INTRODUCTION
Cellular injury resulting in inflammation, tissue repair, and fibrosis has been hypothesized to play a functional role in cancer development since Virchow’s original observations[1]. Key somatic changes caused by viral or chemical carcinogens establishing a “subthreshold neoplastic state” (initiation) is often supplemented by a subsequent stimulus from the local niche or microenvironment, such as chronic inflammation (promotion). These synergistic pathways complement each other to augment tumor invasiveness[2,3]. In certain types of cancers, inflammatory conditions are present before a malignant change occurs and investigators have sought to define a causal relationship between inflammation, innate immunity and cancer development. Certainly, epidemiological studies have shown that chronic inflammation predisposes individuals to various types of cancer including bladder, cervical, gastric, intestinal, esophageal, ovarian, prostate, liver, and thyroid tumors. Perhaps the most convincing evidence is the fact that adoptive transfer of inflammatory cells from the local tumor environment or overexpression of inflammatory cytokines promotes tumor development[4]. Despite these observations, the fundamental mechanisms by which inflammation leads to cancer or supports the progression of cancer remain unclear.
In this editorial, we will discuss the critical components of the inflammatory milieu or tumor microenvironment (TME) that promote a neoplastic and/or metastatic phenotype. The canonical physiology of inflammation will be examined within the context of cancer development highlighting similarities and differences between the two analogous processes. Finally, hepatitis and chronic liver cirrhosis will be discussed as a model of fibrogenesis and hepatic carcinogenesis with potential therapeutic targets.
INFLAMMATION, TISSUE REPAIR, TUMOR PROGRESSION AND METASTASIS
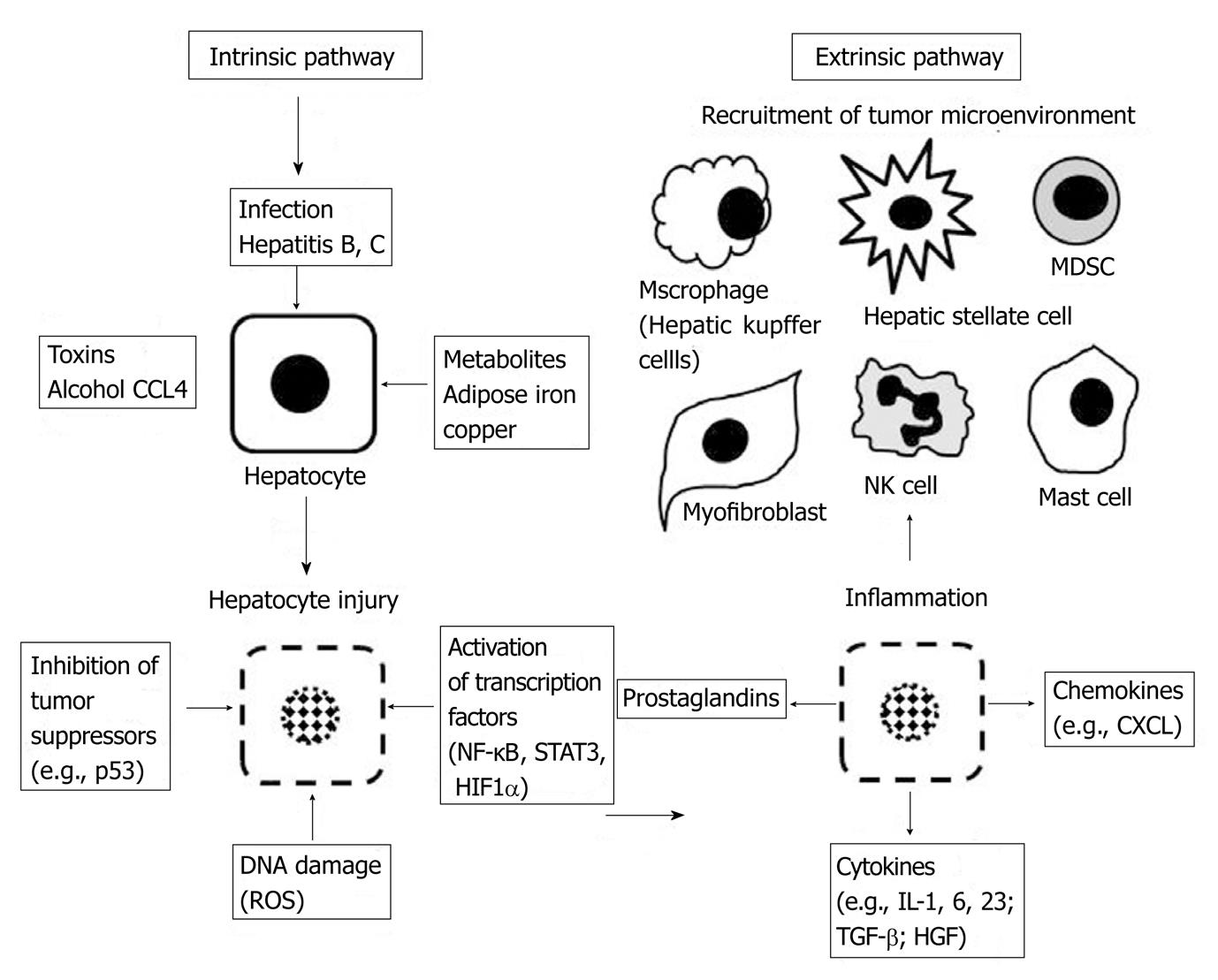
Mantovani and colleagues defined the complementary oncogenic and inflammatory processes in cancer development as the “intrinsic” and “extrinsic” pathways[5]. The intrinsic pathway activates oncogenes and inhibits tumor suppressors by mutation, chromosomal rearrangement or amplification and drives transformation within targeted cells. Tumor cells generated in this fashion subsequently produce cytokines that recruit and populate the inflammatory TME. The extrinsic pathway derives from inflammatory or infectious conditions that amplify the cancer risk (e.g., inflammatory bowel disease, hepatitis, Helicobacter pylori). These two mutually dependent pathways eventually converge, appropriatingnecessary components and signals from the other while also supplying reciprocally useful building blocks to fuel transformation and metastasis in a cooperated fashion. Oncogenes that can produce signals for inflammatory cell recruitment include the RET proto-oncogene in papillary thyroid cancer, K-RAS in pancreatic cancer, and BRAF-MAPK in melanoma[5]. It is no coincidence that inflammation and wound healing physiology parallels the tissue remodeling processes that occur in cancer progression. Dvorak[6] recognized that the composition of the tumor stroma strongly resembles the granulation tissue of healing skin wounds. These important, essential inflammatory cascades promote cell proliferation, migration, invasion through the extracellular matrix, angiogenesis, and ultimately provide the necessary components for host tissue repair and survival. In many types of cancer, these attributes can be subverted by nascent tumor cells as tools for cancer progression and metastasis.
The steps of the inflammatory cascade associated with tissue repair are well characterized.Tissue injury created by toxins, infection, or a chronic inflammatory stimulus results in a host response focused on recruiting cells that initiate healing (Figure 1). Critical members of this microenvironment include neutrophils, monocytes, macrophages, mast cells, dendritic cells, fibroblasts and endothelial cells. The wound healing process often involves partially overlapping phases: blood clotting, inflammation, new tissue formation, and tissue remodeling[7] with key cell types recruited to the niche during specific phases. Important pro-inflammatory signals produced during this cascade include IL-1β, IL-6, IL-23, TNF-α, and TGF-β1. Activation of the selectin family of adhesion molecules (L-, P-, and E-selectin) facilitates leukocyte "rolling" along the injured vascular endothelium, activating integrin binding and immobilization (α4β1 and α4β7 binding to VCAM-1 and MadCAM-1), and ultimately transmigration through the endothelium into the site of injury[7]. Release of cytokines, chemokines, and prostaglandins to recruit additional inflammatory cells, the production of reactive oxygen species (ROS) to destroy infectious vectors, the generation of pro-angiogenic factors, and modulation of apoptosis represent other essential,activated functions.
Figure 1 The intrinsic and extrinsic pathways combine to create a local microenvironment around the injured and transformed hepatocyte to augment tumor promoting mechanisms.
ROS: Reactive oxygen species; HIF1α: Hypoxia inducible factor 1 alpha; NK cell: Natural killer cell; MDSC: Myeloid derived suppressor cell; IL: Interleukin; TGF-β: Transforming growth factor Beta; HGF: Hepatocyte growth factor; NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells; CXCL: Chemokine ligand; STAT3: Signal transducer and activator of transcription 3.
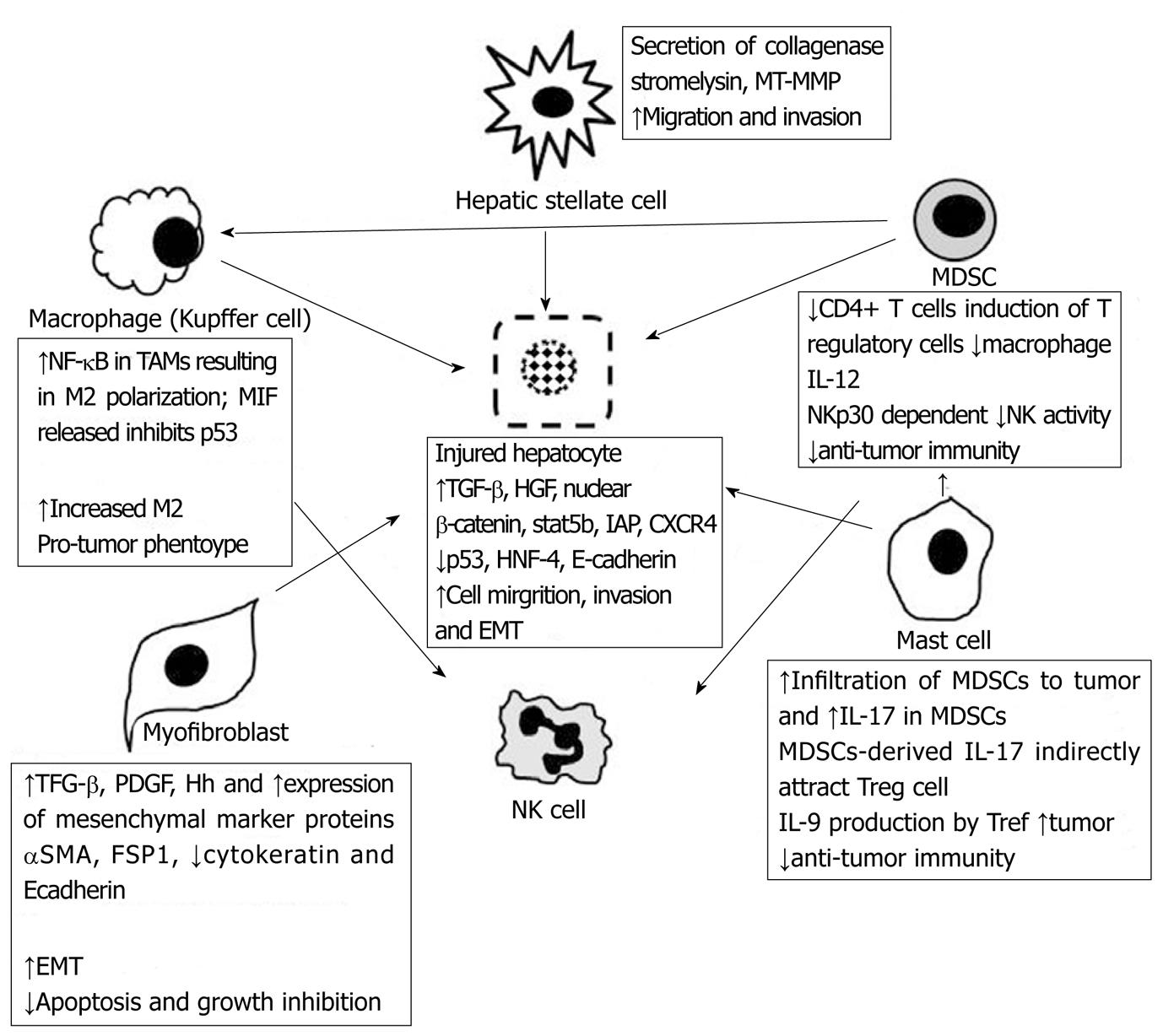
Physiological inflammation is often self-limiting through downstream release of anti-inflammatory regulators (IL-10, IL-11, IL-13) which temper the pro-inflammatory cascade. However, cancer-associated inflammation can often persist, or be driven without regulation,to elicit pathologically persistent signals for cellular proliferation, migration, basement membrane invasion and angiogenesis. In this context,tumors have been comparatively described as “wounds that do not heal”[6]. The key cells residing in the TME are the same constituents that facilitate wound healing and inflammation as described above. However, the tumor-associated cells recruited often display altered functions that lend themselves to cancer development. This alteration in function derives from the upregulated expression of pro-tumor cytokines. For example, dendritic cells in neoplastic infiltrates are regulated by tumor-derived granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 and are frequently immature, less effective at capturing antigens, and defective in T-cell stimulatory capacity[2]. IL-10 released into the TME is a potent inhibitor of dendritic cell activation and differentiation allowing evasion of host adaptive immunity[5]. Similar processes occur in tumor-associated macrophages (TAMs) which produce a number of potent angiogenic and lymphangiogenic growth factors, cytokines and proteases that mediate neoplastic progression. For example, in human cervical carcinogenesis, TAMs express VEGF-C, VEGF-D, and VEGF receptor-3, to facilitate angiogenesis[8]. In a murine mammary cancer metastasis model, CSF-1 regulates tumor growth by supporting and cultivating the TME. In CSF-1-/- mice, advanced mammary tumors and pulmonary metastases fail to develop due to decreased TAMs recruitment into the neoplastic tissue[9]. CSF-1 has been shown to promote progression of mammary tumors to malignancy as replacement of transgenic CSF-1 into mammary epithelium restores macrophage recruitment, primary tumor development, and metastatic potential[9]. A powerful stimulus for tumor progression within the TME includes the ROS derived from infiltrating leukocytes. In the presence of chronic inflammation and repetitive injury, leukocytes and other phagocytic cells induce DNA damage in proliferating cells through the generation of reactive oxygen and nitrogen species such as peroxynitrite. Irreversible DNA mutations generated by these reactive species can provide the critical trigger for neoplastic transformation. In addition to these mechanisms, the inhibition of tumor-suppressor pathways represent yet another strategy for promoting tumor growth. Macrophage migration inhibitory factor (MIF) released from TAMs and T lymphocytes is a potent cytokine that suppresses p53 transcriptional activity. MIF released into the TME creates a niche with a deficient response to DNA damage[10]. TAMs will be diverted into the M2 phenotype in human tumors so that macrophage functions will be focused on promoting tumor growth, remodeling tissues, promoting angiogenesis, and suppressing adaptive immunity[11,12]. Another class of cells that are recruited to the TME include the myeloid-derived suppressor cells (MDSCs). These cells are abundant in tumors and strongly inhibit anti-tumor immunity[7]. MDSCs represent an immature population of myeloid cells that inhibit both innate and adaptive immunity and are present in cancer patients and in experimental animals with sizable tumor burden[13]. Although no definitive molecular characterization exists, many investigators have found human MDSCs to express CD33, CD11b and CD15 cell surface markers[13]. MDSC inhibition of anti-tumor immunity is mediated by suppression of CD4+ T-cells[14], inducing T regulatory cells[15], by down-regulating macrophage production of the type 1 cytokine, IL-12[16], and potentially suppressing natural killer cell cytotoxicity[17]. In hepatocellular carcinoma models, trafficking and accumulation of MDSCs appears to be gp130 dependent and downregulation of NK cell cytokine production to be NKp30 dependent[18]. Recent studies have also focused on the myofibroblast as another cell type that is commonly found in wounds and in the TME and has been implicated in tumor progression. The presence of large numbers of fibroblasts and myofibroblasts is a hallmark of cancer with many tumors producing a desmoplastic response[7]. Although tumor fibroblasts can be derived from the stroma surrounding tumors, there is evidence to suggest that cells recruited from the bone-marrow also ‘home in’ on the TME[19]. Auto- and paracrine PDGF and TGF-β dependent signaling centered on the myofibroblast is considered fundamental to tumor progression, the development of epithelial-mesenchymal transition (EMT),and generation of cancer stem cells (CSCs). CSCs exhibit a CD44high/CD24lowantigenic phenotype, demonstrate upregulation of the mesenchymal markers and the transcription factors, N-cadherin, fibronectin, vimentin, FOXC2, SIP1, Hedgehog (Hh), Snail, and Twist, and possess self-renewal capability enabling CSCs to exit tissue reservoirs, enter and survive in the circulation, and exit into secondary tissue sites (“stemness”)[20]. In the liver, cancer-associated fibroblasts are important contributors to the TME[21] and their precise origin continue to be unclear with a variety of hepatic cells able to generate stem-cell characteristics including hepatocytes, oval cells/hepatic progenitor cells, and bone marrow-derived cells[22].
HEPATIC FIBROGENESIS AND TUMOR PROGRESSION
Constituents of the cancer-associated inflammatory stroma vary between different tumor types suggesting that TMEs can be disease-specific. Distinguishing mechanistic pathways that are conserved versus pathways that are tissue- and tumor- specific is important. Friedman and colleagues described the difference between “core” and “non-core” pathways leading to end-stage inflammation or fibrosis with “core” pathways maintaining a dominant role through different organ systems, processes, and species[23]. Non-core pathways may regulate core pathways, but are not necessarily fundamental to the fibrotic process, and may be specific to one model system without correlation to other disease states. Discovery of universal anti-fibrotic cures may require specific attention to core pathways. However, such integral proteins and signals (e.g., TGF-β) may be required in normal tissue function and may not be appropriate targets for inhibition. In this context, regulatory signaling pathways that are tissue-specific or even disease/pathology-specific may provide realistic targets that can be candidates for therapeutic intervention.
Hepatic fibrosis is a reversible wound-healing response to liver injury and is characterized by inflammation, accumulation of extracellular matrix (ECM), and ultimately scarring, as described above. If the injury is self-limiting, the inflammatory changes are transient and the liver tissue is restored to its normal configuration. However, when the injury or the resultant inflammatory response is persistent, the liver architecture is irreversibly transformed leading to progressive fibrosis and cirrhosis. Agents that injure the liver in such a way include toxins (CCL4, alcohol, or bile from biliary stasis), chronic infections (hepatitis B, hepatitis C), or remodeling processes (adipose tissue in non-alcoholic fatty liver disease). Chemical toxicity, viral infection, and metabolic derangements damage hepatocytes and this injury triggers a cascade designed to contain the injury by removing or repairing damaged cells, defense against further infection or injury, tissue regeneration and repair. Chronic inflammation due to repetitive injury (toxin) or inability to remove the offending agent (viral infection) results in a deranged, decompensated response (Figure 1). The liver is composed of constituent cells capable of mounting a robust inflammatory response. The space of Disse is a subendothelial space that separates the hepatic sinusoids from the hepatocytes. It contains a low density basal membrane-like matrix that is essential for maintaining the differentiated function of parenchymal cells but can become porous enough to enable metabolic exchange between the bloodstream and hepatocytes[24]. Inflammatory cells that can be activated to secrete cytokines and regulate ECM in this space include the hepatic macrophages (Kupffer cells), hepatic stellate cells (HSCs) (that can be activated to become fibroblasts), dendritic cells, natural killer cells, hepatocytes and cholangiocytes. Progression to cirrhosis leads to the formation of nodules of regenerative parenchyma surrounded by sheets of fibrotic septae. During chronic liver injury, extracellular matrix (ECM) deposition is upregulated. Interactions between the ECM and its surrounding cells then become mutually dependent with decorin and biglycan binding of TGF-β; fibronectin and laminin binding of TNF-α; and collagen binding to PDGF, HGF, and IL-2[24]. HSCs become activated from a quiescent vitamin A-rich cell to a contractile, highly fibrogenic, myofibroblast cell-type. Activated HSCs leads to upregulation of integrin receptors α2β1, α6β4, αvβ8, αvβ6, and α5β1, regulation of TGF-β1, PDGF, and Hedgehog (Hh) signaling pathways, expression of ADAMSTS-13, ADAMSTS-1, and secretion of collagenase-1 and -3, stromelysin-1 and -2, gelatinases, metalloelastases (MT-MMP1) and tissue inhibitor of metalloproteinase-1 (TIMP-1)[24] (Figure 2).
Figure 2 The complex cellular network in the tumor microenvironment mediated by chemokines, cytokines, and cellular transcription factors.
NK cell: Natural killer cell; MDSC: Myeloid derived suppressor cell; IL: Interleukin; TGF- β:Transforming growth factor Beta; αSMA: Alpha smooth actin; FSP-1: Fibroblast specific protein; PDGF: Platelet derived growth factor; Hh: Hedgehog; NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells; CXCL: Chemokine ligand; STAT3: Signal transducer and activator of transcription 3; Treg: T regulatory cells; MT-MMP: Membrane type matrix metalloproteinase; HNF-4: Hepatocyte nuclear factor-4; EMT: Epithelial-mesenchymal-transition; MIF: migration inhibitory factor.
Accumulating evidence suggests that hepatocellular epithelial mesenchymal transition (EMT) plays a pivotal role in the dissemination of malignant hepatocytes during HCC progression[25]. Complete EMT often occurs in cancer cells as they lose cell-cell contacts, acquire a fibroblast-like morphology, and express mesenchymal marker proteins such as alpha smooth muscle actin (αSMA) and fibroblast-specific protein 1 (FSP1)[7], while shedding the epithelial cell markers cytokeratin and E-cadherin. This process often allows a firmly attached epithelial cell with appropriate apical-basal polarity to migrate into the interstitium and acquire characteristics of mesenchymal cells (highly motile, invasive, resistant to apoptosis, produce ECM, transmigrate through basement membrane, and intravasate into endothelium). Hepatic cancer cells that undergo EMT appear to acquire the properties of stem cells[20]. After liver injury occurs, the major trigger of EMT is the release of chemokines, MMPs, and other growth factors with PDGF, TGF-β and Hh occupying principal roles in this process (Figure 2).
TGF-β
TGF-β is secreted by a variety of cell types and exists as the following isoforms: TGF-β1, TGF-β2, and TGF-β3. The predominant isoform in inflammation and fibrosis is TGF-β1 and signaling is mediated through the type II receptor. PDGF receptors transduce their signals through the PI3K/Akt pathways while TGF-β mediates signaling through the Smad proteins. Inhibition of PDGF signaling decreases migration in vitro and efficient tumor suppression in vivo indicating that TGF-β mediated EMT of neoplastic hepatocytes is PDGF-dependent[25,26]. Activation of the type II receptor results in dimerization with the type I receptor and promotes binding to Smad2 and Smad3. The phosphorylated Smad2/Smad3 complex associates with Smad4 and activates transcription. TGF-β is a tumor suppressor protein that is frequently involved in tumor progression of human cancer and also in tumor-promoting inflammation. Many liver cell types, including HSCs, hepatocytes, and liver sinusoidal endothelial cells are regulated by TGF-β[27]. Interestingly, as in other cancer cell types, TGF-β has been shown to have a dual role in HCC with TGF-β displaying antitumor effects initially. For example, loss-of-function of TGF-β type II receptor results in enhanced susceptibility to HCC, suggesting that TGF-β retains some tumor-suppressor functions[28]. Alternatively, transgenic mice with upregulated Smad7 expression restricted to hepatocytes demonstrate significantly diminished liver damage and fibrosis, demonstrating that TGF-β signaling in hepatocytes is required for fibrogenesis progression[29]. The significance of the dual nature of these effects is unclear but are reflected across other cancer cell types and suggest that the effects of TGF-β may be time-, and context-dependent. For example, inactivation of type II TGF-β receptor in an animal model of breast carcinoma increases CXCL5- and CXCL12-mediated recruitment of myeloid-derived suppressor cells (MDSCs) which are potent suppressors of the adaptive immune response to tumors[5]. Smad7 activation or RNA interference against Smad4 decreases TGF-β signaling and attenuates the expression of pro-fibrotic genes[29,30]. However, hepatocytes isolated from livers exposed to high TGF-βin vivo demonstrate elongated, fibroblastoid hepatocytes expressing vimentin and collagen I in comparison to healthy mouse livers[31].Other evidence for pro-tumor TGF-β-mediated downstream effects include studies by Morris et al[32] that show loss of TGF-β receptor type II in the context of loss of p53 decreased the incidence of HCC in a murine model of liver cancer. Cumulative evidence from the Fabregat group demonstrates that TGF-β signaling regulates seemingly contradictory processes in normal liver cells and in HCC. TGF-β-mediated growth inhibition and apoptosis (tumor-suppressor characteristics) occur in non-transformed human fetal hepatocytes while transdifferentiation into a mesenchymal-stem cell-like phenotype with increased expression of Snail, decreased E-cadherin expression, increased Vimentin and N-cadherin expression (pro-tumor) is also TGF-β-mediated[33]. Indeed, parallel experiments using siRNA-mediated down regulation of Snail showed that hepatocytes became sensitized to TGF-β mediated apoptosis and that Snail and induction of the EMT phenotype impairs TGF-β apoptosis in HCC cells[34].
Nuclear factor-kappa B
Nuclear factor-kappa B (NF-κB) is an important regulator of innate immunity,inflammation and also of tumor progression. NF-κB is activated downstream from Toll-like receptor (TLR)-MyD88 signaling and by signaling through the TNF-α and IL-1β pathways. NF-κB can also be activated as a result of genetic alterations (amplification, mutations, or deletions) in tumor cells. NF-κB activation is controlled by multiple factors and its transcriptional activity is linked to various inflammatory or pro-tumor states. Deficiency of TIR8, an inhibitor of Toll/IL-1 (TIR)-mediated NF-κB signaling, results in increased NF-κB activation with increased susceptibility to intestinal inflammation and carcinogenesis[35,36]. The causative role of NF-κB in inflammation and cancer is further supported by studies in TAMs where the p50 homodimers confer a pro-tumor phenotype through constitutively activated NF-κB[37]. Inhibition of IKK-α releases inhibition of maspin, a tumor suppressor, and reduces metastatic spread in malignant prostate epithelial cells[38]. Evidence also suggests that NF-κB determines the balance between pro- and anti-tumor effects. When NF-κB signaling is inhibited specifically in TAMs, they switch to a "classically" activated M1 phenotype (IL-12high; IL-10low) and TAMs become cytotoxic to tumor cells again[39]. Clearly, the mechanisms involved in NF-κB activation are complex and contradictory functions occur in liver physiology and hepatic carcinogenesis. In Mdr2-knockout mouse (a murine model of chronic inflammation induced-HCC), NF-κB inhibition with inducible IκB super-repressor resulted in decreased tumor progression[40]. In contrast, Maeda and colleagues demonstrated that IKK-β knockout in hepatocytes during an acute liver injury model with diethylnitrosamine (DEN) resulted in mice with an absence of chronic inflammation but increased hepatocarcinogenesis[41]. The timing and context of NF-κB activation or inhibition determines the associated phenotype.
Signal transducer and activator of transcription
The signal transducer and activator of transcription (STAT) family of transcription factors also play a critical role in tumor and immune cells. STAT1 and STAT3 play a key role in liver fibrosis, antiviral defense, liver inflammation, and liver regeneration. STAT1 confers a protective effect and functions in down-regulating pro-fibrotic mechanisms in the liver by inhibiting HSC proliferation, suppression of β-PDGF receptor expression, inhibition of TGF-β/Smad3 signaling, and stimulation of NK cell cytotoxicity[42]. In these loss of function studies, STAT1-/- mice demonstrated accelerated CCL4-induced liver fibrosis and HSC proliferation.
STAT3 is one of the main signaling proteins activated by HGF and EGF receptors, and is involved in oncogenesis, inhibition of apoptosis, inhibition of dendritic cells, and increased evasion of the immune system[43]. Mice that lack STAT3 in the epidermis suffer from reduced wound re-epithelization, and are resistant to carcinogen-induced skin cancer development. Conversely, mice that overexpress a constitutively active form of STAT3 develop skin cancers with a shorter latency period[44].
Chemokines
Tumor cells can regulate their chemokine expression profile to recruit inflammatory cells, but also use these factors to enhance tumor growth and expression[2]. The chemokines CXCL1, CXCL2, CXCL3, and CXCL8 have been extensively studied in melanoma and they play a role in the regulation of tumor growth. Inhibition of the CXCR2 receptor attenuates melanoma cell proliferation[45], whereas overexpression of CXCL1, CXCL2, CXCL3 enhances tumor cell colony-forming activity and tumorigenicity in nude mice[46,47]. Macrophage pro-inflammatory chemokine 3α (CCL20), is a CC chemokine where the two cysteine (C) residues lie adjacent to each other, is overexpressed in pancreatic carcinoma cells and infiltrating macrophages adjacent to tumor cells, and enhances migration of TAMs[48]. Angiogenesis is associated with chronic inflammatory states including arthritis, infections, tumor growth and metastasis. The Glutamic acid-Leucine-Arginine (ELR) motif upstream of the CXC domain in the chemokine family can stimulate endothelial cell chemotaxis and retains pro-angiogenic function. Metastatic potential also appears in part to be governed by chemokine receptors and chemokine signaling. CXCL12 [stromal-cell derived factor-1 (SDF-1)] is a product of resting cells in multiple organs and also myofibroblasts. Binding to its receptor CXCR4 appears to play a critical role in cancer metastasis. Use of CXCR4 antibodies can limit CXCL12-mediated chemotaxis of mammary cells to distant organs during metastasis[49]. Indeed the amount of CXCR4 expression in primary human tumors correlates with the extent to which metastasis to the lymph nodes occurs in colorectal, breast, liver and esophageal cancer[50-52]. The critical threshold of pro-inflammatory cytokines reached by the myriad of contributing cells within the local tumor environment may augment the invasive capacity of transforming cells in a para- and auto-crine fashion. For example, autocrine TNF-α upregulates the expression of CXCR4 in ovarian cancer[53].
IAP
Recently, the inhibitor of apoptosis (IAP) family of proteins have been shown to play a significant role in cancer-related inflammation and metastasis[54]. Alterations in IAPs are found in many types of human cancer including HCC and are associated with chemoresistance, disease progression, and poor prognosis[55]. IAPs function by regulating caspases (cysteine proteases that are involved in apoptosis) and through ubiquitin (Ub)-dependent activation of NF-κB transcription factors. IAP1 (cIAP1; encoded by BIRC2), cIAP2 (encoded by BIRC3) and XIAP (encoded by BIRC4) have particular roles that have been demonstrated in tumor maintenance and progression and deserve particular attention. Using a model of breast cancer metastasis with MDA-MB-231 cells and also MCF-7 cells stably transfected with survivin, Mehrotra and colleagues demonstrated that the survivin-XIAP complex activates NF-κB and increases metastasis in a splenic injection model of hepatic metastasis[56]. Amplification of chromosome 11q22 containing the BIRC2 and BIRC3 exons, occurs at high frequency in hepatocellular carcinoma with cIAP1 and cIAP2 functioning as key mediators of TNF-α-induced activation of NF-κB[55]. TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in hepatocellular cancer in a cIAP1-dependent fashion[54].
Other signals
Hepatic EMT defined as loss of cell polarity and decrease in cell-cell adhesion was shown to be associated with reduced hepatocyte nuclear factor (HNF)-4 expression while restoration of HNF-4α1 expression in aggressive HCC cells re-programmed the cells into resting hepatocytes[57]. Analysis of Wnt/β-catenin signaling demonstrated that nuclear accumulation of β-catenin results in loss of epithelial markers and increases expression of hepatic mesenchymal markers such as M2-pyruvate kinase (M2-PK) and cytokeratin (CK19)[58]. In vivo loss of E-cadherin, upregulation of Twist (a negative inhibitor of E-cadherin transcription) and reduction of cytosolic β-catenin correlate with more invasive HCC phenotypes, increases metastasis and reduces patient survival[59-61]. Recent studies have also shown that HCV core proteins lower Smad3 expression and decrease E-cadherin expression promoting EMT in human HCC cells[62], while hepatitis B virus encoded HBX upregulates STAT5b in HCC cell lines to augment EMT and cell invasion by repressing E-cadherin[63].