Published online Dec 10, 2011. doi: 10.5306/wjco.v2.i12.397
Revised: November 24, 2011
Accepted: December 1, 2011
Published online: December 10, 2011
AIM: To evaluate neurovascular uncoupling (NVU) associated with low grade gliomas (LGG) using blood oxygen level dependent (BOLD) cerebrovascular reactivity mapping.
METHODS: Seven patients with low grade gliomas referred by neurosurgeons for presurgical mapping were included in this pilot study. Cerebrovascular reactivity (CVR) mapping was performed by acquiring BOLD images while patients performed a block-design breath-hold (BH) hypercapnia task. CVR mapping was expressed as BOLD percentage signal change (PSC) from baseline associated with performance of the BH hypercapnia task. Standard T2* Dynamic Susceptibility Contrast perfusion imaging was performed and relative cerebral blood volume (rCBV) and relative cerebral blood flow (rCBF) maps were generated. Structural T1 weighted MR images were also acquired. A correlation analysis between intratumoral normalized (via ratio with contralateral homologous regions) BOLD BH PSC [referred to as (nCVR)] and intratumoral normalized resting state rCBV (rCBF) values (i.e., nCBV and nCBF, respectively) was performed.
RESULTS: No significant correlation was seen between the normalized BOLD BH PSC (i.e., nCBV) and nCBV or nCBF. However, the average nCVR (median = 0.50, z = -2.28, P = 0.01) was significantly less than 1.0, indicating abnormally reduced vascular responses in the tumor regions relative to normal contralesional homologous regions, whereas the average nCBV (median = 0.94, z = -0.92, P = 0.375) and nCBF (median = 0.93, z = -1.16, P = 0.25) were not significantly higher or lower than 1.0, indicating iso-perfusion in the tumor regions relative to normal contralesional homologous regions. These findings suggest that in LGG, hyperperfusion that is seen in high grade gliomas is not present, but, nevertheless, abnormally decreased regional CVR is present within and adjacent to LGG. Since the patients all demonstrated at least some residual function attributable to the cortical regions of impaired CVR, but were incapable of producing a BOLD response in these regions regardless of the tasks performed, such regionally decreased CVR is indicative of NVU. The low nCVR ratios indicate high prevalence of NVU in this LGG cohort, which is an important consideration in the interpretation of clinical presurgical mapping with functional magnetic resonance (MR) imaging.
CONCLUSION: Our preliminary study shows that BH CVR mapping is clinically feasible and demonstrates an unexpectedly high prevalence of NVU in patients with LGG.
- Citation: Pillai JJ, Zacá D. Clinical utility of cerebrovascular reactivity mapping in patients with low grade gliomas. World J Clin Oncol 2011; 2(12): 397-403
- URL: https://www.wjgnet.com/2218-4333/full/v2/i12/397.htm
- DOI: https://dx.doi.org/10.5306/wjco.v2.i12.397
Presurgical localization of brain sensorimotor, visual and language regions in patients with brain tumors or epilepsy who are candidates for surgical resection currently represents the most mature clinical application of blood oxygen level dependent (BOLD) functional MR imaging (fMRI)[1-3]. Presurgical mapping with fMRI can assist neurosurgeons by providing useful information for: (1) preoperative risk assessment; (2) planning the safest surgical trajectory; (3) selection of patients for asleep vs awake craniotomy; and most importantly; and (4) optimization of efficiency, exposure and technique of intraoperative mapping. However, despite its many advantages in presurgical planning that have resulted in widespread clinical utilization over the last decade, some limitations of clinical fMRI do exist[4-9]. One such limitation is the frequent inability to distinguish essential from nonessential participatory activated (i.e., “eloquent”) cortex involved in performance of a particular cognitive, sensorimotor or visual task, thus leading to lower than ideal specificity of activation maps[10].
Another limitation is the problem of decreased sensitivity for detection of actual electrically active eloquent cortex in areas of impaired cerebrovascular reactivity (CVR); this phenomenon is referred to as neurovascular uncoupling (NVU)[11,12]. NVU can result in false negative BOLD activation, which constitutes a major hazard with respect to interpretation of clinical fMRI examinations; such false negatives within or adjacent to tumor boundaries can result in undesirable resection (in the absence of intraoperative electrophysiologic confirmation) of essential electrically active eloquent cortex that is incapable of demonstrating a BOLD response due to impaired CVR. Such eloquent cortical resection may lead to serious permanent postoperative neurological deficits. Thus, the phenomenon of NVU is not merely a theoretical issue of scientific interest, but is rather an issue of considerable clinical relevance and importance. NVU has been documented in the immediate vicinity of high grade gliomas (HGG), mostly due to tumor angiogenesis, which is associated with abnormal vascular permeability, abnormal hyperperfusion (elevated relative cerebral blood volume [rCBV]) related to increased vascular density, and impaired regional CVR[13,14]. However, it is not clear how high the prevalence of impaired CVR (and resultant NVU) is in low grade gliomas (LGG), in which hyperperfusion is unusual. In this study, we investigated regional CVR, using a BOLD breath-hold (BH) hypercapnia task, within LGG, which are known to infiltrate, rather than destroy or displace, eloquent cortex, in order to determine whether the same NVU potential exists in these tumors as in HGG. In this study we report our initial experience using BH CVR mapping in 7 patients with LGG (6 patients with grade II gliomas and 1 patient with grade I glioma) as a quality control tool for detecting risk of NVU, and we compare these results to those of T2* DSC perfusion imaging that was also performed during the same scan sessions as part of a comprehensive clinical presurgical mapping protocol. The findings of this study are discussed in the context of current literature pertaining to brain tumor-related NVU.
