is highly specific and sensitive for melanocytic tumors but does not react with other non-melanoma malignancies such as carcinomas, lymphomas and sarcomas and normal melanocytes, may be used for the pathological diagnosis to distinguish poorly differentiated melanoma subtypes of other tumor types[69,71]. The immunohistochemical analysis of the vimentin expression in primary melanoma tissues, which is frequently overexpressed in primary melanoma patients with hematogenous metastasis, also may help to establish the melanoma patients with a high risk to develop hematogenous metastasis[72]. Although this importance advance, few biomarkers in melanoma stem/progenitor cells and their progenies have been validated in the clinics to use in combination in screening methods for an early and non-invasive detection of cutaneous melanomas and the establishment of the risk of the disease progression, metastases at near lymph nodes and distant sites and relapse. Consequently, the identification and validation of novel molecular biomarkers associated with the melanoma initiation and progression to locally invasive and metastatic disease states and response of melanoma patients to the clinical treatment is of great interest for improving the efficacy of current diagnostic and prognostic methods and therapeutic management of melanoma patients.
Numerous cytogenetic analyses in malignant melanoma tissues and serum samples vs benign melanocytic naevi and normal tissues and serum samples using microarray, immunohistochemical and polymerase chain reaction (PCR)-based techniques have led to the discovery of novel deregulated genes in melanoma cells[18,23,42,73-83]. The gene products often altered during melanoma progression constitute potential biomarkers for a more early diagnosis and accurate prognosis of melanoma patients and effective personalized medicine. The potential biomarkers that may be detected in malignant tissues and/or serum samples, either alone or in combination, to establish the risk of disease progression and as prognostic indicator of melanoma patients include different oncogenic products. Among the more promising molecular biomarkers, there are EGFR, activated pAkt phosphorylated form, microphthalmia-associated transcription factor (MITF), serum amyloid, MIC-1 also designated as growth and differentiation factor-15 (GDF-15), VEGF, interleukin-8 (IL-8) and/or twist[18,23,42,73-79,84-88].
More specifically, it has been observed that the EGFR expression was enhanced in primary and metastatic melanoma tissues from patients relative to non-malignant tissues suggesting that the detection of EGFR could be used as a prognostic indicator to predict the risk of disease progression to metastatic disease states and poor outcome of melanoma patients[86,87]. Moreover, the overexpression of MITF protein has also been detected in 62 of 104 tumor tissues obtained from metastatic melanoma patients and correlated with the chemotherapeutic response and reduced disease-specific survival of melanoma patients[73]. Importantly, the secreted MIC-1 cytokine has also been observed to be overexpressed in 66% of 53 melanoma cell lines analyzed as compared to normal melanocytes[76]. Moreover, the immunohistochemical analyses have indicated that the MIC-1 protein was expressed at low levels in primary melanoma biopsies (15 of 22) while all metastatic melanoma biopsies examined (16 of 16) exhibited strong expression of MIC-1[76]. The results from another study have also indicated that MIC-1 was overexpressed in approximately 67% cases of advanced melanomas and secreted MIC-1 protein levels detected in serum samples of melanoma patients were 5-6 fold higher as compared with serum samples from normal individuals[42]. In this matter, it has been reported that the enhanced MIC-1 expression in melanoma cells may be induced at least in part through the constitutively active mutant B-RafV600E and activation of MAPKs, and to a lesser extent via the activated PI3K/Akt pathway[42,76]. The stimulation of SCF receptor KIT, which may contribute to the activation of MAPK pathway and the phosphorylation of MITF, also may result in an up-regulation of the MIC-1 expression[76]. Hence, together these results combined with the fact that the secreted MIC-1 cytokine has been observed to promote the tumorigenicity of melanoma cells in vivo[42,76], support the clinical interest to detect MIC-1 in melanoma tissue biopsies or serum samples for improving the diagnosis and prognosis of melanoma patients.
On the other hand, the occurrence of polymorphisms in the melanocortin-1-receptor (MC1R), which may lead to the MC1R variants encoding a non-functional MC1R protein and the acquisition of a red hair color (RHC) phenotype, fair skin, freckles and poor tanning ability of individuals, has also been associated with a high risk of developing melanoma[89]. Interestingly, the combined immunohistochemical analyses of expression levels of different cell cycle modulators (p21, p27, p53 and retinoblastoma proteins) and pro-apoptotic factors (Bax and Bak) in primary cutaneous melanoma tissues at stage IIa from 31 patients performed during a 10-year follow-up period have also indicated that the down-regulation of these markers may be more appropriate than the detection of a single molecular marker for assessing the risk of melanoma progression and metastases[83].
Of particular therapeutic interest, a multicenter phase II trial has also been undertaken in order to investigate the efficacy of a sensitivity-directed, first-line chemotherapy in patients with metastasized melanomas by performing an in vitro assay using an ATP-based luminescence viability test for evaluating the chemosensibility of viable melanoma cells obtained from metastatic lesions to seven single drugs and five drug combinations[90]. The results have revealed that among the 53 patients evaluable for all study end points, 22 (42%) were chemosensitive and 31 (58%) chemoresistant patients and the chemosensitive patients showed an increased overall survival of 14.6 months compared with 7.4 mo in chemoresistant patients[90]. In the same way, the results from a recent study have also indicated the possibility to establish the B-RafE600V mutation status in the tissue biopsies and circulating free DNA samples from melanoma patients to assess the patients that could be susceptible to respond to the pharmacological agents targeting oncogenic B-RafE600V mutant[91]. In addition, it has also been noted that the serum concentrations of diverse angiogenic factors such as VEGF, basic fibroblast factor (bFGF) and IL-8 were increased in melanoma patients relative to healthy individuals and associated with advanced stages and poor overall and progression-free survival of melanoma patients[18,23]. More particularly, a study carried out with 35 patients with stage IV melanoma has indicated that 15 patients who responded to chemotherapy showed a significant decrease in the serum IL-8 level while non-responders with progressive disease did not[92]. These data suggest that the detection of serum IL-8 level could serve as an indicator of the potential response of melanoma patients to the chemotherapeutic treatment.
Of great clinical interest, the results from recent studies have also indicated the possibility to detect the stem cell-like markers such as ABCB5, nestin, CD133 and CD166 in primary and metastatic melanoma tissue specimens and/or circulating melanoma stem/progenitor cells in combination with current clinical biomarkers to predict the risk of the metastasis formation and overall survival of melanoma patients[51,55,59,63,93-95]. For instance, it has been observed that highly tumorigenic circulating melanoma cells isolated from the peripheral circulation of melanoma patients expressing the stem cell-like marker, ABCB5 multidrug transporter were tumorigenic and able to form the metastases in animal model in vivo[63]. Furthermore, it has been observed that the expression of ABCB5 protein was enhanced in primary and metastatic melanoma specimens as compared to normal skin and benign nevi[51,55,94]. Then, these data support the interest to detect the ABCB5 multidrug transporter in primary melanoma tissue specimens and circulating melanoma cells to predict the risk of progression to metastatic disease states.
The immunohistochemical analysis of nestin, which is a neuroepithelial intermediate filament expressed in proliferative neuroectodermal progenitor cells during embryonic development and adult bulge areas-resident stem cells in hair follicle, has also indicated that its expression was significantly enhanced in primary and metastatic melanoma tissue specimens as compared to benign and normal melanocytes[96-101]. Nestin was also co-expressed with SOX9 and SOX10, which may contribute to its transcriptional up-regulation, in primary and secondary melanoma specimens and associated with a poor survival of melanoma patients[98-101]. The analyses by flow cytometry and quantitative reverse transcription-PCR (qRT-PCR) of the expression level of nestin performed on 23 tissue specimens from patients with stage III-IV melanoma has also indicated that this stem cell-like marker was expressed at a higher level in stage IV patients compared to stage III/IV with no evidence of disease[93]. It has also been noted that the expression of nestin positively correlated with the tumor burden and tyrosinase and melan-A co-expression in malignant tissues[93]. Nestin has also been detected with tyrosinase in a proportion of circulating melanoma cells enriched from peripheral blood samples while no cells expressing nestin were detected in peripheral blood of healthy volunteers[93].
Additionally, it has also been reported that the percentage of circulating melanoma cells expressing stem cell-like markers, nestin and CD133, detected in 32 melanoma patients correlated with tumor burden and number of metastatic sites, and was associated with a shorter overall survival of patients[59]. The immunohistochemical analyses of co-expression of different stem cell-like markers, including nestin, CD133, ABCB5 and CD166 have also indicated that these biomarkers were significantly enhanced in primary and metastatic melanoma specimens as compared to melanocytic nevi[102,103]. On the other hand, a higher proportion of melanoma cells coexpressing stem cell-like markers, CD271 and SOX10, has also been detected within melanoma biopsies of primary tumors, melanoma metastases and melanoma cell lines and associated with higher metastatic potential and poor tumor-specific survival of melanoma patients[64].
Collectively, the recent advancements on the identification of distinct potential biomarkers in melanoma stem/progenitor cells and their differentiated progenies offer now the possibility to assess their expression levels in primary and metastatic melanoma tissue specimens, serum samples and/or circulating melanoma cells detected in peripheral circulation from patients in the clinics. The simultaneous analyses of the expression of these novel molecular biomarkers could be exploited to develop more effective and non-invasive screening tests for improving the current diagnostic and prognostic methods. Moreover, these novel molecular biomarkers could be used to predict the potential response of melanoma patients to the inhibitory agents targeting these deregulated signaling elements, and thereby lead to an optimization of the choice of cytotoxic drugs for their therapeutic treatment in the clinics. In this matter, we review data from recent in vitro and in vivo studies and clinical trials carried out to validate new potential therapeutic targets in melanoma stem/progenitor cells and their progenies for improving current treatments of patients diagnosed with aggressive melanomas.
Recent investigations in melanoma research have led to the identification of several molecular pathways and specific gene products that are often deregulated during melanoma initiation and progression to locally advanced and metastatic disease states. The oncogenic products constitute new potential therapeutic targets to eradicate the total melanoma cell mass, including melanoma stem/progenitor cells, and prevent disease progression and relapse. These deregulated gene products include B-RafV600E, N-RasG61K, different receptor tyrosine kinases (RTKs) such as EGFR, KIT, MET, PDGFRs and VEGFRs as well as sonic hedgehog, Wnt/β-catenin, Notch, Nodal/Cripto, HA/CD44 and SDF-1/CXCR4 and their downstream signaling effectors such as PI3K/Akt, NF-κB and MIC-1 as well as ABC multidrug resistance transporters (Figure 1; Table 1)[13,17,22,24-46]. The blockade of these tumorigenic pathways and targeting of drug resistance-associated molecules by using specific inhibitory agents has been shown to suppress the growth, invasion and/or metastases of melanoma cells and angiogenesis process in vitro and in vivo[17,24-46,104]. For instance, a combination of EGFR tyrosine kinase inhibitor, erlotinib plus adenoviral vector-mediated IL-24 expression was more effective as individual agents at inhibiting growth and inducing apoptosis of different melanoma cell lines in vitro[105]. In the same way, the combined treatment with erlotinib and a monoclonal antibody (mAb) termed bevacizumab that binds to and inhibits VEGF, also induced supra-additive inhibitory effect on the tumor growth of melanoma cell-derived xenografts and reduced the metastatic spread of melanoma cells to lymph nodes and lungs in mice as compared to single agents[106]. The antitumoral effects of the combined drugs was mediated in part through the inhibition of proliferation and increase of apoptosis of melanoma cells as well as a reduction in tumor angiogenesis[106]. Moreover, it has been reported that the activation of Ras/MAPK and PI3K/Akt pathways may contribute to the up-regulation of GLI transcriptional effector of hedgehog cascade in melanoma cells and the inhibition of smoothened (SMO) co-receptor for sonic hedgehog ligand using cyclopamine reduced the growth of melanoma cell-derived xenografts and metastases in mice and prevented disease recurrence (Figure 1)[104].
| Targeted deregulated element | Name of inhibitory agent |
| mAb against stem cell-like surface marker | |
| CD133 | Anti-CD133 mAb |
| ABCB5 | Anti-ABCB5 mAb |
| Growth factor signaling inhibitor | |
| EGFR (erbB1) antibody | mAb-C225, cetuximab (IMC-C225), IMC-1121B |
| EGFR-TKI | Gefitinib, erlotinib, AG1478, PD153035 |
| Anti-EGF antibody | ABX-EGF |
| Pan-erbB1/erbB2/erbB3/erbB4-TKI | Cl1033 |
| MET | SUI1274 |
| Hedgehog | Anti-SHH antibody, SMO inhibitor (cyclopamine, GDC-0449, BMS-833923, NVP-LDE225, IPI-926 IPI-269609) |
| Wnt/β-catenin | Anti-Wnt antibody, WIF-1 |
| Notch | γ-secretase inhibitor (DAPT, MK-0752,GSI-18) |
| Nodal/Cripto | LEFTY, Anti-Cripto mAb |
| KIT | Imatinib mesylate, dasatinib |
| HA/CD44 | Anti-CD44 mAb, soluble CD44 protein |
| VEGF | Anti-VEGF antibody (bevacizumab) |
| VEGFR2 | Anti-VEGFR-2 mAb (DC101) |
| VEGFR2/EGFR/RET | Vandetanib (ZD6474) |
| VEGFRs, PDGFRs, KIT | Sunitinib |
| B-Raf, C-Raf, KIT, PDGFRs, VEGFR2 and 3 | Sorafenib |
| ECM component/integrin | Anti-integrin antibody |
| CXCR4 | AMD3100 |
| Intracellular signaling inhibitor | |
| B-RafE600V | PLX 4032, PLX4720 |
| MEK1/2 | AZD6244 (ARRY-142886), PD0325901 |
| PI3K | LY294002 |
| mTOR | Rapamycin, CCl-779, |
| PI3K/mTOR | PI-103 |
| NF-κB | IkBα inhibitor, sulfasalazine, bortezomib (PS-341) salinosporamides A (NPI-0052), parthenolide |
| COX-2 | NS-396, etodolax, celecoxib, rofecoxib |
| Immunomodulatory agent | |
| Immune and/or vascular systems | Imiquimod, INF-α, IL-2, IL-21, anti-CTLA-4, mAb (ipilimumab “MDX 010”and tremelimumab “CP-675, 206”) |
Importantly, the targeting of the stem cell-like marker CD133 using mAbs has also been reported to induce the cytotoxic effects in FEMX-I melanoma cells in vitro and reduce their metastatic spread in mice in vivo[57]. Moreover, the inhibition of ABCB5 multidrug transporter using a mAb also inhibited the tumor growth of CD133+/ABCB5+ melanoma stem cell-derived xenografts in vivo[55]. A combination of a CXCR4 inhibitor AMD3100 plus current chemotherapeutic drug, darcarbazine was also more effective at reducing the tumor growth and metastases of chemoresistant CD133+/CXCR4+ melanoma cells in vivo as compared to single drugs[65]. Additional studies, however, are necessary to further establish the molecular mechanisms at the basis of the cytotoxic effects of these therapeutic agents, alone or in combination therapies with current chemotherapeutic drug, dacarbazine on different melanoma cell models.
In addition, several clinical trials have also been carried out or are undergoing to investigate the anticarcinogenic efficacy of new chemopreventive and anticarcinogenic agents and diverse immunosuppressive therapeutic strategies such as the use of dentritic cells, high-doses of interferon-α (IFN-α) and/or IL-2 and anti- cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) antibody, alone or in combination with current therapies for treating locally advanced, metastatic and recurrent melanomas[33,66,107-119]. The cytotoxic drugs include the specific inhibitors of B-RafE600V, N-RasG61K, KIT, EGFR and hedgehog signaling elements. Importantly, the results from phase I clinical studies with a orally active inhibitor of oncogenic B-RafV600E product, PLX4032 carried out with 32 patients with metastatic melanomas harboring the B-RafV600E mutation have revealed that this treatment led to a substantial tumor regression including 24 patients that showed a partial response and 2 had a complete response[120]. The trials are now undergoing to determine the long-term effect of PLX4032, alone or in combination with other agents such as MEK inhibitor, on the survival of melanoma patients[121,122]. In regard with this, the clinical responses have also been observed in a phase II study with orally active and highly selective inhibitor of MEK1/2, AZD6244 (ARRY-142886) or temozolomide performed with 200 patients with advanced melanoma harboring B-RafE600V mutation[123,124]. On the other hand, it has also been reported that the melanoma patients harboring the activating mutations in the KIT receptor exhibited a partial or complete response to imatinib mesylate[33]. It has however been noted that dasatinib was more effective than imatinib at reducing the viability of melanoma cells in two melanoma patients harboring the KITL576P mutation which is the most frequent KIT mutation occurring in approximately 30%-40% cases of melanoma[44]. Furthermore, the data from a multi-institutional phase II trial with an oral multikinase inhibitor termed sorafenib, which targets different tyrosine protein kinases, including wild-type and mutant B-Raf and C-Raf kinases, PDGFRs, KIT, VEGFR2 and VEGFR3, performed with 36 patients with advanced melanomas have indicated that 1 patient showed a partial response for 175 d and 3 patients had stable disease with a mean duration of 37 wk[125].
Among other promising experimental strategies, the results from the clinical trials with a experimental treatment consisting to a topical application of a cream containing 5% an immunomodulatory agent, imiquimod (Aldara) after surgical excision of tumors have revealed that this treatment reduced some melanocytic nevi and melanoma-in-situ (lentigo maligna)[9,10,107-109,126,127]. Moreover, the data from a Phase I/II study of a combination of topical imiquimod and intralesional IL-2 have also revealed that its treatment induced a significant clinical response in patients with multiple accessible melanoma metastases by increasing the activated lymphocytes and the production of IFN-γ by peripheral blood mononuclear cells as well as by restoring the Th1/Th2 balance[110,111]. In addition, a therapeutic treatment consisting of an adjuvant immunotherapy with high doses of immunosuppressive agents, IL-2 and/or IFN-α, alone or in combination with chemotherapy or adoptive cell therapy, has also been observed to result in a complete and long-lasting remission in a small subset of melanoma patients[1,9,10,66,113,116,117,128-130]. In particular, it has been reported that the melanoma cell density in metastases and angiogenesis was significant reduced after a treatment with IFN-α[131]. Moreover, the results of phase II trials with 28 patients with stage IV melanoma without brain metastases have revealed that a combination of dacarbazine plus pegylated IFN-α2a was well tolerated and associated with a response rate of 24% in 25 patients evaluable for response, including 2 long-lasting complete responses[132]. Interestingly, the results of a phase II trial with an oncolytic herpes simplex virus type 1 encoding granulocyte macrophage-colony stimulating factor (GM-CSF), designated as Oncovex (GM-CSF), have also indicated a 28% objective response rate occurred in patients with melanomas which was accompanied by a tumor regression of both injected and non-injected lesions[126,127]. These data suggest that the treatment with Oncovex (GM-CSF) can induce a direct oncolytic effect in injected tumors as well as a secondary immune-mediated anti-tumor effect on non-injected tumors[126,127].
Significant advancements made in last few years have provided important information on the molecular signaling pathways and gene products that are frequently deregulated in melanoma stem/progenitor cells and their progenies during melanoma formation and progression to locally advanced and metastatic disease states. Consequently, the combination of different molecular biomarkers or cytotoxic agents targeting distinct gene products altered during melanoma development may constitute more promising therapeutic strategies as the use a single biomarker or monotherapy for improving the accurate of current diagnostic and prognostic methods and efficacy of the treatment of melanoma patients.
Peer reviewer: Simone Mocellin, MD, PhD, Department of Oncological and Surgical Sciences, University of Padova, via Giustiniani 2, 35128 Padova, Italy
S- Editor Yang XC L- Editor A E- Editor Yang XC
| 1. | Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 589] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 2. | Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1007] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 3. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10002] [Cited by in RCA: 10453] [Article Influence: 696.9] [Reference Citation Analysis (0)] |
| 4. | Garbe C, Leiter U. Melanoma epidemiology and trends. Clin Dermatol. 2009;27:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 415] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 5. | Houghton AN, Polsky D. Focus on melanoma. Cancer Cell. 2002;2:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 187] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A, Kirkwood JM. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635-3648. [PubMed] |
| 7. | Francken AB, Bastiaannet E, Hoekstra HJ. Follow-up in patients with localised primary cutaneous melanoma. Lancet Oncol. 2005;6:608-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Bastiaannet E, Beukema JC, Hoekstra HJ. Radiation therapy following lymph node dissection in melanoma patients: treatment, outcome and complications. Cancer Treat Rev. 2005;31:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Buzaid AC. Management of metastatic cutaneous melanoma. Oncology (Williston Park). 2004;18:1443-150; discussion 1443-150;. [PubMed] |
| 10. | Danson S, Lorigan P. Improving outcomes in advanced malignant melanoma: update on systemic therapy. Drugs. 2005;65:733-743. [PubMed] |
| 11. | Comis RL. DTIC (NSC-45388) in malignant melanoma: a perspective. Cancer Treat Rep. 1976;60:165-176. [PubMed] |
| 12. | Hill GJ, Metter GE, Krementz ET, Fletcher WS, Golomb FM, Ramirez G, Grage TB, Moss SE. DTIC and combination therapy for melanoma. II. Escalating schedules of DTIC with BCNU, CCNU, and vincristine. Cancer Treat Rep. 1979;63:1989-1992. [PubMed] |
| 13. | Tawbi HA, Buch SC. Chemotherapy resistance abrogation in metastatic melanoma. Clin Adv Hematol Oncol. 2010;8:259-266. [PubMed] |
| 14. | Liu ZJ, Xiao M, Balint K, Smalley KS, Brafford P, Qiu R, Pinnix CC, Li X, Herlyn M. Notch1 signaling promotes primary melanoma progression by activating mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating N-cadherin expression. Cancer Res. 2006;66:4182-4190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 216] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Mehnert JM, McCarthy MM, Jilaveanu L, Flaherty KT, Aziz S, Camp RL, Rimm DL, Kluger HM. Quantitative expression of VEGF, VEGF-R1, VEGF-R2, and VEGF-R3 in melanoma tissue microarrays. Hum Pathol. 2010;41:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Gangjee A, Kurup S, Ihnat MA, Thorpe JE, Shenoy SS. Synthesis and biological activity of N(4)-phenylsubstituted-6-(2,4-dichloro phenylmethyl)-7H-pyrrolo[2,3-d]pyrimidine-2,4-diamines as vascular endothelial growth factor receptor-2 inhibitors and antiangiogenic and antitumor agents. Bioorg Med Chem. 2010;18:3575-3587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Mimeault M, Bonenfant D, Batra SK. New advances on the functions of epidermal growth factor receptor and ceramides in skin cell differentiation, disorders and cancers. Skin Pharmacol Physiol. 2004;17:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Rofstad EK, Halsør EF. Vascular endothelial growth factor, interleukin 8, platelet-derived endothelial cell growth factor, and basic fibroblast growth factor promote angiogenesis and metastasis in human melanoma xenografts. Cancer Res. 2000;60:4932-4938. [PubMed] |
| 19. | Pinnix CC, Herlyn M. The many faces of Notch signaling in skin-derived cells. Pigment Cell Res. 2007;20:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Fernandez F, Caygill CP, Kirkham JS, Northfield TC, Savalgi R, Hill MJ. Faecal bile acids and bowel cancer risk in gastric-surgery patients. Eur J Cancer Prev. 1991;1 Suppl 2:79-82. [PubMed] |
| 21. | Mimeault M, Batra SK. Recent advances on skin-resident stem/progenitor cell functions in skin regeneration, aging and cancers and novel anti-aging and cancer therapies. J Cell Mol Med. 2010;14:116-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Ohshima Y, Yajima I, Takeda K, Iida M, Kumasaka M, Matsumoto Y, Kato M. c-RET molecule in malignant melanoma from oncogenic RET-carrying transgenic mice and human cell lines. PLoS One. 2010;5:e10279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19:577-583. [PubMed] |
| 24. | Iivanainen E, Lauttia S, Zhang N, Tvorogov D, Kulmala J, Grenman R, Salven P, Elenius K. The EGFR inhibitor gefitinib suppresses recruitment of pericytes and bone marrow-derived perivascular cells into tumor vessels. Microvasc Res. 2009;78:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Djerf EA, Trinks C, Abdiu A, Thunell LK, Hallbeck AL, Walz TM. ErbB receptor tyrosine kinases contribute to proliferation of malignant melanoma cells: inhibition by gefitinib (ZD1839). Melanoma Res. 2009;19:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Amin DN, Bielenberg DR, Lifshits E, Heymach JV, Klagsbrun M. Targeting EGFR activity in blood vessels is sufficient to inhibit tumor growth and is accompanied by an increase in VEGFR-2 dependence in tumor endothelial cells. Microvasc Res. 2008;76:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Chin L, Garraway LA, Fisher DE. Malignant melanoma: genetics and therapeutics in the genomic era. Genes Dev. 2006;20:2149-2182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 373] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 28. | Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340-4346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1178] [Cited by in RCA: 1122] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 29. | Guo Y, Ma J, Wang J, Che X, Narula J, Bigby M, Wu M, Sy MS. Inhibition of human melanoma growth and metastasis in vivo by anti-CD44 monoclonal antibody. Cancer Res. 1994;54:1561-1565. [PubMed] |
| 30. | Ahrens T, Sleeman JP, Schempp CM, Howells N, Hofmann M, Ponta H, Herrlich P, Simon JC. Soluble CD44 inhibits melanoma tumor growth by blocking cell surface CD44 binding to hyaluronic acid. Oncogene. 2001;20:3399-3408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 142] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 683] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 32. | Smalley KS, Contractor R, Nguyen TK, Xiao M, Edwards R, Muthusamy V, King AJ, Flaherty KT, Bosenberg M, Herlyn M. Identification of a novel subgroup of melanomas with KIT/cyclin-dependent kinase-4 overexpression. Cancer Res. 2008;68:5743-5752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Hodi FS, Friedlander P, Corless CL, Heinrich MC, Mac Rae S, Kruse A, Jagannathan J, Van den Abbeele AD, Velazquez EF, Demetri GD. Major response to imatinib mesylate in KIT-mutated melanoma. J Clin Oncol. 2008;26:2046-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 302] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 34. | Bartolazzi A, Peach R, Aruffo A, Stamenkovic I. Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J Exp Med. 1994;180:53-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 261] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 35. | Knight LA, Di Nicolantonio F, Whitehouse P, Mercer S, Sharma S, Glaysher S, Johnson P, Cree IA. The in vitro effect of gefitinib ('Iressa') alone and in combination with cytotoxic chemotherapy on human solid tumours. BMC Cancer. 2004;4:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Kim Y, Lee YS, Choe J, Lee H, Kim YM, Jeoung D. CD44-epidermal growth factor receptor interaction mediates hyaluronic acid-promoted cell motility by activating protein kinase C signaling involving Akt, Rac1, Phox, reactive oxygen species, focal adhesion kinase, and MMP-2. J Biol Chem. 2008;283:22513-22528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 37. | Qiu L, Wang Q, Di W, Jiang Q, Schefeller E, Derby S, Wanebo H, Yan B, Wan Y. Transient activation of EGFR/AKT cell survival pathway and expression of survivin contribute to reduced sensitivity of human melanoma cells to betulinic acid. Int J Oncol. 2005;27:823-830. [PubMed] |
| 38. | Kumano K, Masuda S, Sata M, Saito T, Lee SY, Sakata-Yanagimoto M, Tomita T, Iwatsubo T, Natsugari H, Kurokawa M. Both Notch1 and Notch2 contribute to the regulation of melanocyte homeostasis. Pigment Cell Melanoma Res. 2008;21:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Postovit LM, Seftor EA, Seftor RE, Hendrix MJ. Targeting Nodal in malignant melanoma cells. Expert Opin Ther Targets. 2007;11:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Postovit LM, Margaryan NV, Seftor EA, Hendrix MJ. Role of nodal signaling and the microenvironment underlying melanoma plasticity. Pigment Cell Melanoma Res. 2008;21:348-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Nickoloff BJ, Hendrix MJ, Pollock PM, Trent JM, Miele L, Qin JZ. Notch and NOXA-related pathways in melanoma cells. J Investig Dermatol Symp Proc. 2005;10:95-104. [PubMed] |
| 42. | Huh SJ, Chung CY, Sharma A, Robertson GP. Macrophage inhibitory cytokine-1 regulates melanoma vascular development. Am J Pathol. 2010;176:2948-2957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Puri N, Ahmed S, Janamanchi V, Tretiakova M, Zumba O, Krausz T, Jagadeeswaran R, Salgia R. c-Met is a potentially new therapeutic target for treatment of human melanoma. Clin Cancer Res. 2007;13:2246-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | Woodman SE, Trent JC, Stemke-Hale K, Lazar AJ, Pricl S, Pavan GM, Fermeglia M, Gopal YN, Yang D, Podoloff DA. Activity of dasatinib against L576P KIT mutant melanoma: molecular, cellular, and clinical correlates. Mol Cancer Ther. 2009;8:2079-2085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 45. | Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, Bremer R, Gillette S, Kong J, Haass NK. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041-3046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 1037] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 46. | Halaban R, Zhang W, Bacchiocchi A, Cheng E, Parisi F, Ariyan S, Krauthammer M, McCusker JP, Kluger Y, Sznol M. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010;23:190-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 272] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 47. | Frank NY, Schatton T, Kim S, Zhan Q, Wilson BJ, Ma J, Saab KR, Osherov V, Widlund HR, Gasser M. VEGFR-1 expressed by malignant melanoma-initiating cells is required for tumor growth. Cancer Res. 2011;71:1474-1485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 48. | Ruiter D, Bogenrieder T, Elder D, Herlyn M. Melanoma-stroma interactions: structural and functional aspects. Lancet Oncol. 2002;3:35-43. [PubMed] |
| 49. | Merighi S, Simioni C, Gessi S, Varani K, Mirandola P, Tabrizi MA, Baraldi PG, Borea PA. A(2B) and A(3) adenosine receptors modulate vascular endothelial growth factor and interleukin-8 expression in human melanoma cells treated with etoposide and doxorubicin. Neoplasia. 2009;11:1064-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328-9337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 923] [Cited by in RCA: 934] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 51. | Frank NY, Margaryan A, Huang Y, Schatton T, Waaga-Gasser AM, Gasser M, Sayegh MH, Sadee W, Frank MH. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res. 2005;65:4320-4333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 414] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 52. | Dou J, Pan M, Wen P, Li Y, Tang Q, Chu L, Zhao F, Jiang C, Hu W, Hu K. Isolation and identification of cancer stem-like cells from murine melanoma cell lines. Cell Mol Immunol. 2007;4:467-472. [PubMed] |
| 53. | Monzani E, Facchetti F, Galmozzi E, Corsini E, Benetti A, Cavazzin C, Gritti A, Piccinini A, Porro D, Santinami M. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer. 2007;43:935-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 407] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 54. | Keshet GI, Goldstein I, Itzhaki O, Cesarkas K, Shenhav L, Yakirevitch A, Treves AJ, Schachter J, Amariglio N, Rechavi G. MDR1 expression identifies human melanoma stem cells. Biochem Biophys Res Commun. 2008;368:930-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C. Identification of cells initiating human melanomas. Nature. 2008;451:345-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1140] [Cited by in RCA: 1034] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 56. | Grichnik JM, Burch JA, Schulteis RD, Shan S, Liu J, Darrow TL, Vervaert CE, Seigler HF. Melanoma, a tumor based on a mutant stem cell? J Invest Dermatol. 2006;126:142-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 57. | Rappa G, Fodstad O, Lorico A. The stem cell-associated antigen CD133 (Prominin-1) is a molecular therapeutic target for metastatic melanoma. Stem Cells. 2008;26:3008-3017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 58. | Kupas V, Weishaupt C, Siepmann D, Kaserer ML, Eickelmann M, Metze D, Luger TA, Beissert S, Loser K. RANK is expressed in metastatic melanoma and highly upregulated on melanoma-initiating cells. J Invest Dermatol. 2011;131:944-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Fusi A, Reichelt U, Busse A, Ochsenreither S, Rietz A, Maisel M, Keilholz U. Expression of the stem cell markers nestin and CD133 on circulating melanoma cells. J Invest Dermatol. 2011;131:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 60. | Boonyaratanakornkit JB, Yue L, Strachan LR, Scalapino KJ, LeBoit PE, Lu Y, Leong SP, Smith JE, Ghadially R. Selection of tumorigenic melanoma cells using ALDH. J Invest Dermatol. 2010;130:2799-2808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 61. | Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, Butler PD, Yang GP, Joshua B, Kaplan MJ. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 601] [Cited by in RCA: 558] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 62. | Zabierowski SE, Herlyn M. Learning the ABCs of melanoma-initiating cells. Cancer Cell. 2008;13:185-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Ma J, Lin JY, Alloo A, Wilson BJ, Schatton T, Zhan Q, Murphy GF, Waaga-Gasser AM, Gasser M, Stephen Hodi F. Isolation of tumorigenic circulating melanoma cells. Biochem Biophys Res Commun. 2010;402:711-717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 64. | Civenni G, Walter A, Kobert N, Mihic-Probst D, Zipser M, Belloni B, Seifert B, Moch H, Dummer R, van den Broek M. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011;71:3098-3109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 65. | Kim M, Koh YJ, Kim KE, Koh BI, Nam DH, Alitalo K, Kim I, Koh GY. CXCR4 signaling regulates metastasis of chemoresistant melanoma cells by a lymphatic metastatic niche. Cancer Res. 2010;70:10411-10421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 66. | Algazi AP, Soon CW, Daud AI. Treatment of cutaneous melanoma: current approaches and future prospects. Cancer Manag Res. 2010;2:197-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 67. | Patnana M, Bronstein Y, Szklaruk J, Bedi DG, Hwu WJ, Gershenwald JE, Prieto VG, Ng CS. Multimethod imaging, staging, and spectrum of manifestations of metastatic melanoma. Clin Radiol. 2011;66:224-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Nonaka D, Chiriboga L, Rubin BP. Differential expression of S100 protein subtypes in malignant melanoma, and benign and malignant peripheral nerve sheath tumors. J Cutan Pathol. 2008;35:1014-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 69. | Mahmood MN, Lee MW, Linden MD, Nathanson SD, Hornyak TJ, Zarbo RJ. Diagnostic value of HMB-45 and anti-Melan A staining of sentinel lymph nodes with isolated positive cells. Mod Pathol. 2002;15:1288-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Ben-Izhak O, Stark P, Levy R, Bergman R, Lichtig C. Epithelial markers in malignant melanoma. A study of primary lesions and their metastases. Am J Dermatopathol. 1994;16:241-246. [PubMed] |
| 71. | Gown AM, Vogel AM, Hoak D, Gough F, McNutt MA. Monoclonal antibodies specific for melanocytic tumors distinguish subpopulations of melanocytes. Am J Pathol. 1986;123:195-203. [PubMed] |
| 72. | Li M, Zhang B, Sun B, Wang X, Ban X, Sun T, Liu Z, Zhao X. A novel function for vimentin: the potential biomarker for predicting melanoma hematogenous metastasis. J Exp Clin Cancer Res. 2010;29:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 73. | Ugurel S, Houben R, Schrama D, Voigt H, Zapatka M, Schadendorf D, Bröcker EB, Becker JC. Microphthalmia-associated transcription factor gene amplification in metastatic melanoma is a prognostic marker for patient survival, but not a predictive marker for chemosensitivity and chemotherapy response. Clin Cancer Res. 2007;13:6344-6350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 74. | Matharoo-Ball B, Ratcliffe L, Lancashire L, Ugurel S, Miles AK, Weston DJ, Rees R, Schadendorf D, Ball G, Creaser CS. Diagnostic biomarkers differentiating metastatic melanoma patients from healthy controls identified by an integrated MALDI-TOF mass spectrometry/bioinformatic approach. Proteomics Clin Appl. 2007;1:605-620. [PubMed] |
| 75. | Utikal J, Schadendorf D, Ugurel S. Serologic and immunohistochemical prognostic biomarkers of cutaneous malignancies. Arch Dermatol Res. 2007;298:469-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 76. | Boyle GM, Pedley J, Martyn AC, Banducci KJ, Strutton GM, Brown DA, Breit SN, Parsons PG. Macrophage inhibitory cytokine-1 is overexpressed in malignant melanoma and is associated with tumorigenicity. J Invest Dermatol. 2009;129:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 77. | Ugurel S, Thirumaran RK, Bloethner S, Gast A, Sucker A, Mueller-Berghaus J, Rittgen W, Hemminki K, Becker JC, Kumar R. B-RAF and N-RAS mutations are preserved during short time in vitro propagation and differentially impact prognosis. PLoS One. 2007;2:e236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 78. | Findeisen P, Zapatka M, Peccerella T, Matzk H, Neumaier M, Schadendorf D, Ugurel S. Serum amyloid A as a prognostic marker in melanoma identified by proteomic profiling. J Clin Oncol. 2009;27:2199-2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 79. | Busch C, Geisler J, Knappskog S, Lillehaug JR, Lønning PE. Alterations in the p53 pathway and p16INK4a expression predict overall survival in metastatic melanoma patients treated with dacarbazine. J Invest Dermatol. 2010;130:2514-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 80. | Jönsson G, Busch C, Knappskog S, Geisler J, Miletic H, Ringnér M, Lillehaug JR, Borg A, Lønning PE. Gene expression profiling-based identification of molecular subtypes in stage IV melanomas with different clinical outcome. Clin Cancer Res. 2010;16:3356-3367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 81. | Grafström E, Egyházi S, Ringborg U, Hansson J, Platz A. Biallelic deletions in INK4 in cutaneous melanoma are common and associated with decreased survival. Clin Cancer Res. 2005;11:2991-2997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 82. | Blokx WA, van Dijk MC, Ruiter DJ. Molecular cytogenetics of cutaneous melanocytic lesions - diagnostic, prognostic and therapeutic aspects. Histopathology. 2010;56:121-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 83. | Tchernev G, Orfanos CE. Downregulation of cell cycle modulators p21, p27, p53, Rb and proapoptotic Bcl-2-related proteins Bax and Bak in cutaneous melanoma is associated with worse patient prognosis: preliminary findings. J Cutan Pathol. 2007;34:247-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | de Wit NJ, Rijntjes J, Diepstra JH, van Kuppevelt TH, Weidle UH, Ruiter DJ, van Muijen GN. Analysis of differential gene expression in human melanocytic tumour lesions by custom made oligonucleotide arrays. Br J Cancer. 2005;92:2249-2261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 85. | Dai DL, Martinka M, Li G. Prognostic significance of activated Akt expression in melanoma: a clinicopathologic study of 292 cases. J Clin Oncol. 2005;23:1473-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 86. | Udart M, Utikal J, Krähn GM, Peter RU. Chromosome 7 aneusomy. A marker for metastatic melanoma? Expression of the epidermal growth factor receptor gene and chromosome 7 aneusomy in nevi, primary malignant melanomas and metastases. Neoplasia. 2001;3:245-254. [PubMed] |
| 87. | Rákosy Z, Vízkeleti L, Ecsedi S, Vokó Z, Bégány A, Barok M, Krekk Z, Gallai M, Szentirmay Z, Adány R. EGFR gene copy number alterations in primary cutaneous malignant melanomas are associated with poor prognosis. Int J Cancer. 2007;121:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 88. | Hoek K, Rimm DL, Williams KR, Zhao H, Ariyan S, Lin A, Kluger HM, Berger AJ, Cheng E, Trombetta ES. Expression profiling reveals novel pathways in the transformation of melanocytes to melanomas. Cancer Res. 2004;64:5270-5282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 380] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 89. | Williams PF, Olsen CM, Hayward NK, Whiteman DC. Melanocortin 1 receptor and risk of cutaneous melanoma: a meta-analysis and estimates of population burden. Int J Cancer. 2011;129:1730-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 90. | Ugurel S, Schadendorf D, Pföhler C, Neuber K, Thoelke A, Ulrich J, Hauschild A, Spieth K, Kaatz M, Rittgen W. In vitro drug sensitivity predicts response and survival after individualized sensitivity-directed chemotherapy in metastatic melanoma: a multicenter phase II trial of the Dermatologic Cooperative Oncology Group. Clin Cancer Res. 2006;12:5454-5463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 457] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 91. | Board RE, Ellison G, Orr MC, Kemsley KR, McWalter G, Blockley LY, Dearden SP, Morris C, Ranson M, Cantarini MV. Detection of BRAF mutations in the tumour and serum of patients enrolled in the AZD6244 (ARRY-142886) advanced melanoma phase II study. Br J Cancer. 2009;101:1724-1730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 92. | Brennecke S, Deichmann M, Naeher H, Kurzen H. Decline in angiogenic factors, such as interleukin-8, indicates response to chemotherapy of metastatic melanoma. Melanoma Res. 2005;15:515-522. [PubMed] |
| 93. | Fusi A, Ochsenreither S, Busse A, Rietz A, Keilholz U. Expression of the stem cell marker nestin in peripheral blood of patients with melanoma. Br J Dermatol. 2010;163:107-114. [PubMed] |
| 94. | Vásquez-Moctezuma I, Meraz-Ríos MA, Villanueva-López CG, Magaña M, Martínez-Macias R, Sánchez-González DJ, García-Sierra F, Herrera-González NE. ATP-binding cassette transporter ABCB5 gene is expressed with variability in malignant melanoma. Actas Dermosifiliogr. 2010;101:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 95. | Laga AC, Zhan Q, Weishaupt C, Ma J, Frank MH, Murphy GF. SOX2 and nestin expression in human melanoma: an immunohistochemical and experimental study. Exp Dermatol. 2011;20:339-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 96. | Flørenes VA, Holm R, Myklebost O, Lendahl U, Fodstad O. Expression of the neuroectodermal intermediate filament nestin in human melanomas. Cancer Res. 1994;54:354-356. [PubMed] |
| 97. | Brychtová S, Fiurásková M, Brychta T, Hirnák J. [The role of intermedial filament nestin in malignant melanoma progression]. Cesk Patol. 2005;41:143-145. [PubMed] |
| 98. | Piras F, Perra MT, Murtas D, Minerba L, Floris C, Maxia C, Demurtas P, Ugalde J, Ribatti D, Sirigu P. The stem cell marker nestin predicts poor prognosis in human melanoma. Oncol Rep. 2010;23:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 99. | Kanoh M, Amoh Y, Tanabe K, Maejima H, Takasu H, Katsuoka K. Nestin is expressed in HMB-45 negative melanoma cells in dermal parts of nodular melanoma. J Dermatol. 2010;37:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 100. | Tanabe K, Amoh Y, Kanoh M, Takasu H, Sakai N, Sato Y, Katsuoka K. Prognostic significance of the hair follicle stem cell marker nestin in patients with malignant melanoma. Eur J Dermatol. 2010;20:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 101. | Bakos RM, Maier T, Besch R, Mestel DS, Ruzicka T, Sturm RA, Berking C. Nestin and SOX9 and SOX10 transcription factors are coexpressed in melanoma. Exp Dermatol. 2010;19:e89-e94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 102. | Klein WM, Wu BP, Zhao S, Wu H, Klein-Szanto AJ, Tahan SR. Increased expression of stem cell markers in malignant melanoma. Mod Pathol. 2007;20:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 230] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 103. | Sharma BK, Manglik V, Elias EG. Immuno-expression of human melanoma stem cell markers in tissues at different stages of the disease. J Surg Res. 2010;163:e11-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 104. | Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, Beermann F, Ruiz I Altaba A. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci U S A. 2007;104:5895-5900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 423] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 105. | Deng WG, Kwon J, Ekmekcioglu S, Poindexter NJ, Grimm EA. IL-24 gene transfer sensitizes melanoma cells to erlotinib through modulation of the Apaf-1 and Akt signaling pathways. Melanoma Res. 2010;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 106. | Schicher N, Paulitschke V, Swoboda A, Kunstfeld R, Loewe R, Pilarski P, Pehamberger H, Hoeller C. Erlotinib and bevacizumab have synergistic activity against melanoma. Clin Cancer Res. 2009;15:3495-3502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 107. | Turza K, Dengel LT, Harris RC, Patterson JW, White K, Grosh WW, Slingluff CL. Effectiveness of imiquimod limited to dermal melanoma metastases, with simultaneous resistance of subcutaneous metastasis. J Cutan Pathol. 2010;37:94-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 108. | Buettiker UV, Yawalkar NY, Braathen LR, Hunger RE. Imiquimod treatment of lentigo maligna: an open-label study of 34 primary lesions in 32 patients. Arch Dermatol. 2008;144:943-945. [PubMed] |
| 109. | Wolf IH, Cerroni L, Kodama K, Kerl H. Treatment of lentigo maligna (melanoma in situ) with the immune response modifier imiquimod. Arch Dermatol. 2005;141:510-514. [PubMed] |
| 110. | Green DS, Dalgleish AG, Belonwu N, Fischer MD, Bodman-Smith MD. Topical imiquimod and intralesional interleukin-2 increase activated lymphocytes and restore the Th1/Th2 balance in patients with metastatic melanoma. Br J Dermatol. 2008;159:606-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 111. | Green DS, Bodman-Smith MD, Dalgleish AG, Fischer MD. Phase I/II study of topical imiquimod and intralesional interleukin-2 in the treatment of accessible metastases in malignant melanoma. Br J Dermatol. 2007;156:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 112. | Krauze MT, Tarhini A, Gogas H, Kirkwood JM. Prognostic significance of autoimmunity during treatment of melanoma with interferon. Semin Immunopathol. 2011;33:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 113. | Weide B, Eigentler TK, Pflugfelder A, Leiter U, Meier F, Bauer J, Schmidt D, Radny P, Pföhler C, Garbe C. Survival after intratumoral interleukin-2 treatment of 72 melanoma patients and response upon the first chemotherapy during follow-up. Cancer Immunol Immunother. 2011;60:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 114. | Bedikian AY, Johnson MM, Warneke CL, Papadopoulos NE, Kim KB, Hwu WJ, McIntyre S, Rohlfs M, Homsi J, Hwu P. Does complete response to systemic therapy in patients with stage IV melanoma translate into long-term survival? Melanoma Res. 2010;. [PubMed] |
| 115. | Ridolfi L, Petrini M, Fiammenghi L, Granato AM, Ancarani V, Pancisi E, Scarpi E, Guidoboni M, Migliori G, Sanna S. Unexpected high response rate to traditional therapy after dendritic cell-based vaccine in advanced melanoma: update of clinical outcome and subgroup analysis. Clin Dev Immunol. 2010;2010:504979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 116. | Finkelstein SE, Carey T, Fricke I, Yu D, Goetz D, Gratz M, Dunn M, Urbas P, Daud A, DeConti R. Changes in dendritic cell phenotype after a new high-dose weekly schedule of interleukin-2 therapy for kidney cancer and melanoma. J Immunother. 2010;33:817-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 117. | Schadendorf D, Algarra SM, Bastholt L, Cinat G, Dreno B, Eggermont AM, Espinosa E, Guo J, Hauschild A, Petrella T. Immunotherapy of distant metastatic disease. Ann Oncol. 2009;20 Suppl 6:vi41-vi50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 118. | Becker JC, Bröcker EB, Schadendorf D, Ugurel S. Imatinib in melanoma: a selective treatment option based on KIT mutation status? J Clin Oncol. 2007;25:e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 119. | Agarwala SS. Novel immunotherapies as potential therapeutic partners for traditional or targeted agents: cytotoxic T-lymphocyte antigen-4 blockade in advanced melanoma. Melanoma Res. 2010;20:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 120. | Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O'Dwyer PJ, Lee RJ, Grippo JF, Nolop K. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809-819. [PubMed] |
| 121. | Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, Spevak W, Zhang C, Zhang Y, Habets G. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1536] [Cited by in RCA: 1439] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 122. | Ledford H. Rare victory in fight against melanoma. Nature. 2010;467:140-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 123. | Dummer R, Robert C, Chapman PB, Sosman JA, Middleton M, Bastholt L, Kemsley K, Cantarini MV, Morris C, Kirkwood JM. AZD6244 (ARRY-142886) vs temozolomide (TMZ) in patients (pts) with advanced melanoma: an open-label, randomized, multicenter, phase II study. J Clin Oncol. 2008;26:abstr 9033. |
| 124. | Banerji U, Camidge DR, Verheul HM, Agarwal R, Sarker D, Kaye SB, Desar IM, Timmer-Bonte JN, Eckhardt SG, Lewis KD. The first-in-human study of the hydrogen sulfate (Hyd-sulfate) capsule of the MEK1/2 inhibitor AZD6244 (ARRY-142886): a phase I open-label multicenter trial in patients with advanced cancer. Clin Cancer Res. 2010;16:1613-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 125. | Ott PA, Hamilton A, Min C, Safarzadeh-Amiri S, Goldberg L, Yoon J, Yee H, Buckley M, Christos PJ, Wright JJ. A phase II trial of sorafenib in metastatic melanoma with tissue correlates. PLoS One. 2010;5:e15588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 126. | Kaufman HL, Bines SD. OPTIM trial: a Phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future Oncol. 2010;6:941-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 127. | Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17:718-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 410] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 128. | Dréau D, Foster M, Hogg M, Swiggett J, Holder WD, White RL. Angiogenic and immune parameters during recombinant interferon-alpha2b adjuvant treatment in patients with melanoma. Oncol Res. 2000;12:241-251. [PubMed] |
| 129. | Fateh S, Schell TD, Gingrich R, Neves RI, Drabick JJ. Unsuccessful high dose IL-2 therapy followed immediately by near continuous low dose temozolomide can result in rapid durable complete and near-complete remissions in metastatic melanoma. Cancer Biol Ther. 2010;10:1091-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 130. | Hong JJ, Rosenberg SA, Dudley ME, Yang JC, White DE, Butman JA, Sherry RM. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clin Cancer Res. 2010;16:4892-4898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 131. | Håkansson A, Gustafsson B, Krysander L, Håkansson L. Effect of IFN-alpha on tumor-infiltrating mononuclear cells and regressive changes in metastatic malignant melanoma. J Interferon Cytokine Res. 1998;18:33-39. [PubMed] |
| 132. | Hauschild A, Dummer R, Ugurel S, Kaehler KC, Egberts F, Fink W, Both-Skalsky J, Laetsch B, Schadendorf D. Combined treatment with pegylated interferon-alpha-2a and dacarbazine in patients with advanced metastatic melanoma: a phase 2 study. Cancer. 2008;113:1404-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |