INTRODUCTION
Natural products, useful in preventing and treating various diseases, have been sought after for centuries. The investigation of β-glucans began during the 1960s and 1970s. Two gradually converging lines can be traced in the history of β-glucans. The first took place mainly in Europe and the United States and the second in Japan. Research on β-glucans in the Euro-American milieu was based on knowledge of the immunomodulatory effects of zymosan-a mixture of polysaccharides isolated from the cell walls of Saccharomyces cerevisiae. Zymosan was prepared and investigated for the first time by Pillemer et al[1] in the 1940s, and since that time it has been used in numerous studies. Zymosan is a potent stimulator especially of macrophages and it also induces the release of a series of cytokines from neutrophils. For many years, it was not clear which part of this crude composition was responsible for its activities. When zymosan was examined in detail, β-glucan was identified as the component which had the primary effect. Glucan was subsequently isolated and its immunological effects were further investigated.
β-glucan was approached differently in Japan. In Far Eastern medicine, the consumption of medicinal mushrooms has been a long tradition. An interest in these actions led to detailed studies of the biological effects of these mushrooms. As a result of these studies, β-glucans were again found to be a main cause of non-specific immunomodulation[2].
A major problem in characterizing virtually all natural products is that they represent a complex mixture of ingredients-each one of which may contribute to their bioactivity and, at the same time, some might act against each other. β-glucans from fungi, yeast and seaweed are well-known biologic response modifiers that function as immunostimulants against infectious diseases and cancer[3,4]. Unlike most other natural products, highly purified β-glucans retain their bioactivity which permits their characterization at the cellular and molecular level.
Based on the multiple biological effects of β-glucan, it is not surprising that this immunomodulator is also involved in the fight against cancer. Despite the fact that most tumors are recognized by the immune system, the antibody response is usually not strong enough to kill cancer growth.
Since the first direct scientific study forty years ago, the anti-tumor activity of β-glucan has been clearly demonstrated. Since these pioneering studies, numerous animal as well as human trials have shown remarkable anti-tumor activity against a wide variety of tumors including breast, lung, and gastrointestinal cancer. Since the 1980s, two types of β-glucan have been successfully used as traditional medicine for cancer therapy in Japan[5-7]. Another activity demonstrated by β-glucan in the mid 1980s was its ability to stimulate hematopoiesis in an analogous manner as granulocyte monocyte-colony stimulating factor[8]. Both particulate and soluble β-glucans caused significantly enhanced recovery of blood cell counts after gamma radiation[9]. It has also been shown that β-glucan could reverse myelosuppression produced by chemotherapeutic drugs[10].
The mechanism of action of β-glucan on cancer includes the C3 fragment of complement and antibodies. Our investigation showed that the majority of malignant cells in mammary carcinomas are naturally targeted with C3 for cytotoxicity by natural killer (NK) cells bearing CR3 receptors that have been primed with β-glucan. Both freshly excised human mammary tumors and established breast cancer cell lines were examined, and published reports of both circulating antibodies to tumors and tumor opsonization with immunoglobulins and C3 were confirmed. Furthermore, whereas older investigations tested tissue sections using immunohistochemistry, our investigation examined single cell suspensions of tumors using flow cytometry, thus allowing full quantification of antibodies and the C3 fragment of complement. Our results suggested that while the majority of malignant cells within tumors bore IgM, IgG and C3, the surrounding normal breast epithelium was devoid of these molecules.
Numerous recent studies have shown that β-glucan is extremely active in cooperation with antibodies which naturally occur in cases of cancer. Subsequent experiments showed that daily therapy with soluble or insoluble β-glucan for two weeks resulted in a 70%-95% reduction in tumor weight as compared to the control group. Following the binding of antibodies on the surface of cancer cells, C3 fragments of complement coat the cancer cells. The β-glucan-primed cells, such as blood neutrophils, macrophages, and NK cells, then specifically recognize these complement-antibody complexes and kill the tumor cells. β-glucan cooperation with antibodies is therefore a most promising combination therapy.
GLUCAN AS AN IMMUNOSTIMULANT
The effects of β-glucan on immune reactions are well established. Traditionally, β-glucan is considered to be a stimulator of cellular immunity. Binding of β-glucan to a specific receptor (especially CR3 and Dectin-1) activates macrophages. This activation consists of several interconnected processes including increased chemokinesis, chemotaxis, migration of macrophages to particles which then undergo phagocytosis, degranulation that leads to increased expression of adhesive molecules on the macrophage surface, adhesion to the endothelium, and migration of macrophages to tissues. In addition, β-glucan binding also triggers intracellular processes, characterized by the respiratory burst after phagocytosis of invading cells (formation of reactive oxygen species and free radicals-hydrogen peroxide, super oxide radical, NO, HClO, HIO, etc.), increasing the content and activity of hydrolytic and metabolic enzymes, and signaling processes leading to activation of other phagocytes and the secretion of cytokines and other substances initiating inflammatory reactions [e.g. interleukin (IL)-1, IL-9 and TNF-α]. For an excellent review on the interactions of β-glucans with macrophages see[11].
In addition, β-glucan has been extensively used as protection against infection. Using several experimental models, β-glucan has been shown to protect against both bacterial and protozoan infections and enhances antibiotic efficacy in infections with antibiotic-resistant bacteria. The protective effect of β-glucans was shown in experimental infections with Leishmania major[12] and L. donovani[13], C. albicans[14], Toxoplasma gondii[15], Streptococcus suis[16], Plasmodium berghei[17], Staphylococcus aureus[18], Escherichia coli[19], Mesocestoides corti[20], Trypanosoma cruzi[21], Eimeria vermiformis[22] and Bacillus anthracis[23].
Based on the multiple biological effects of β-glucan, it is not surprising that it has also been involved in the fight against cancer. Since the first direct scientific study forty years ago, the anti-tumor activity of β-glucan has been clearly demonstrated[24]. Since then, numerous animal as well as human studies have shown remarkable anti-tumor activity against a wide variety of tumors. Additionally, in Japan, the β-glucans (β1,3-D-glucans) such as Lentinan derived from the Shiitake mushroom and Polysaccharide K derived from Coriolus versicolor are already licensed as drugs effective in cancer treatment and their properties are routinely improved. Recent studies have repeatedly shown that β-glucan is extremely active in cooperation with antibodies which naturally occur in cases of cancer. Subsequent experiments showed that daily therapy with β-glucan resulted in a 70%-95% reduction in tumor weight as compared to the control group[25,26]. However, we must keep in mind that antibodies alone cannot make tumor cells disappear. The mechanisms of these effects are based on the close cooperation of β-glucan with antitumor antibodies. Following the binding of antibodies on the surface of cancer cells, the C3 fragments of complement coat the cancer cells. The β-glucan-primed cells, such as blood neutrophils, macrophages, and NK cells, then specifically recognize these complement-antibody complexes and kill the coated tumor cells. Immunotherapy with β-glucan substantially enhances the therapeutic efficacy of anti-tumor antibodies. β-glucan cooperation with antibodies is therefore a most promising combination immunotherapy[27-29].
GLUCAN AS A DRUG DELIVERY VEHICLE
There is already substantial research on glucan use in drug delivery systems either as an actual drug carrier, an adjuvant, or in combination with other materials to form suitable drug delivery systems. One example is curdlan, which has been investigated for use in the preparation of tablets containing theophylline-a drug used in the treatment of respiratory diseases such as asthma. These formulations were prepared from spray-dried particles of curdlan and theophylline[30]. Scleroglucan has been applied as a coating in liposome formulations and in the preparation of hydrogels.
Recently, research has been focused on yeast-derived glucans. β-glucan derived from Baker’s yeast (Saccharomyces cerevisiae) can be processed into hollow, highly-porous microparticles. Their use was described more that 15 years ago when Alpha-Beta developed β-glucan carbohydrate microcapsules (Adjuvax) for specific antigen and drug delivery[31]. The use of Adjuvax resulted in more than a 1000-fold increase in antibody response[32]. Recently, glucan particles have been successfully used to prepare encapsulated polyplexes for DNA, siRNA, protein and nanoparticles. A recent study by Aouadi’s group showed the highly effective use of β-glucan encapsulated siRNA particles as an orally-delivered vehicle to silence genes in mouse macrophages in vivo[33]. Each of these, despite offering a wide range of individual approaches, is based on one single characteristic of glucan-being readily phagocytosed by numerous cell types. β-glucan thus represents a natural, inexpensive and safe vehicle for entering these omnipotent and omnipresent cells. An exciting future will surely capitalize on the intersection of the unique immunomodulatory properties of these polysaccharides to develop drug formulations targeting tissues and organs rich in phagocytosing cells.
β-GLUCAN AND RESVERATROL
Several studies in the last decade have suggested a possible synergy of glucan with additional bioactive substances. First, numerous scientific studies[34,35] have shown some beneficial effects when β-glucan was given in combination with vitamin C. The main reason why vitamin C shows synergistic effects is due to the fact that this vitamin has been shown to stimulate the exact same immune responses as β-glucan, i.e. macrophage activities-NK cell activity and specific antibody formation. A mouse study showed significant healing abilities of a β-glucan-vitamin C combination in the treatment of Mesocestoides corti infection. The treatment resulted in positive modulation of liver fibrosis and pathophysiological changes[36]. Earlier, the same group found that yeast-derived glucan was a promising agent against several helminthic parasites. With respect to liver disease, schizophyllan β-glucan was shown to help against an ischemia-reperfusion injury of the liver. The mechanisms of these effects are most likely due to the β-glucan-caused decrease in the expression of immediate early genes following injury to the liver[37].
Our laboratory studied the possible effects of a combination of β-glucan and resveratrol. Resveratrol (trans-3,4’,5-trihydroxystilbene) is a non-flavonoid polyphenol found in various fruits and vegetables and is abundant in the skin of grapes. In addition to various biochemical, biological, and pharmacological activities, resveratrol has been found to exhibit numerous immunomodulatory effects such as suppression of lymphocyte proliferation, changes in cell-mediated cytotoxicity, cytokine production, and induction of apoptosis. In addition, resveratrol has been reported to have a cancer-chemopreventive activity. As resveratrol is prominent primarily in red wine grapes, its beneficial effects are often suggested to be responsible for the so called “Mediterranean paradox”.
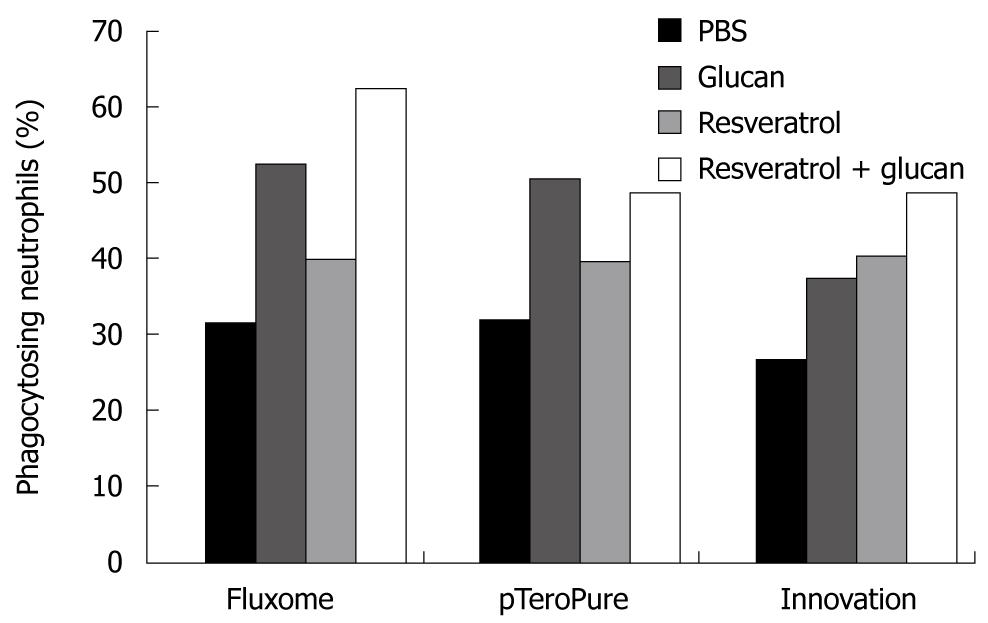
Our study was based on a recent observation that seaweed-derived β-glucan elicited defense responses in wine and induced protection against Botrytis cinerea and Plasmopara viticola via the induction of production of two phytoalexins including resveratrol[38]. This led us to evaluate the possible synergetic effects of β-glucan and resveratrol on immune reactions. This study showed that both glucan and resveratrol stimulated the phagocytosis of blood leukocytes, caused increased expression of some membrane markers (such as CD4) on spleen cells, and showed higher restoration of spleen recovery after experimentally induced leucopenia. In all cases, strong synergetic effects were observed. When we measured the effects of these substances on the expression level of some important genes (such as NF-κB2, Cdc42 and Bcl-2) in breast cancer cells, the up-regulation of Cdc42 expression was evident only with the use of both immunomodulators (glucans and resveratrol) in combination[39]. The up-regulation of NF-κB2 gene expression is considered significant since the members of this family are important regulators of cell cycle progression, cell survival, cell adhesion/angiogenesis, invasion and inflammatory responses. Studies have shown the positive role of the NF-κB family proteins in regulating the expression of adhesion molecules and angiogenic factors that are known to increase the invasion and metastasis of cancer cells. However, not every preparation of resveratrol has identical effects on the immune system, particularly in synergy with β-glucan. We compared the synergetic effects of three different preparations of resveratrol with yeast-derived β-glucan and found significant differences (Figure 1). The differences in activities of the resveratrol samples were most probably caused by biochemical and biological differences in individual resveratrols. Most of the resveratrols represent various mixtures of trans-resveratrol and resveratrol glycosides.
Figure 1 Stimulation of phagocytosis of synthetic microspheres by the combination of yeast-derived glucan with different types of resveratrol injected ip.
Twenty-four hours before the test. Peripheral blood neutrophils with three and more Hydroxy ethyl methacrylate particles were considered positive. PBS: Phosphate buffered saline.
CONCLUSION
β-glucans occupy a prominent position among many known and tested immunomodulators. The effects of β-glucans on a variety of diseases, such as infections, immunosuppression and most importantly on neoplastic growth have been investigated. The flood of various food additives and “alternative remedies”, usually offered by partially enlightened non-specialists, is a holdover from those pioneer years. Not surprisingly, the wave of enthusiasm slowly dissipated. β-glucans were, and often incompetently, criticized by many authorities, the main reasons being insufficiently defined preparations and non-specific and/or complex effects. Fortunately, in the last decade, research in reputable laboratories has reached the stage where the basic mechanisms of β-glucan effects are known and the relationship between structure and activity has been clearly established. Therefore, it is possible that β-glucan will finally hold the position that was ascribed more than several decades ago.
Peer reviewer: Qi-Nong Ye, PhD, Professor, Beijing Institute of Biotechnology, Beijing 100850, China
S- Editor Cheng JX L- Editor Webster JR E- Editor Ma WH