Seven patients (mean age 34 ± 11 year, 5M/2F) with histopathologically proved gradeIand II intra-axial primary brain tumors (Table 1) underwent our institutional clinical BOLD fMRI protocol for presurgical planning which included multiple T2* BOLD fMRI sequences during performance of motor, language or visual tasks and a BH task, a T2* Dynamic Susceptibility Contrast perfusion sequence and a structural T1-weighted 3D MPRAGE sequence after Gadolinium injection. Details of these three sequences are reported in Table 2. Images were acquired on a 3T MRI scanner (Magnetom Trio, Siemens Medical Solutions, Erlangen, Germany). The block design BH task consisted of four cycles of 40 s each of normal breathing (baseline) alternating with blocks of 4 s of inhalation and 16 s of breath-holding[15]. Instructions for task performance were delivered visually using Prism Acquire Software (Prism Clinical Imaging, Elm Grove, WI, United States).
| Age | Sex | Tumor location | Histology/tumor grade |
| 25 | F | Left frontal lobe | Oligoastrocytoma grade II |
| 27 | M | Right cingulate gyrus | Oligodendroglioma grade II |
| 42 | M | Left temporal lobe | Astrocytoma grade II |
| 25 | M | Right temporal lobe | Diffuse astrocytoma grade II |
| 54 | M | Left frontal | Oligodendroglioma grade II |
| 27 | F | Left hemispheric (primarily insular and inferior frontal) | Pilocytic astrocytoma gradeI |
| 41 | M | Left insular | Oligoastrocytoma grade II |
| Sequence | TR (ms) | TE (ms) | FA | FOV (cm2) | Acquisition matrix | Slice thickness (mm) |
| T1 MPRAGE | 7 | 3.5 | 9° | 24 × 24 | 256 × 256 | 1 |
| T2* DSC | 2450 | 45 | 90° | 24 × 24 | 128 × 128 | 4 |
| T2* BOLD | 2000 | 30 | 90° | 24 × 24 | 64 × 64 | 4 |
The study was approved by our Institutional Review Board. Images for each patient were first transferred to an external workstation. Perfusion and raw BOLD BH images were coregistered to the T1 MPRAGE images using DynaSuiteNeuro software (DynaSuiteNeuro, InVivo Corporation, Pewaukee, WI, United States). Perfusion image analysis included the generation of rCBV and rCBF maps. rCBV was calculated by adding a correction factor to take into account the contrast leakage through the disrupted blood-brain barrier[16]. BOLD BH data analysis was carried out using AFNI software (afni.nimh.nih.gov) and included slice timing correction, realignment, spatial smoothing followed by generation of PSC maps[17]. Subsequently, a Region of Interest (ROI) analysis was carried out using MIPAV (mipav.cit.nih.gov) (Medical Image Processing, Analysis and Visualization) software. For each patient, two independent raters [JJP (a board-certified neuroradiologist with 14 years of neuroimaging experience) and DZ (an imaging scientist with a PhD in functional imaging and 3 years of postdoctoral neuroimaging experience)] selected on the high resolution T1 MPRAGE images a ROI that included the tumor entirety, defined as the entire hypointense component. This ROI encompassing the entire lesion shall be referred to as the “ipsilesional ROI.” This T1 hypointense region corresponded exactly to the areas of tumor T2/FLAIR hyperintensity seen on other sequences acquired as part of the overall clinical fMRI examination, but T1 MPRAGE images were selected for ROI delineation because of their higher resolution compared to the standard FSE T2 and T2 FLAIR sequences. A mirror homologous contralateral hemispheric (referred to as “contralesional”) ROI was generated in a semi-automated fashion, with particular attention paid to trying (to the greatest extent possible) to ensure a similar degree of contribution from gray and white matter structures in the contralesional ROI as in the ipsilesional ROI, considering the degree of anatomic distortion resulting from the tumor. The following metrics were then calculated: a normalized rCBV (nCBV), expressed as the ratio between the mean rCBV value (of all included voxels) in the ipsilesional ROI and the mean rCBV value in the contralesional ROI; a similarly computed normalized rCBF (nCBF), defined as the ratio between the mean rCBF value in the ipsilesional ROI and the mean rCBF value in the contralesional ROI; a normalized PSC (nCVR) expressed as the ratio between the mean BOLD PSC in the ipsilesional ROI and the mean BOLD PSC in the contralesional ROI.
Correlation analysis was performed between nCBV and nCVR as well as between nCBF and nCVR. Mean values among the raters were used. A one sample Wilcoxon test was also performed to assess whether the PSC normalized ratio was significantly lower than 1.0. An identical statistical test was also perfomed on nCBV and nCBF to determine whether there were any significant differences in perfusion metrics between the ipsilesional and contralesional ROIs. Statistical analysis was performed using OriginPro 8.0 software.
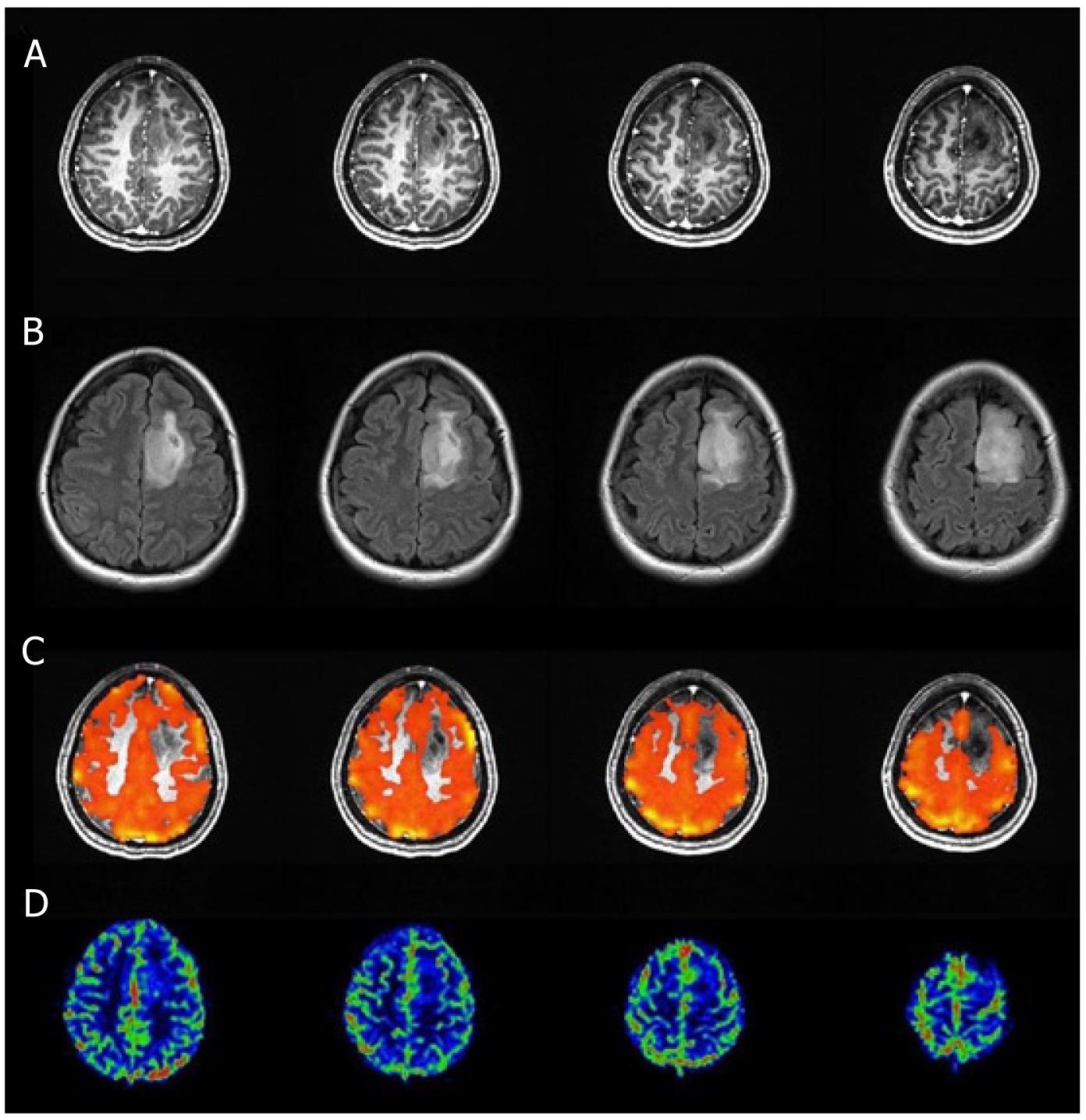
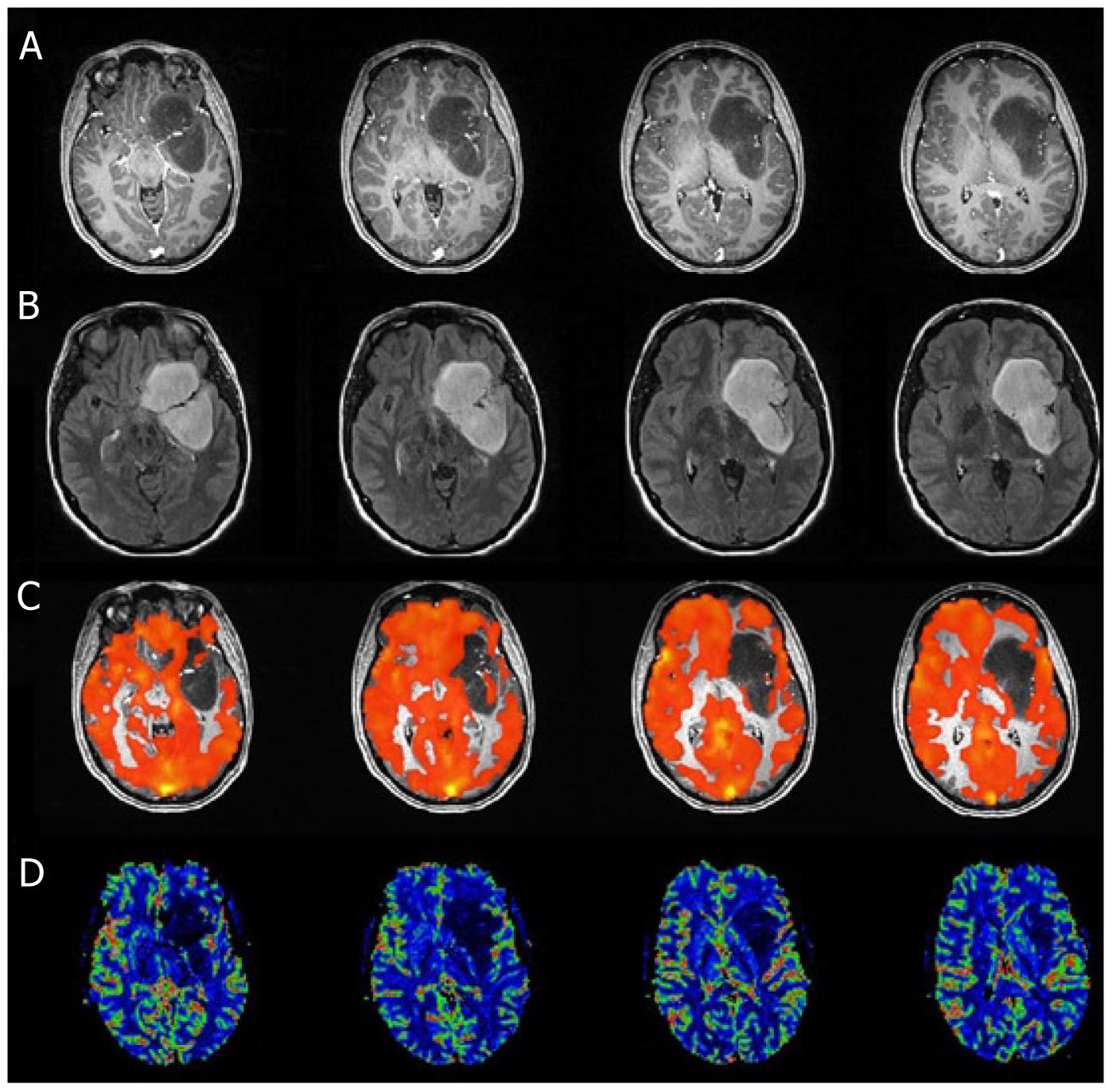
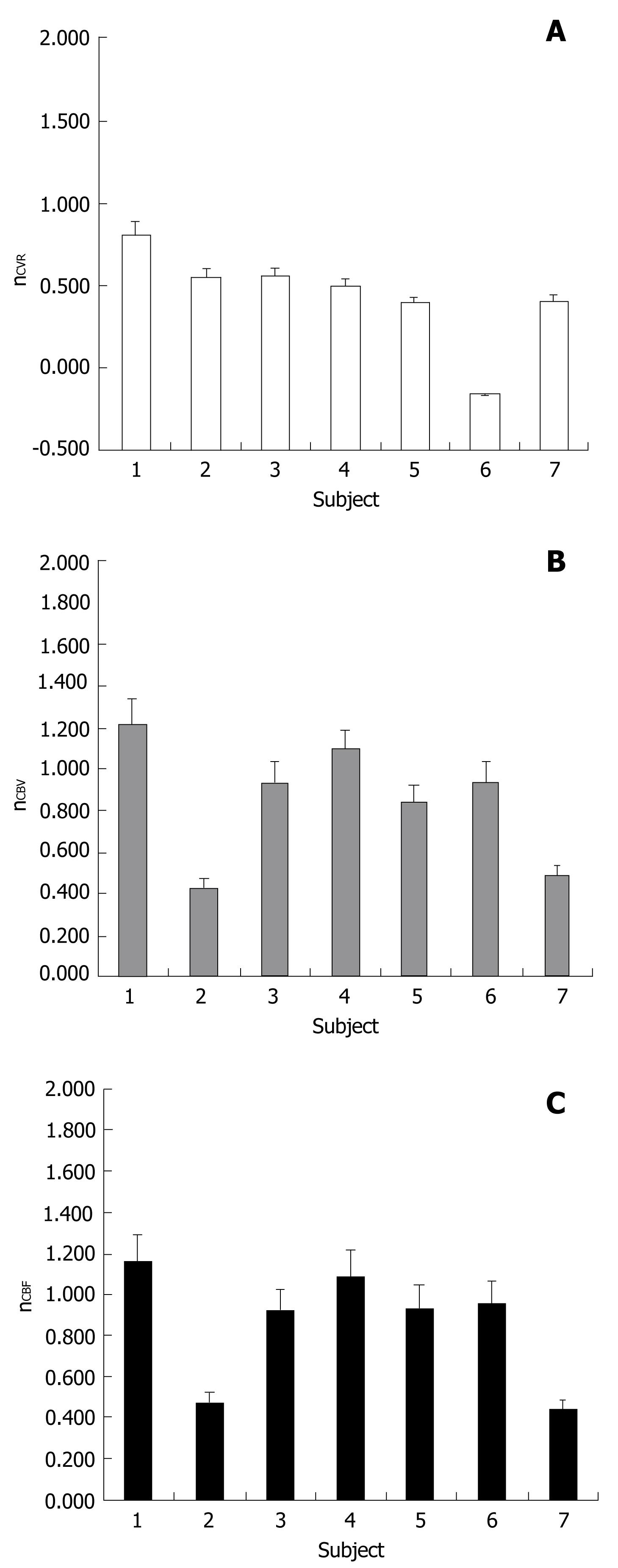
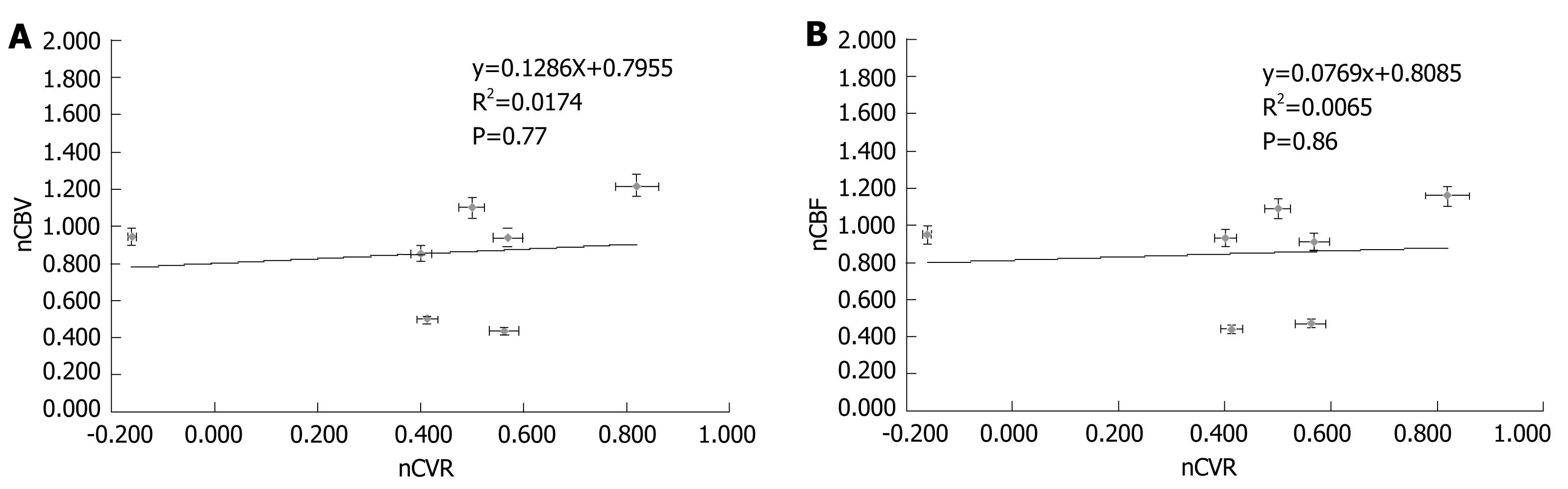
T1 MPRAGE and T2 FLAIR images, perfusion maps and BH PSC maps for two cases are shown in Figures 1 and 2. Reduced PSC is clearly visible in the ipsilesional ROI compared to the contralesional ROI, whereas in both cases the lesion appears iso-perfused relative to the contralesional ROIs. Intraclass Correlation Coefficients (ICC) among the raters were excellent (0.88 for nCVR, 0.98 for nCBV, 0.98 for n nCBF). In this group of 7 patients, none of the cases demonstrated a nCVR that was greater than 1.0 (Figure 3A). The fact that all 7 cases demonstrated nCVR values less than 1.0 indicated that every one of the LGG cases demonstrated abnormally decreased CVR in the ipsilesional ROI compared to the contralesional ROI, suggesting a high risk of NVU in all cases in areas of cortex where regionally decreased CVR were noted. Correlation with the patients’ clinical status confirmed that these areas of regionally decreased cortical CVR corresponded to areas of actual NVU, since preservation of residual motor and language function was noted clinically despite absent expected task-based activation in these regions of decreased CVR on task-based fMRI activation maps obtained as part of the concurrent clinical fMRI examinations. Based on group analysis, the overall distribution of nCVR in this cohort of patients was statistically significantly lower than 1.0 (median = 0.50, z = -2.28, P = 0.01). nCBV (median = 0.94, z = -0.92, P = 0.375) and nCBF (median = 0.93, z = -1.16, P = 0.25) were not significantly higher or lower than 1.0 at a group level, indicating the absence of any substantial hyperperfusion or hypoperfusion in the ipsilesional ROI compared to the contralesional ROI (Figure 3B and C). We did not find any significant correlation between the perfusion and CVR metrics (Figure 4A and B), indicating that perfusion imaging by itself is not a valid predictor of vascular reactivity, and therefore an indicator of NVU in this particular cohort of LGG patients.
The use of a semi-automated approach to contralesional ROI placement helped to ensure that similar contributions to the contralesional ROI from gray and white matter structures were obtained as in the ipsilesional ROIs. This avoided spuriously high contralateral perfusion and CVR values related to greater contributions from normal gray matter in the contralesional ROIs. This was especially important considering that all of the lesions were gliomas, and as such involved mostly white matter rather than cortical gray matter. None of the 7 LGGs demonstrated any appreciable contrast enhancement on Gadolinium-enhanced T1 weighted images, and none demonstrated any internal areas of necrosis by histopathology or imaging features, although some very small regions of internal cystic change were noted in some of the oligodendrogliomas in this group. It is important to note that no internal necrosis or enhancement was present, since such features may result in spuriously decreased or increased mean perfusion, respectively, in ipsilesional ROIs. In the patients with oligodendrogliomas in our cohort, the known propensity toward relatively higher tumor perfusion than comparable low grade astrocytomas is balanced by propensity for internal cystic change, thus not resulting in overall mean voxel hyperperfusion within the ipsilesional ROIs. By using overall mean perfusion metrics computed from all voxels within the ROI rather than simply voxels with maximal perfusion metrics within the ROI, we avoided the risk of spuriously high perfusion values contributing to artifactually high perfusion ratios.
All of the LGG cases in our study demonstrated reduced CVR in the tumor (i.e., ipsilesional) ROI compared to the normal contralateral hemispheric mirror (i.e., contralesional) ROI. Such regionally abnormally reduced CVR, despite the presence of clinically intact, albeit impaired, motor or language function in all of these patients is direct evidence of tumor-related NVU. The fact that no substantial corresponding regional perfusion abnormality was present in any of these cases is reflected in the absence of significant correlation between the normalized (i.e., ipsilesional to contralesional) perfusion ratios and normalized CVR ratios. The absence of tumor hyperperfusion is expected in this cohort of LGG, since such tumors, unlike HGG, are not associated with angiogenesis[18,19]. Although reports of NVU associated with hyperperfusion in HGG exist in the literature[20,21], few reports of NVU related to LGG exist[22,23]. We have shown in our study that the phenomenon of NVU, as detected by regionally decreased CVR, in LGG is much more prevalent than previously thought[24]. We have also shown the clinical feasibility of the performance of such BH CVR mapping in such a patient population. Our results suggest that BH CVR mapping should be considered in all brain tumor patients regardless of tumor grade.
The coupling mechanism between neuronal firing and blood flow changes results from a complex sequence (which we can consider as the NVU cascade) of cellular, metabolic and vascular processes involving neurons, glial/astrocytic components, neurotransmitters, chemical mediators and eventually vascular smooth muscle cells. The currently accepted explanation is that during neuronal activity, synaptic release of neurotransmitters, such as glutamate, that bind to receptors on other neurons, may trigger the secondary release of vasodilatory mediators such as nitric oxide, which in turn increase CBF and CBV. These neurotransmitters such as glutamate can also act on astrocytes through different receptors, thus resulting in the release of compounds such as arachidonic acid and prostaglandin E2, which in turn result in vasoconstriction or vasodilatation, respectively, by acting on arteriolar smooth muscle[25]. It is possible that while in HGG, aberrant neovascularity with abnormal permeability and vasoactivity may be primarily responsible for the NVU, in LGG, abnormal astrocytic function or dysfunction involving other elements of the NVU cascade may be responsible. However, little is known about the pathophysiologic mechanisms underlying such NVU in LGG.
The need to detect NVU, when present, is critical in the interpretation of clinical fMRI examinations because regional cortical NVU will result in an inability to elicit BOLD activation in the affected cortex regardless of the nature of the particular fMRI task performed. Thus, false negative activation in these cortical regions may result during performance of sensorimotor, visual, language or other cognitive tasks that are expected to activate such regions based on a priori knowledge of functional anatomy. Such false negatives may not only result in incorrect language lateralization, but also incorrect localization or underestimation of the true extent of localization of eloquent cortex, as well as possibly incorrect inferences regarding tumor-induced cortical functional reorganization[11]. Such erroneous interpretations of task-related fMRI activation maps can result in unexpected and tragic postoperative neurological deficits related to inadvertent resection of eloquent cortex that is “BOLD-silent” directly due to NVU. The added value of BH CVR mapping in this setting lies in the additional confidence in assignment of function to areas of activation on fMRI maps in cases where no regional CVR abnormality results, as well as in proper exercising of caution in cases where functional activation is expected on a particular task in a particular cortical region where absent activation is seen on task-based fMRI with corresponding abnormally decreased regional CVR. In the latter case, such as in this LGG cohort, one needs to acknowledge the limitation of clinical fMRI and needs to inform the referring neurosurgeon that complementary intraoperative electrophysiologic mapping will be necessary to exclude eloquent cortex in these regions of impaired CVR adjacent to or within the LGG. Such knowledge is very useful, in our clinical experience, in neurosurgical planning as well as in counseling of patients regarding the potential risks of tumor resection.
Thus, in conclusion, although our results are preliminary and based on a fairly small sample size, they suggest that BH CVR mapping in patients with LGG is both clinically feasible and capable of detecting NVU, which is a critical limitation of clinical fMRI. We furthermore note an unexpectedly high prevalence of NVU in LGG, suggesting that NVU is a commonly encountered phenomenon in brain tumors of all grades, and not just in HGG as previously thought.
Cerebrovascular reactivity (CVR) mapping using a breath-hold (BH) technique is a method of evaluating how responsive the microvasculature in the brain is to external stimuli. Although the mechanism for BH CVR mapping is related to transient mild increases in pCO2 (i.e., hypercapnia), resulting in vasodilatation, this can be applied to the evaluation of standard clinical blood oxygen level dependent (BOLD) functional MRI (fMRI) examinations, where sensorimotor, visual or language/cognitive stimuli result in transient blood flow changes in brain microvasculature adjacent to activated neurons. If CVR is impaired for any reason, such as aberrant tumor neovascularity or astrocytic dysfunction due to tumor infiltration, then no BOLD response is possible on standard clinical fMRI activation studies because the BOLD response relies on intact CVR.
It has been determined that CVR is impaired within or adjacent to low grade gliomas (LGG), thus compromising our ability to accurately map eloquent cortex for surgical planning using fMRI. It has already been established that such impaired CVR is present in high grade gliomas (HGG) due to tumor angiogenesis as reflected in hyperperfusion on MR perfusion imaging.
Very little is currently known about CVR in LGG. Most of the work to date relating to CVR mapping in brain tumor patients relates to applications in HGG. It is clear that tumor hyperperfusion, as detected on T2* DSC (standard clinical) MR perfusion imaging, is related to tumor angiogenesis, but in LGG, angiogenesis typically does not occur. Astrocytic dysfunction, however, is known to occur due to tumor infiltration and primary astrocytic and/or oligodendrocyte involvement by all LGGs.
The findings of a high prevalence (100% in our cohort) of abnormal CVR in LGG is of immense clinical value, because this is a potentially serious limitation of standard clinical BOLD fMRI examinations that may result in false negatives which adversely impact surgical planning. The recognition of this pitfall of fMRI is critical for proper surgical planning and counseling of patients prior to surgical resection of LGG. Further studies with larger sample sizes will need to be performed to evaluate the true prevalence of such findings in LGG of different histologic subtypes.
Cerebrovascular reactivity mapping and BOLD fMRI have been described in the Background section above. BOLD fMRI is a method of noninvasively evaluating sensorimotor, visual, language and other cognitive functions and mapping eloquent cortical regions prior to neurosurgical intervention, particularly in brain tumor patients and patients with other conditions such as epilepsy.
The manuscript is well written and authors are reporting a valuable research. Methods and material section is well described and results are defined well.
Peer reviewer: Ali Syed Arbab, MD, PhD, Associate Scientist and Director, Cellular and Molecular Imaging Laboratory, Department of Radiology, Henry Ford Hospital, 1 Ford Place, 2F, PO Box 82, Detroit, MI 48202, United States
S- Editor Yang XC L- Editor Webster JR E- Editor Yang XC
| 1. | Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868-9872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4233] [Cited by in RCA: 3784] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 2. | Norris DG. Principles of magnetic resonance assessment of brain function. J Magn Reson Imaging. 2006;23:794-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Sunaert S. Presurgical planning for tumor resectioning. J Magn Reson Imaging. 2006;23:887-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Pillai JJ, Zaca D, Choudhri A. Clinical impact of integrated physiologic brain tumor imaging. Technol Cancer Res Treat. 2010;9:359-380. [PubMed] |
| 5. | Petrella JR, Shah LM, Harris KM, Friedman AH, George TM, Sampson JH, Pekala JS, Voyvodic JT. Preoperative functional MR imaging localization of language and motor areas: effect on therapeutic decision making in patients with potentially resectable brain tumors. Radiology. 2006;240:793-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Håberg A, Kvistad KA, Unsgård G, Haraldseth O. Preoperative blood oxygen level-dependent functional magnetic resonance imaging in patients with primary brain tumors: clinical application and outcome. Neurosurgery. 2004;54:902-914; discussion 914-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Vlieger EJ, Majoie CB, Leenstra S, Den Heeten GJ. Functional magnetic resonance imaging for neurosurgical planning in neurooncology. Eur Radiol. 2004;14:1143-1153. [PubMed] |
| 8. | Medina LS, Bernal B, Dunoyer C, Cervantes L, Rodriguez M, Pacheco E, Jayakar P, Morrison G, Ragheb J, Altman NR. Seizure disorders: functional MR imaging for diagnostic evaluation and surgical treatment--prospective study. Radiology. 2005;236:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Wengenroth M, Blatow M, Guenther J, Akbar M, Tronnier VM, Stippich C. Diagnostic benefits of presurgical fMRI in patients with brain tumours in the primary sensorimotor cortex. Eur Radiol. 2011;21:1517-1525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Giussani C, Roux FE, Ojemann J, Sganzerla EP, Pirillo D, Papagno C. Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery. 2010;66:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 11. | Ulmer JL, Krouwer HG, Mueller WM, Ugurel MS, Kocak M, Mark LP. Pseudo-reorganization of language cortical function at fMR imaging: a consequence of tumor-induced neurovascular uncoupling. AJNR Am J Neuroradiol. 2003;24:213-217. [PubMed] |
| 12. | Zaca D, Hua J, Pillai JJ. Cerebrovascular reactivity mapping for brain tumor presurgical planning. World J Clin Oncol. 2011;2:289-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Holodny AI, Schulder M, Liu WC, Wolko J, Maldjian JA, Kalnin AJ. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. AJNR Am J Neuroradiol. 2000;21:1415-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Chen CM, Hou BL, Holodny AI. Effect of age and tumor grade on BOLD functional MR imaging in preoperative assessment of patients with glioma. Radiology. 2008;248:971-978. [PubMed] |
| 15. | Magon S, Basso G, Farace P, Ricciardi GK, Beltramello A, Sbarbati A. Reproducibility of BOLD signal change induced by breath holding. Neuroimage. 2009;45:702-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol. 2006;27:859-867. [PubMed] |
| 17. | Birn RM, Smith MA, Jones TB, Bandettini PA. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage. 2008;40:644-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 601] [Cited by in RCA: 509] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 18. | Provenzale JM, Wang GR, Brenner T, Petrella JR, Sorensen AG. Comparison of permeability in high-grade and low-grade brain tumors using dynamic susceptibility contrast MR imaging. AJR Am J Roentgenol. 2002;178:711-716. [PubMed] |
| 19. | Law M, Yang S, Babb JS, Knopp EA, Golfinos JG, Zagzag D, Johnson G. Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol. 2004;25:746-755. [PubMed] |
| 20. | Hou BL, Bradbury M, Peck KK, Petrovich NM, Gutin PH, Holodny AI. Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex. Neuroimage. 2006;32:489-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Lüdemann L, Förschler A, Grieger W, Zimmer C. BOLD signal in the motor cortex shows a correlation with the blood volume of brain tumors. J Magn Reson Imaging. 2006;23:435-443. [PubMed] |
| 22. | Schreiber A, Hubbe U, Ziyeh S, Hennig J. The influence of gliomas and nonglial space-occupying lesions on blood-oxygen-level-dependent contrast enhancement. AJNR Am J Neuroradiol. 2000;21:1055-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Ulmer JL, Hacein-Bey L, Mathews VP, Mueller WM, DeYoe EA, Prost RW, Meyer GA, Krouwer HG, Schmainda KM. Lesion-induced pseudo-dominance at functional magnetic resonance imaging: implications for preoperative assessments. Neurosurgery. 2004;55:569-579; discussion 580-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Jiang Z, Krainik A, David O, Salon C, Troprès I, Hoffmann D, Pannetier N, Barbier EL, Bombìn ER, Warnking J. Impaired fMRI activation in patients with primary brain tumors. Neuroimage. 2010;52:538-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |