Published online Jan 10, 2011. doi: 10.5306/wjco.v2.i1.28
Revised: October 20, 2010
Accepted: October 27, 2010
Published online: January 10, 2011
Imaging of gastroenteropancreatic neuroendocrine tumors can be broadly divided into anatomic and functional techniques. Anatomic imaging determines the local extent of the primary lesion, providing crucial information required for surgical planning. Functional imaging, not only determines the extent of metastatic disease spread, but also provides important information with regard to the biologic behavior of the tumor, allowing clinicians to decide on the most appropriate forms of treatment. We review the current literature on this subject, with emphasis on the strengths of each imaging modality.
- Citation: Tan EH, Tan CH. Imaging of gastroenteropancreatic neuroendocrine tumors. World J Clin Oncol 2011; 2(1): 28-43
- URL: https://www.wjgnet.com/2218-4333/full/v2/i1/28.htm
- DOI: https://dx.doi.org/10.5306/wjco.v2.i1.28
Neuroendocrine tumors (NETs) are a rare and heterogeneous group of neoplasms, which as the name suggests, are derived from cells of the neuroendocrine system. It was Lubarsch et al[1] in 1888, a German pathologist at the University of Munich, who is generally credited with the first report of a carcinoid tumor. He gave the classical microscopic description of multiple ileal carcinoids in two patients, termed “little carcinomata”, which he thought originated in the intestinal crypts of Lieberkuhn.
Later in 1907, Ciacco M C coined the term “enterochromaffin” to describe the cells that were thought to give rise to the tumor, and this was further expanded by Feyrter et al[2], who proposed the concept of the diffuse neuroendocrine system in an attempt to unify tumors in diverse sites that had similar histological features.
The term neuroendocrine is derived from the similarity of such cells to neural cells in the expression of certain proteins, such as synaptophysin, neuron-specific enolase and chromogranin A. Currently, the following criteria proposed by Langley[3] are generally accepted as defining neuroendocrine cells: (1) The production of a neurotransmitter, neuromodulator or neuropeptide hormone; (2) The presence of dense-core secretory granules from which the hormones are released by exocytosis in response to an external stimulus; and (3) The absence of axons and synapses.
The morphologic appearance of well-differentiated NETs is fairly typical, demonstrating an organoid-type pattern under light microscopy, and diagnosis can be fairly confidently made on the basis of such morphology. In cases of poorly differentiated tumors or neuroendocrine origin or variant morphology, electron microscopy or immunohistochemical assessments might be necessary[4,5].
Several commonly used immunohistochemical markers include synaptophysin, chromogranin, vasoactive monoamine transporter 2, serotonin and substance P[6]. Chromogranin appears to be the most consistent general marker, and has been found to have high sensitivity and specificity in diagnosing NETs[7]. In addition, circulating levels of chromogranin A have been found to correlate with tumor volume and are related to disease extent, and could potentially play a role in disease monitoring and prognostication[8,9].
Historically, the diverse and widespread nature of disease presentation meant that a large number of descriptions have been used for NETs in different body regions. Also, certain descriptive terms have been used loosely with different connotations between physicians, surgeons and pathologists, leading to further confusion and miscommunication. In response, attempts have been made to organize and categorize the tumors that comprise the neuroendocrine disease spectrum. In 2000, the World Health Organization (WHO) published a classification for NETs of the gastroenteropancreatic system that categorized tumors into 3 broad categories[10]: (1) Well-differentiated neuroendocrine tumor (benign or uncertain malignant potential); (2) Well-differentiated neuroendocrine carcinoma (low grade malignancy); and (3) Poorly differentiated neuroendocrine carcinoma (high grade malignancy). The criteria used for differentiating the various grades include tumor size, angioinvasion, proliferative activity, histological differentiation, presence of metastasis/local invasion, association with certain syndromes, and hormonal/functional activity.
In an attempt to clarify terminology, the term “carcinoid” was reserved to describe well-differentiated NETs, and the term “malignant carcinoid” was used to describe well-differentiated neuroendocrine carcinomas.
Several publications have supported the clinical effectiveness of the WHO criteria in management decision support (Table 1)[11-14], but there was a need for improved prognostication assessment of NETs.
| Behaviour | Metastasis | Muscularis propria invasion | Differentiation | Size (cm) | Angioinvasion | Ki-67 (%) | Hormonal index |
| WHO criteria (gastrointestinal) | |||||||
| Benign | - | - | Well-differentiated | ≤ 1 | - | < 2 | - |
| Benign/Low-grade malignant | - | - | Well-differentiated | 1-2 | -/+ | < 2 | - |
| Low-grade malignant | + | + | Well-differentiated | > 2 | + | 2-20 | + |
| High-grade malignant | + | + | Poorly-differentiated | Any | + | > 20 | - |
| WHO criteria (pancreas) | |||||||
| Benign | - | - | Well-differentiated | ≤ 1 | - | < 2 | -/+ |
| Benign/Low-grade malignant | - | - | Well-differentiated | > 2 | -/+ | < 2 | -/+ |
| Low-grade malignant | + | + | Well-differentiated | > 4 | + | 2-20 | + |
| High-grade malignant | + | + | Poorly-differentiated | Any | + | > 20 | - |
In response, the European Neuroendocrine Tumor Society has attempted to address the staging of NETs. Their staging system addressed 2 issues; the cell characteristics/proliferation capacity of the tumor, and an adapted tumor node metastasis (TNM) staging system[15-17].
The TNM staging system was sub-divided into specific areas such as the stomach, duodenum/proximal jejunum, lower jejunum/ileum, and pancreas, and follows conventional grading criteria assessing for tumor size/invasion, nodal and distant spread.
With regard to cellular grading, three tumor grade categories were identified. Grade I tumors show a low proliferative index (Ki67 </= 2% or < 2 mitoses per HPF), Grade II tumors show a moderate proliferative index (Ki67 3%-20% or 2-20 mitoses per HPF), and Grade III tumors show a high proliferative index (Ki67 > 20% or > 20 mitoses per HPF). In general, Grade 1 and 2 tumors should refer to well-differentiated NETs while Grade 3 tumors indicate poorly differentiated neuroendocrine carcinomas.
Publications have supported the utility of this TNM classification system for prognostication stratification (Table 2a, 2b and 2c)[18], but further validation is required.
| TNM staging | Gastric | Duodenum/ampulla/proximal jejunum | Pancreas | Lower jejunum/Ileum |
| Tx | Primary tumor cannot be assessed | Primary tumor cannot be assessed | Primary tumor cannot be assessed | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor | No evidence of primary tumor | No evidence of primary tumor | No evidence of primary tumor |
| Tis | In situ tumor/dysplasia (> 0.5 mm) | - | - | - |
| T1 | Tumor invades lamina propria or submucosa and </= 1 cm | Tumor invades lamina propria or submucosa and </= 1 cm | Tumor limited to pancreas and size < 2 cm | Tumor invades mucosa or submucosa and size </= 1 cm |
| T2 | Tumor invades muscularis propria or subserosa or > 1 cm | Tumor invades muscularis propria or > 1 cm | Tumor limited to pancreas and size 2-4 cm | Tumor invades muscularis propria or size > 1 cm |
| T3 | Tumor penetrates serosa | Tumor invades pancreas or retroperitoneum | Tumor limited to pancreas and size > 4 cm or invading duodenum or bile duct | Tumor invades subserosa |
| T4 | Tumors invade adjacent structures (for any T, add M for multiple tumors) | Tumor invades peritoneum or other structures (for any T, add m for multiple tumors) | Tumor invading adjacent organs (stomach, spleen, colon, adrenal gland) or the wall or large vessels (celiac or superior mesenteric artery) | Tumor invades peritoneum/other organs (for any T, add m for multiple tumors) |
| Nx | Regional lymph nodes cannot be assessed | Regional lymph nodes cannot be assessed | Regional lymph nodes cannot be assessed | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis | No regional lymph node metastasis | No regional lymph node metastasis | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis | Regional lymph node metastasis | Regional lymph node metastasis | Regional lymph node metastasis |
| Mx | Distant metastasis cannot be assessed | Distant metastasis cannot be assessed | Distant metastasis cannot be assessed | Distant metastasis cannot be assessed |
| M0 | No distant metastasis | No distant metastasis | No distant metastasis | No distant metastasis |
| M1 | Distant metastasis | Distant metastasis | Distant metastasis | Distant metastasis |
| Appendix | Colon/rectum | |
| Tx | Primary Tumor cannot be assessed | Primary Tumor cannot be assessed |
| T0 | No evidence of primary tumor | No evidence of primary tumor |
| T1 | Tumor invades lamina propria or submucosa and </= 1 cm | Tumor invades mucosa or submucosa, T1a < 1 cm, T1b 1-2 cm |
| T2 | Tumor invades submucosa, muscularis propria and/or minimally (up to 3 mm) invading subserosa/mesoappendix and </= 2 cm | Tumor invades muscularis propria or > 2 cm |
| T3 | Tumor > 2 cm and/or invasion (more than 3 mm) of the serosa/ mesoappendix | Tumor invades subserosa/pericolic/perirectal fat |
| T4 | Tumors invade peritoneum/other organs | Tumor directly invades other organs and/or perforates visceral peritoneum |
| Nx | Regional lymph nodes cannot be assessed | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis | Regional lymph node metastasis |
| Mx | Distant metastasis cannot be assessed | Distant metastasis cannot be assessed |
| M0 | No distant metastasis | No distant metastasis |
| M1 | Distant metastasis | Distant metastasis |
| Disease stage | T | N | M |
| Gastric, duodenum, ampulla, jejunum, ileum, pancreas | |||
| Stage I | T1 | N0 | M0 |
| Stage IIa | T2 | N0 | M0 |
| Stage IIb | T3 | N0 | M0 |
| Stage IIIa | T4 | N0 | M0 |
| Stage IIIb | Any T | N1 | M0 |
| Stage IV | Any T | Any N | M1 |
| Appendix | |||
| Stage I | T1 | N0 | M0 |
| Stage IIa | T2 | N0 | M0 |
| Stage IIb | T3 | N0 | M0 |
| Stage IIIa | T4 | N0 | M0 |
| Stage IIIb | Any T | N1 | M0 |
| Stage IV | Any T | Any N | M1 |
| Colon/rectum | |||
| Stage Ia | T1a | N0 | M0 |
| Stage Ib | T1b | N0 | M0 |
| Stage IIa | T2 | N0 | M0 |
| Stage IIb | T3 | N0 | M0 |
| Stage IIIa | T4 | N0 | M0 |
| Stage IIIb | Any T | N1 | M0 |
| Stage IV | Any T | Any N | M1 |
Anatomic imaging of NETs still plays a crucial role in the diagnosis and management of this condition, largely due to its ability to provide anatomical information for surgical planning. The widespread availability of ultrasound (US) and computed tomography (CT), and in most large centers, magnetic resonance imaging (MRI), has led to a number of publications on the imaging detection of NET. Due to the relative paucity of this condition, most of the published data describing the efficacy of each modality, and consequently studies directly comparing between modalities, suffer from small sample sizes with wide variability in results. Nevertheless, for gastroenteropancreatic (GEP) NETs, it is generally agreed upon that CT and MRI are superior to US, both in terms of lesion detection, and characterization.
The use of transabdominal ultrasound (TAUS) in GEP NETs is largely confined to the solid viscera. This is due to the fact that sound waves are heavily attenuated by air, and US is therefore not usually suitable for assessment of lesions within the gastrointestinal tract or mesentery. The use of US in tumor diagnosis and staging is further limited by inter-operator variability. Nevertheless, newer techniques, such as contrast enhanced US (CEUS) and endoscopic US (EUS), have found a greater role for US in the management of GEP NETs.
The use of TAUS for assessment of pancreatic lesions is limited, especially in the body and tail region, which are commonly obscured by air and ingested material in the overlying stomach. Therefore, the patient should ideally have fasted for several hours prior to scanning. Using the stomach and proximal duodenum as an acoustic window by drinking water is recommended[19]. Characteristic features of pancreatic NETs on US would be a homogenously hypoechoic mass that may sometimes have a hyperechoic halo. Use of CEUS shows promise but requires validation. Detection rate of US for pancreatic NET varies widely and ranges from 0%-66%[20].
EUS is a more invasive method of imaging assessment. Its advantage over TAUS lies in the fact that the US probe is positioned much closer to the organ of interest. This allows the use of higher frequency probes 7.5-12 MHz which provide better spatial resolution in the order of millimeters. Rösch et al[21] reported a sensitivity of 82% and a specificity of 95% for EUS in localizing pancreatic NET lesions. In a similar study by McAuley et al[20] on insulinomas, for lesions smaller than 2 cm in diameter, EUS carried a sensitivity of 80%-90%, leading the authors to recommend EUS as a screening tool for patients with a known diagnosis of multiple endocrine neoplasia (MEN) type 1.
While previously considered to represent the gold standard of assessment of NETs, preoperative imaging by CT and MRI has largely superseded intraoperative US (IOUS). This is partly because IOUS entails a longer operating time, and carries with it the potential risk of iatrogenic injury (e.g. to the splenic vein) during the course of examination.
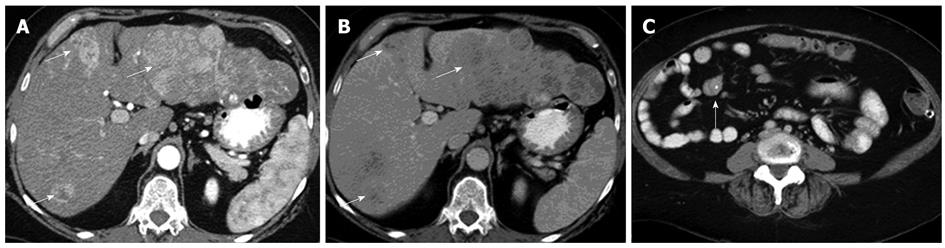
Significant improvements in the spatial and temporal resolution of CT have been made over the past decade, with the advent of multidetector row CT (MDCT). This has allowed for multiphasic contrast enhanced CT (CECT) while achieving spatial resolutions in the order of millimeters. The use of biphasic or triphasic CECT is generally considered a prerequisite for detection and characterization of NETs, both for primary disease involving the pancreas, as well as for liver metastases (Figure 1). The reason for this is that the majority of NET lesions show avid early enhancement. CT is regarded as a first-line imaging modality for detection and staging of NETs.
A recommended protocol for imaging of pancreatic NETs would require the patient to be adequately fasted. Ingestion of water just before the CT scan would act as a negative contrast for visualization of periampullary tumors. An unenhanced scan can initially be performed to look for calcifications, which occur in around 20% of cases, and differentiate this from pancreatic adenocarcinomas, which calcify in only approximately 2% of cases[22]. Thin collimation allows for depiction of submillimeter lesions, and this is usually performed at 1.25 to 2.0 mm section thickness[23]. Multiplanar reconstructions may also help improve lesion assessment.
Typically, intravenous administration of iodinated contrast at a rate of 3-5 mL/s is required to provide an adequate bolus. Most MDCT scanners are equipped with automated bolus tracking capabilities, hence allowing for patient-specific adjustments of scan delay times following bolus injection of contrast. On average, arterial phase imaging is performed at 20-25 s following initiation of contrast injection, while the portal venous phase is timed at approximately 50 s. The pancreatic parenchymal phase, which is the time at which the pancreas enhances maximally, is usually at 35-40 s.
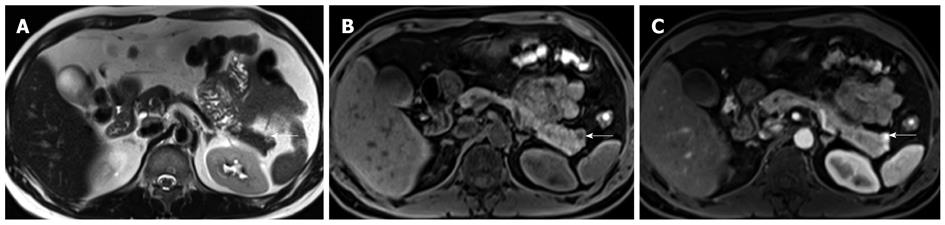
The typical pancreatic NET lesion is isodense on the non-contrast scan but shows homogeneous avid arterial enhancement. Vascular encasement and biliary obstruction are considered rare. Atypical lesions include those that are hypovascular (or hypoenhancing), hyperdense on non-contrast scan, cystic (Figure 2) or calcified[24,25]. Non-functioning lesions tend to be larger, and present with mass effect such as biliary dilatation. They may, therefore, appear as heterogeneous lesions with central necrosis or cystic degeneration. Functioning tumors are usually small, with around 50% of lesions measuring less than 1.3 cm in diameter, and therefore, do not cause deformation of the contour of the gland. The main differential for pancreatic NETs would be metastases from clear cell type renal cell carcinoma, both of which may be present in patients with von Hippel Lindau's disease.
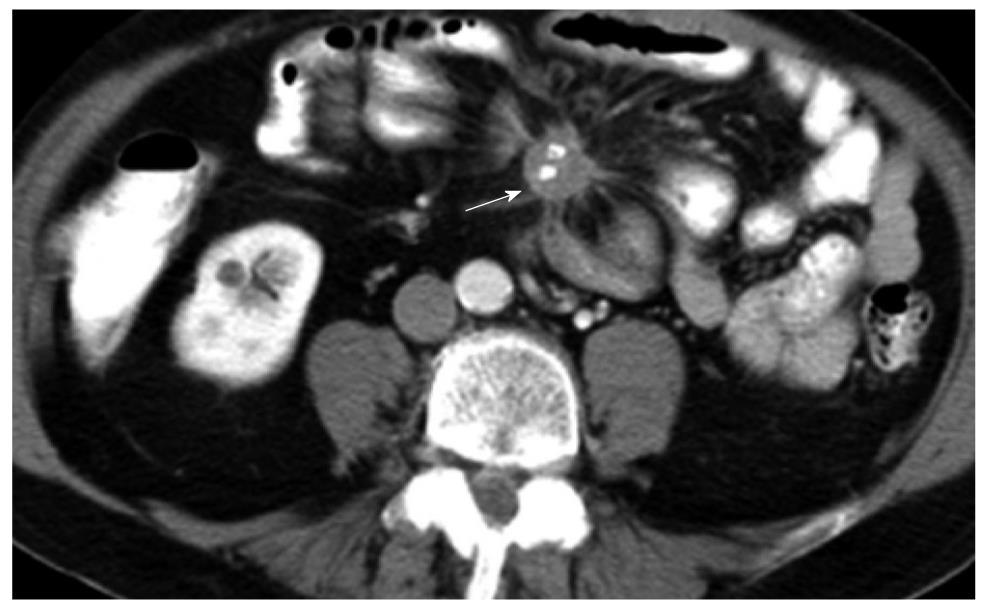
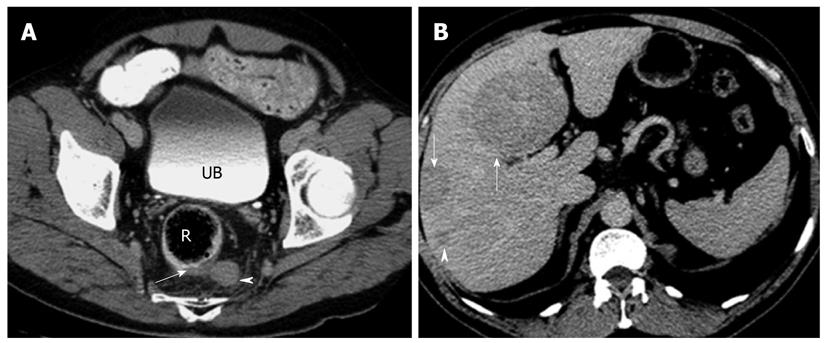
CT has the advantage of a wider field of view than US. It is, therefore, suited for detection of nodal and metastatic disease. In the presence of a NET originating from the bowel, the high contrast to noise ratio between the primary lesion and mesenteric fat allows for excellent depiction of the extent of mesenteric retraction (Figure 3). The secondary lesions, most notably liver metastases, tend to show a similar imaging pattern as the primary lesion itself. Esophageal hyperenhancement and small bowel mural thickening are concomitant findings associated with gastrinomas and best depicted on CT.
In terms of lesion detection, sensitivity of detection with CT increases proportionately with lesion size. In gastric NETs, Binstock et al[26] showed that those lesions that were larger than 1 cm in diameter and presented with focal wall thickening were detected with increased frequency. Similarly, for the small bowel lesions, CT was able to detect around half of the lesions when the size of the mesenteric masses exceeded 1.5 cm[27]. Interestingly, it is not uncommon to find that the sizes of the metastatic lesions far exceed the size of the primary tumor (Figure 4).
Improvements in CT technology over time, probably also due to the use of multiphasic CECT, have led to a concomitant increase in lesion detection of pancreatic NETs. For example, a retrospective study of cases over 13 years by Gouya et al[28] in 2003, showed lesion sensitivity of 94.4% with the use of dual phase thin section CT compared to 28.6% with the use of single slice section CT technology.
Similarly, for metastatic disease to the liver, which can be the most common imaging finding in GI NETs, multiphasic imaging is recommended, with the hepatic arterial phase being best for lesion detection[29]. On standard radiography and CT, bone metastases frequently demonstrate either an osteosclerotic or a mixed osteolytic-osteosclerotic pattern[30].
MRI is considered superior to CT for lesion assessment in the solid visceral organs. In a comparison study between MRI and CT as well as angiography for detection of metastatic lesions, MRI was shown to be superior[31].
As with CT, multiphasic CE MRI is recommended, with fat-suppressed CE T1-weighted (T1W) imaging providing the best accuracy, with an area under the receiver-operating curve (AUROC) of 0.98[32]. This has been corroborated by a more recent study by Herwick et al[33].
The advantages of MRI over CT are the lack of ionizing radiation and the use of gadolinium chelate contrast agents, which have a better safety profile in terms of allergic reactions and nephrotoxicity, although the latter point is slightly mitigated by the concerns of nephrogenic systemic fibrosis. Nonetheless, MRI provides added information about the lesions, such as T1 relaxivity and T2 dephasing.
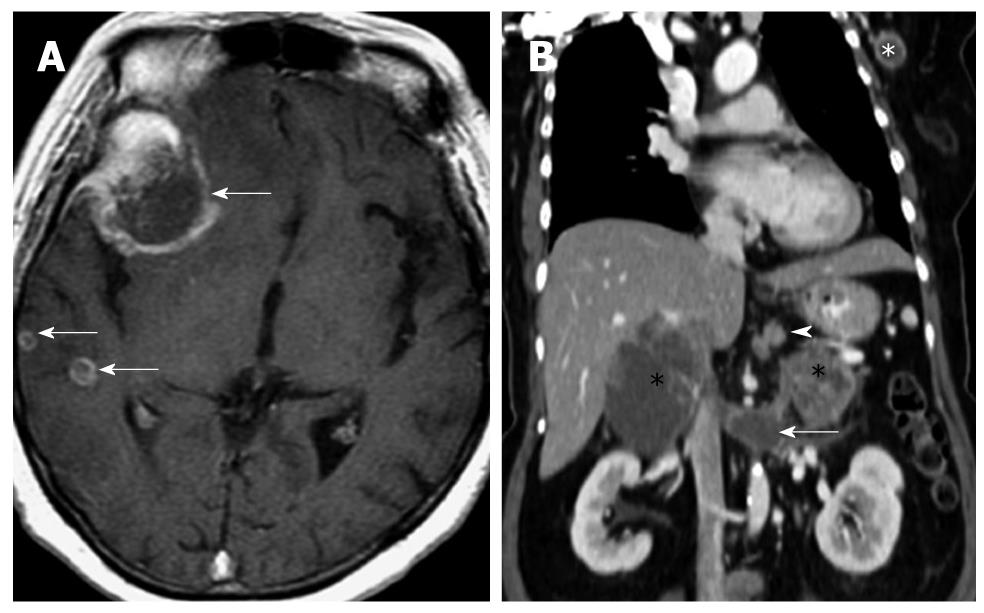
Typically, NET lesions show T2 hyperintensity and T1 hypointensity (Figure 5). In the study by Owen et al[34], 14 out of 29 (48.3%) lesions demonstrated this finding, while only one out of 29 (3.4%) showed a reversal of signal, that is, T1 hyperintensity and T2 hypointensity. The study by Semelka et al[35] showed a positive predictive value of 96% for MRI in pancreatic NETs. Gastrinomas tend to show ring or peripheral enhancement while most other subtypes of NETs demonstrated a diffuse pattern of enhancement.
For GI NETs, MRI is able to detect around two-thirds of lesions[36], with fat-suppressed T1W imaging yielding maximal results. Similarly, hepatic metastases are well depicted on MRI, and MRI is often used to further characterize lesions that are equivocal on CT. Like the primary lesions, hepatic metastases appear as T1 hypointense and T2 hyperintense. Bader et al[36] showed this finding in 75% of cases. Interestingly, 15% of cases showed increased enhancement only in the arterial phase. Furthermore, some of the metastases may display T2 hyperintensity approaching that of hemangiomas[37]. Nevertheless, T2WI and hepatic arterial phase T1WI fat-suppressed imaging have been shown to be most sensitive[38].
Advances in diffusion weighted imaging (DWI) have led to its widespread clinical use in abdominal imaging. Vossen et al[39] showed that there was a statistically significant difference in apparent diffusion coefficient (ADC) values between hemangiomas and NET metastases (as well as other hypervascular liver lesions), with an AUROC of 0.91. An added advantage of using DWI is its ability to reflect lesion changes in treatment response. In the study by Liapi et al[40], ADC values rose concomitantly with response to transarterial chemoembolization (TACE), in tandem with decreased enhancement of the treated lesions. For the primary lesions, DWI may allow for preoperative localization of tumors in the pancreas, especially those which do not demonstrate the typical hypervascular pattern[41,42].
The basis of functional imaging lies with the targeted detection of specific cell targets or receptors, allowing precise localization of lesions. In the context of diagnostic imaging, the concentration of receptor molecules in target tissues may be hard to differentiate from background non-specific binding[43]. As such, molecular imaging techniques have often been confined to nuclear-based modalities such as positron emission tomography (PET) or single photon emission CT (SPECT), which are able to generate images with micromolar to picomolar concentrations of imaging probes[44].
The Delphi consensus with regard to the diagnostic imaging of NETs, has acknowledged that functional imaging in the form of somatostatin receptor scintigraphy (SRS) plays a central role in the diagnosis of NETs[45], and we will explore this and various other functional imaging modalities and techniques in relation to their clinical utility in the diagnosis of NETs. Our discussion will focus largely on the gastroenteropancreatic system, but general principles are likely applicable to NETs in other parts of the body.
Somatostatin receptors are widely distributed in the human nervous system and tissues in the body, including the adrenals, kidneys, pancreas and prostate[46]. Currently, 5 subtypes of somatostatin receptors have been identified in humans (SSRT1, SSRT2, SSRT3, SSRT4, SSRT5), with SSRT2 further classified into subtypes 2A and 2B[47].
Of particular interest, somatostatin receptor expression has been found in a large number of tumors, of which NETs are the archetypical class, and this forms the basis for the molecular imaging of NETs.
The half-life of somatostatin itself is too short (< 2 min) for use in either diagnosis or therapy. As a result, synthetic somatostatin analogues with sufficiently long half-lives have been developed for use in diagnostic imaging or therapeutics.
The first commercially available somatostatin analogue was Octreotide (Sandostatin, Novartis Pharmaceutical Corp), with an approximate half-life of 2 h, and a radiolabeled analogue of octreotide, Octreoscan® (111In-DTPA-Octreotide, D-Phe-Cys-Phe-D-Trp-Lys-Thr-Cys-Thr[ol]), was successfully used to visualize somatostatin receptor positive tumors by gamma camera scintigraphy in the early 1990s[48-50].
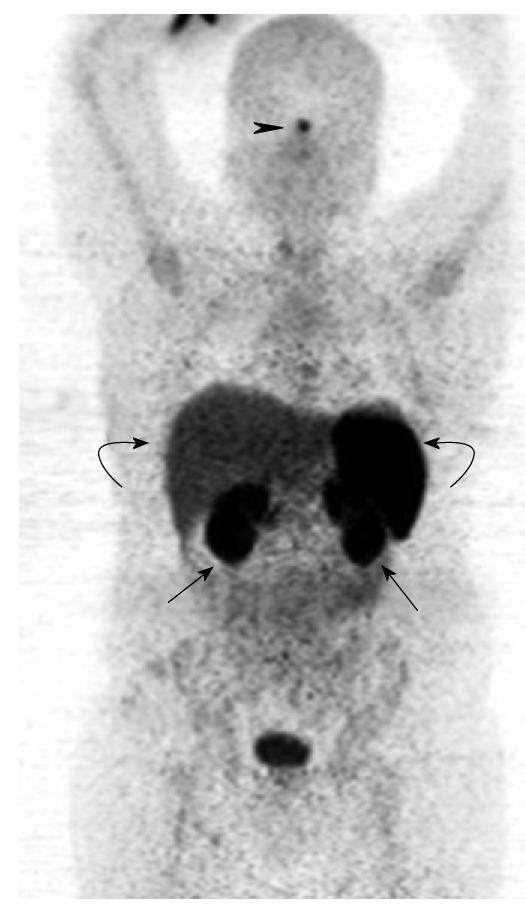
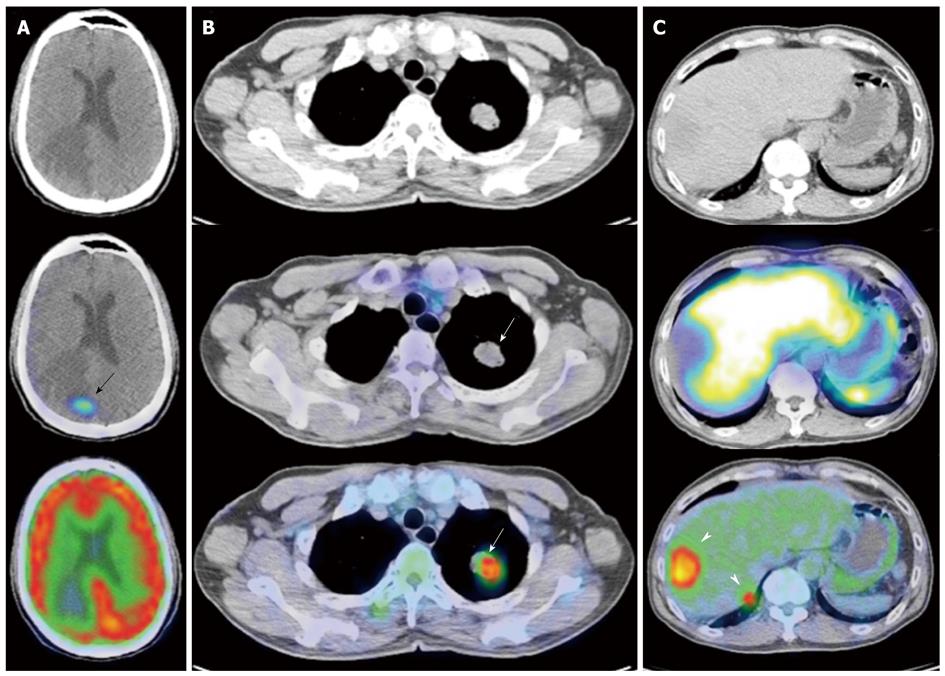
Normal physiological uptake is seen in the thyroid, spleen, liver and pituitary due to receptor binding of the peptides, while tracer uptake in the kidneys is predominantly secondary to reabsorption of filtered peptides, and bowel uptake is presumably secondary to hepatobiliary clearance (Figures 6 and 7).
Gamma-based SRS (Octreoscan®) has proved to be a safe, sensitive imaging agent in the detection of GEP NETs, with an overall sensitivity of approximately 80%-90% in patients with gastrointestinal neuroendocrine neoplasms[51-54]. However, limitations include false negative results in organs with significant physiological uptake (e.g. liver) where background uptake may mask lesions, and small volume diseases that may be below the intrinsic spatial resolution of gamma imaging. Additionally, false positives can occur with a variety of lesions, such as the thyroid gland, accessory spleens, granulomatous or inflammatory tissue, and benign or malignant breast lesions[55]. Other types of neoplasms that demonstrate somatostatin receptor expression include meningiomas and lymphomas.
Nonetheless, SRS is considered the “gold standard” in the diagnosis, staging and follow-up of patients with NETs (Figure 8).
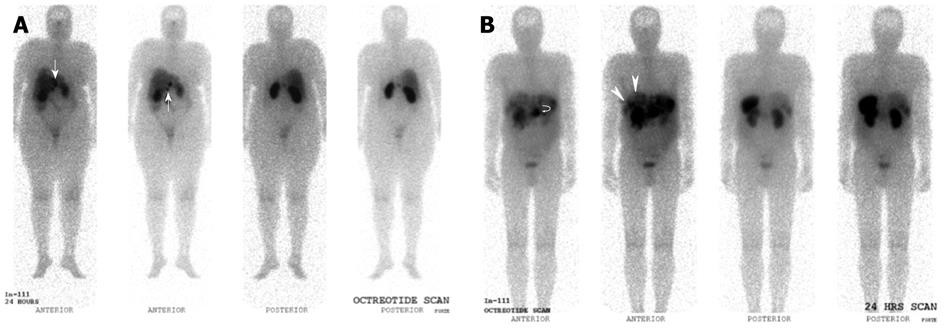
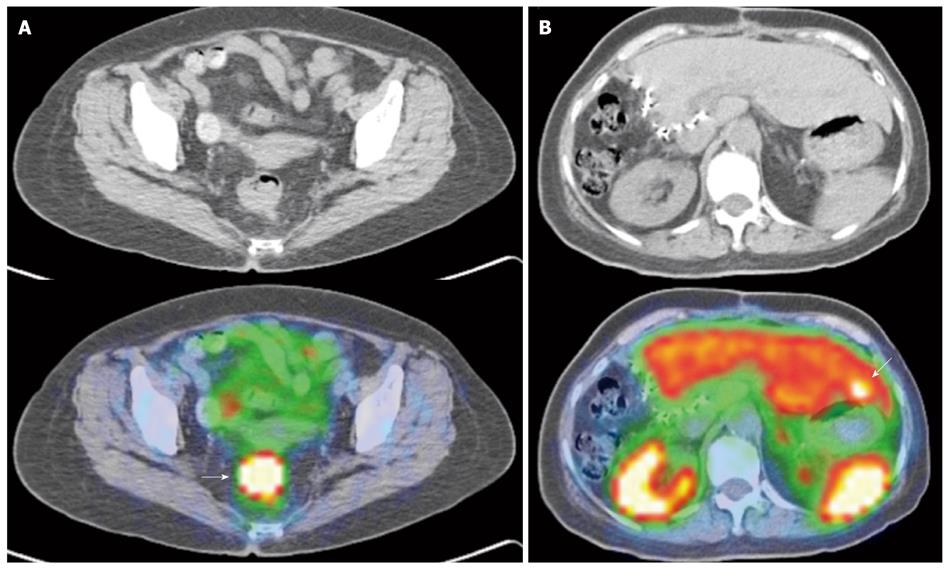
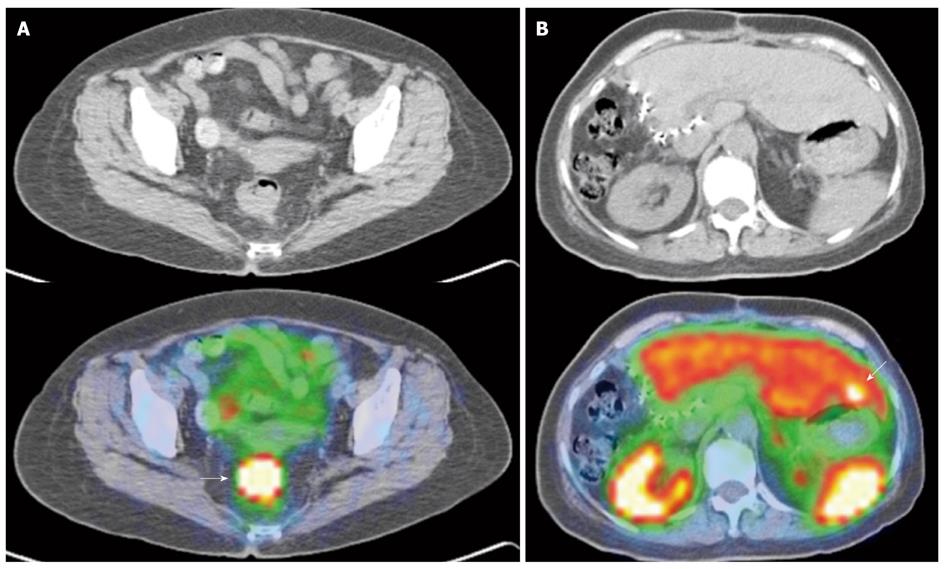
Newer generation somatostatin analogues have since been developed, allowing radiolabeling with positron emitting tracers. Together with the development and adoption of hybrid PET/CT modalities, this potentially addresses several limitations faced with first generation SRS, largely related to the poorer spatial resolution of gamma-based probes and the issue of precise anatomical localization (Figures 9 and 10).
PET-based SRS has shown high sensitivities, specificities and accuracies in the evaluation of NETs. Initial evaluations using PET-based SRS were encouraging. Hofmann et al[56] found higher tumor to non-tumor contrast ratios with significantly higher detection ratios for PET-based SRS, and Kowalski et al[57] also concluded that PET-based SRS was able to detect more lesions and was superior in detecting smaller lesions.
A larger prospective study by Gabriel et al[58] evaluating the diagnostic value of 68Ga-DOTATOC PET in 84 patients with known or suspected NETs demonstrated a sensitivity of 97%, specificity of 92% and an overall accuracy of 96%, showing significantly higher diagnostic efficacy as compared with SPECT imaging using gamma-based SRS and normal diagnostic CT. In addition, PET-based SRS detected more tumor sites in the liver, nodes and bone as compared with the other modalities, and provided further clinically relevant information in 14% of patients compared with gamma-based scintigraphy and 21% as compared with CT.
This was substantiated by Putzer et al[59], who evaluated 51 patients with histologically proven NETs with 68Ga-DOTATOC PET/CT. Reported sensitivity and specificity were 97% and 92%, respectively, higher than CT or bone scan, and detected bone metastasis in patients at a significantly higher rate. This is particularly important as osseous metastasis has a negative prognostic implication on clinical outcomes.
Furthermore, the increased diagnostic accuracy of PET-based SRS has been shown in publications to impact on actual clinical management. Ambrosini et al[60] evaluated the clinical impact of 68Ga-DOTANOC PET/CT imaging in 90 patients with histologically proven NETs. In the subgroup of patients with concordant PET and CT findings (n = 47), PET resulted in a modification of therapeutic management in 36.2% of patients. In the subgroup of patients with discordant PET and CT findings (n = 42), PET resulted in stage modification in 28.6% of patients and a change in management in 76.2% of patients. Overall, PET imaging affected either staging or therapy in 55.5% of patients imaged, with the most frequent management impact being initiation or continuance of peptide receptor radionuclide therapy, initiation or continuance of somatostatin analogue treatment, or referral for surgery. The author also reported that PET prevented unnecessary surgery in 6 patients, and excluded 2 patients with peptide receptor radionuclide treatment who did not show significant somatostatin analogue avidity.
With regard to post-therapy response assessment of NETs following peptide receptor radionuclide therapy, findings are controversial.
Gabriel et al[61] evaluated 46 patients with advanced NETs who underwent peptide receptor radionuclide therapy. 68Ga-DOTATOC PET (dedicated PET) and conventional CT was performed pre- and post-therapy for all patients. RECIST criteria were used to evaluate therapy response, with a reported 30% response rate, 48% stable disease and 22% progressive disease. Concordant findings were noted in 70% of cases. In the 30% discrepant group (n = 14), PET-based SRS outperformed CT in 10 patients, was able to detect lesions not seen on CT in 5 patients and accurately determined disease response in 5 patients. In contrast, CT was able to detect small pulmonary lesions in 1 patient not seen on PET, and in the remaining 3 patients, PET-based SRS showed decreased tracer uptake in the lesions, but these were due to tumor dedifferentiation rather than therapy response, while CT clearly showed tumor size and extent of progression.
The author concluded that PET-based SRS showed no advantages over conventional imaging in response assessment, but several limitations in the study have to be noted. Firstly, the study utilized a dedicated PET scanner, while the majority of newer installations are hybrid PET/CT scanners, and the intrinsic limitations of dedicated PET imaging is accounted. Indeed, based on the 4 discrepant findings reported in the study, if a hybrid PET/CT scanner was utilized, it is expected that such discrepancies would not exist. Secondly, the emergence of non-somatostatin analogue avid lesions on post -therapy assessment scans is of clinical use, as it indicates dedifferentiation of tumor, suggesting the need for alternative treatment from peptide receptor radionuclide therapy or somatostatin analogues (Figures 9 and 10).
Overall, PET-based SRS has been routinely found to demonstrate high diagnostic sensitivity, specificity and accuracy[62], with positive clinical impact during pre-therapy staging. The use of SRS for post-therapy assessment is more indeterminate, and further evaluation needs to be carried out.
Fluorodeoxyglucose (FDG) PET imaging is a molecular imaging technique that addresses the glucose metabolism of tissue. As a rule of thumb, malignant tumors tend to demonstrate significantly higher levels of glucose metabolism as compared with normal physiological tissue, and this has proven true across a wide range of tumor types[63].
The molecular basis of increased glucose metabolism in tumor cells is complex, and there appears a multitude of factors controlling aerobic glycolysis in tumors[64]. However, 2 major factors have been implicated with increased FDG tumor uptake. Firstly, the overexpression of glucose transporters and activity in tumor cells (predominantly GLUT-1, 3 and 5) which actively drive glucose into the cells, and secondly, the overexpression of hexokinase enzymes (predominantly hexokinase-2) that increase glucose metabolism[65,66].
The use of FDG PET in NETs is currently controversial. There are limited sensitivities overall, but there is emerging evidence that the presence of increased glucose metabolism in tumors highlights an increased propensity for invasion and metastasis, and overall poorer prognosis. This correlates with mathematically-based telogenic models and empiric data reviewed by Gillies et al[67], where such increased glucose metabolism confers an “evolutionary advantage” in cancer cells over normal parenchymal tissue.
An early study performed by Adams et al[68] found that FDG PET only demonstrated increased glucose uptake in less differentiated tumors with high proliferative activity. Another small study performed by Pasquali et al[69] evaluated the clinical use of FDG PET against conventional gamma-based SRS and CT, and again found that FDG PET was able to detect NETs characterized by rapid growth or aggressive behavior. Garin et al[70] performed a prospective study evaluating the clinical outcomes of 38 patients with metastatic NETs. FDG PET, SRS and conventional CT were performed for these patients, and patients were tracked to determine progression-free survival and overall survival. Overall 2 year survival and progression-free survival was 73% and 45%, respectively, and it was found that most patients with FDG PET positive lesions had early progressive disease (14/15 for FDG PET positive as compared with 2/23 for FDG PET negative). Furthermore, when only patients with low-grade tumors were considered, FDG PET was able to predict those with early progression. Progression-free survival was 87% ± 7% and 75% ± 10% at 1 and 2 years, respectively, for FDG PET negative lesions, as compared with 7% ± 6% and 0% at 1 and 2 years, respectively, for FDG PET positive patients. Overall, the relative risk of early progression with FDG PET positive lesions was 10.7 (95% CI: 2.8-40.6).
In terms of survival, FDG PET negative patients fared better than patients with FDG avid lesions. Overall survival was 95% ± 5% at both 1 and 2 years, respectively, for FDG PET negative patients, vs 72% ± 12% and 42% ± 13% at 1 and 2 years, respectively, for 18F-FDG PET positive patients.
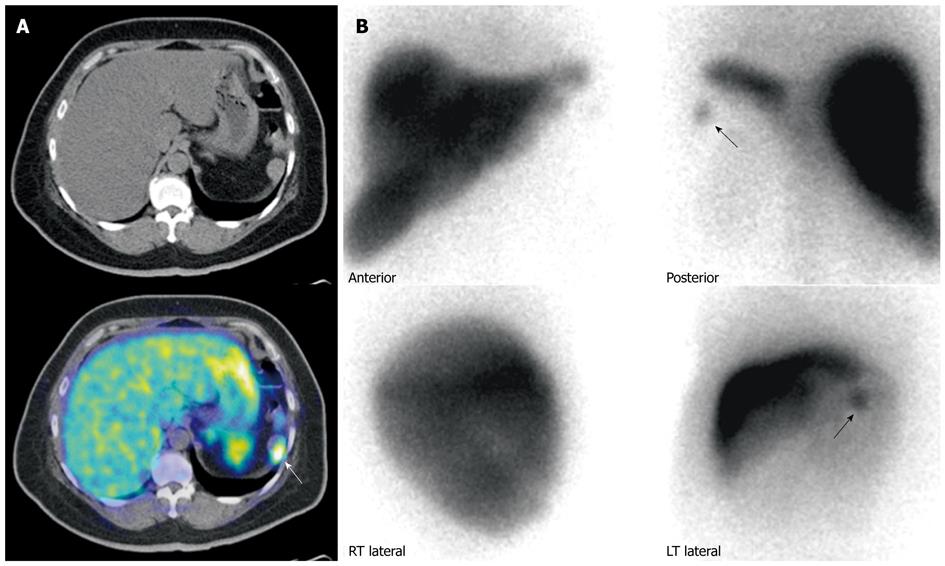
Overall, the use of FDG PET appears promising in disease prognostication, possibly influencing aggressiveness of management. In addition, dual tracer imaging using both FDG and SRS PET might possibly be used in post-therapy assessment following peptide receptor radionuclide therapy to evaluate for tumor dedifferentiation or the “flip-flop” phenomenon[71] (Figure 11).
The APUD by Everson Pearse[72] describes the ability of neuroendocrine type cells to take up and decarboxylate amino acid precursors, and there have been various efforts to evaluate the utility of radiolabeled amine precursors to image NETs. Examples of such precursors include hydroxytryptophan, hydroxyephedrine, dopamine and dihydroxyphenylalanine (DOPA). The radiolabeled DOPA analogues are transported into NETs via the sodium independent system L, and the activity of amino acid decarboxylase in the cells is important for intracellular retention of the metabolized radiolabeled DOPA analogue. Becherer et al[73] in the evaluation of 23 patients with histologically proven NETs concluded that 18F-DOPA PET performed better that gamma-based SRS in visualizing lesions, with the highest sensitivity in visualizing skeletal and mediastinal lesions. Reported sensitivities were 81.3% for the liver, 90.9% for the skeleton and 100% for the mediastinum and lymph nodes.
Koopmans et al[74] evaluated 53 patients in a prospective single-center diagnostic accuracy study using 18F-DOPA PET, conventional CT and SRS without any CT correlation, and reported that 18F-DOPA PET detected more lesions, more positive regions and more lesions per region as compared with the other modalities. Reported sensitivities at the patient, region and lesion levels were 100%, 95% and 96%, respectively.
Kauhanen et al[75] evaluated 82 patients with suspected/known NETs using 18F-DOPA PET, comparing the diagnostic accuracy with histological findings and clinical follow-up. 32 patients were for primary diagnosis and staging, while 61 patients were for restaging. Overall accuracy for gastrointestinal NETs was approximately 89%.
Overall, based on a meta-analysis by Jager et al[76], the radiolabeled DOPA analogues have reported sensitivities in the range of 65%-96% for the detection of individual lesions, with most of the values in the upper half of this range.
The advantages of DOPA PET over conventional anatomic imaging or gamma-based SRS are fairly conclusive, but the role in comparison with PET-based SRS techniques is still uncertain. Accuracies for PET-based SRS as discussed earlier appear to be comparable or better than DOPA PET. In a head to head comparison between 68Ga-DOTATATE and 18F-DOPA PET in the diagnosis of differentiated metastatic NETs, patient-based sensitivities were 96% for 68Ga-DOTATATE compared with 56% for 18F-DOPA, with 68Ga-DOTATATE PET proving clearly superior for detection and staging of NETs[77].
In addition, the clinical impact of PET-based SRS has been established in several publications, and the added advantage of SRS is that it allows the suitability assessment for peptide receptor radionuclide therapy, something that DOPA PET does not allow.
Pancreatic endocrine tumors comprise approximately 2%-10% of all pancreatic tumors[78,79], and are named after the predominant hormone that they secrete.
Insulinomas are the most common, accounting for approximately 60% of pancreatic NETs. They are tumors arising from pancreatic B-cells and are frequently solitary and largely benign. Typically, only 10% of insulinomas are multiple, 10% malignant, and 10% are associated with the Multiple Endocrine Neoplasm (MEN) type 1 syndrome[80]. Gastrinomas are the second most common tumors, accounting for approximately 20% of such tumors. Other rarer types include glucagonomas, somatostatinomas, vasoactive intestinal peptide secreting tumors (VIPomas), adrenocorticotropic secreting tumors (ACTHoma), GRFomas, calcitonin-producing tumors and parathyroid hormone-related peptide tumors.
Such tumors can be broadly classified as functional or non-functional, and although earlier studies estimated non-functional tumors to account for 18%-66% of tumors[81], later large studies have classified 60%-80% of pancreatic NETs as non-functional[82,83].
In approaching functional or molecular imaging of pancreatic neuroendocrine or islet-cell tumors, it is prudent to do so based on 2 separate groups: Insulinomas and non-insulinoma pancreatic NETs.
With regard to insulinomas, the role of SRS is uncertain, as there is generally poor sensitivity in the detection of such tumors. The reasons for this are multifactorial. Firstly, a significant percentage of insulinomas do not express significant densities of somatostatin receptors, especially subtypes 2 and 5. In addition, somatostatin receptors are not significantly expressed in non-malignant insulinomas further limiting SRS sensitivity[84]. However, malignant insulinomas are known to overexpress somatostatin receptors, and SRS has potential imaging roles in such tumors for prognostication and staging[85].
In contrast, gamma-based SRS has reported sensitivity and specificity for non-insulinoma pancreatic NETs of approximately 80%-90%[86,87], and is indicated for use in pre-therapy localization and staging, especially when demonstration of extra-hepatic metastatic lesions is required.
Several reports have established the promising utility of PET-based SRS for imaging non-insulinoma pancreatic NETs[88-90]. The use of PET-based SRS is expected to have improved resolutive capabilities as compared with conventional gamma-based SRS, in keeping with findings from gastrointestinal carcinoid imaging, but further validation is needed.
The utility of FDG PET in the evaluation of pancreatic NETs is indeterminate. FDG PET has generally poor sensitivities in the detection of such tumors (approximately 50%)[91], but may have a role in prognostication as it allows the identification of NETs characterized by aggressive growth or behavior[92-93].
DOPA PET appears to show promise in the evaluation of pancreatic NETs. Koopmans et al[94] in the evaluation of 23 patients with pancreatic islet cell tumors reported a sensitivity of 89% using 18F-DOPA PET, as compared with 78% and 87% for gamma-based SRS and conventional CT, respectively.
In the same study, 5-hydroxytryptophan (5-HTP) was also used as a delivery ligand in the targeted imaging of NETs. 5-HTP is the direct precursor for the serotonin pathway, and thus, is potentially of use in all neuroendocrine type tumors[95]. In relation to pancreatic islet cell tumors, the study reported sensitivities of 100% for 11C-5-HTP in the detection of pancreatic NETs (Table 3).
| Advantages | Disadvantages | Utility | |
| Ultrasound | Widely available modality, dynamic visualization of lesions, no ionizing radiation | Limited to solid organ systems, inter-operator variability | Possible use as a screening tool for assessing the liver and pancreatic head |
| CT | Widely available modality, wide field of view, allowing evaluation of nodal disease and metastasis, good sensitivity | Ionizing radiation, non specific modality, low negative predictive value for small volume nodes | First line imaging modality |
| MRI | Superior to CT for assessment in solid organs, no ionizing radiation, gadolinium contrast agent safety profile better than CT agents in terms of allergic reaction and nephrotoxicity, ability to further characterize lesions using different sequencing | Not as widely available as compared with CT or ultrasound, more specialized diagnostic imaging expertise in interpretation, lower specificity in characterizing neuroendocrine lesions as compared with functional imaging modalities | Local staging of disease, including vascular involvement, use in pediatric age group in which ionizing radiation is of greater concern |
| SRS | Good sensitivity and specificity, able to accurately characterize lesions; single modality staging; allows for dosimetric evaluation of suitability for peptide receptor radionuclide therapy; proven impact on clinical management | Ionizing radiation; not as widely available as CT or ultrasound, requiring nuclear imaging capabilities; more specialized diagnostic imaging expertise in interpretation | Gold standard in the evaluation of neuroendocrine tumors |
| Flurodeoxyglucose PET | Possible use in disease prognostication and management stratification, possible use in post treatment assessment to evaluate for tumor dedifferentiation | Generally poor sensitivity for neuroendocrine tumors, ionizing radiation | Not routinely performed for neuroendocrine tumor assessment, possible utility in prognostication and post therapy assessment |
| Dihydroxyphenylalanine PET | Good sensitivities in the evaluation of neuroendocrine tumors, shows promise especially in assessments of insulinomas | Requires more specialized nuclear facilities (e.g. gaseous F18) for synthesis of the radioisotope, PET based SRS has generally similar or better accuracies in the detection and staging of neuroendocrine tumors, ionizing radiation | Possible clinical utility in the evaluation of insulinomas |
The discussions on the various imaging modalities used in the diagnostic imaging of NETs highlight several of the modalities and various key points, but this is not a comprehensive review. This is partly due to the extensive and complex nature or NETs, and partly due to the explosive growth and developments in medical imaging. In summary, an understanding of the historical and molecular underpinnings of NETs, and the intrinsic uses and limitations of each diagnostic imaging modality, are essential for the physician involved in the management of this complex disease.
Peer reviewers: Tzu-Chen Yen, MD, PhD, Professor and Chairperson, Department of Nuclear Medicine and Molecular Imaging Center, Chang Gung Memorial Hospital and University, Taoyuan, Taiwan, China; Domenico Rubello, MD, Professor, Director of the Department of Nuclear Medicine, PET/CT Centre, Radiology, Medical Physics, Santa Maria della Misericordia Hospital; Via Tre Martiri 140, ZIP 45100, Rovigo, Italy; Ravi Murthy, MD, Interventional Radiology, UT MD Anderson Cancer Center, 1400 Pressler Street, Unit 1471, Houston, TX 77042, United States
S- Editor Cheng JX L- Editor Webster JR E- Editor Ma WH
| 1. | Lubarsch O. Ueber den primären krebs des ileum nebst bemerkungen über das gleichzeitige vorkommen von krebs und tuberculose. Virchows Arch Pathol Anat. 1888;111:280-317. |
| 2. | Feyrter F. Über diffuse endokrine epitheliale Organe. Zentralblatt Innere Medizin. 1938;59:546-556. |
| 3. | Langley K. The neuroendocrine concept today. Ann N Y Acad Sci. 1994;733:1-17. |
| 4. | Said JW, Vimadalal S, Nash G, Shintaku IP, Heusser RC, Sassoon AF, Lloyd RV. Immunoreactive neuron-specific enolase, bombesin, and chromogranin as markers for neuroendocrine lung tumors. Hum Pathol. 1985;16:236-240. |
| 5. | Hainsworth JD, Wright EP, Johnson DH, Davis BW, Greco FA. Poorly differentiated carcinoma of unknown primary site: clinical usefulness of immunoperoxidase staining. J Clin Oncol. 1991;9:1931-1938. |
| 6. | Chetty R. An overview of practical issues in the diagnosis of gastroenteropancreatic neuroendocrine pathology. Arch Pathol Lab Med. 2008;132:1285-1289. |
| 7. | Campana D, Nori F, Piscitelli L, Morselli-Labate AM, Pezzilli R, Corinaldesi R, Tomassetti P. Chromogranin A: is it a useful marker of neuroendocrine tumors? J Clin Oncol. 2007;25:1967-1973. |
| 8. | Zatelli MC, Torta M, Leon A, Ambrosio MR, Gion M, Tomassetti P, De Braud F, Delle Fave G, Dogliotti L, degli Uberti EC. Chromogranin A as a marker of neuroendocrine neoplasia: an Italian Multicenter Study. Endocr Relat Cancer. 2007;14:473-482. |
| 9. | Nobels FR, Kwekkeboom DJ, Coopmans W, Schoenmakers CH, Lindemans J, De Herder WW, Krenning EP, Bouillon R, Lamberts SW. Chromogranin A as serum marker for neuroendocrine neoplasia: comparison with neuron-specific enolase and the alpha-subunit of glycoprotein hormones. J Clin Endocrinol Metab. 1997;82:2622-2628. |
| 10. | Solcia E, Kloppel G, Sobin LH. Histological typing of endocrine tumours. World Health Organization International Histological Classification of Tumours. 2nd ed. Berlin, Germany: Springer 2000; . |
| 11. | Artale S, Giannetta L, Cerea G, Maggioni D, Pedrazzoli P, Schiavetto I, Napolitano M, Veronese S, Bramerio E, Gambacorta M. Treatment of metastatic neuroendocrine carcinomas based on WHO classification. Anticancer Res. 2005;25:4463-4469. |
| 12. | Bajetta E, Catena L, Procopio G, Bichisao E, Ferrari L, Della Torre S, De Dosso S, Iacobelli S, Buzzoni R, Mariani L. Is the new WHO classification of neuroendocrine tumours useful for selecting an appropriate treatment? Ann Oncol. 2005;16:1374-1380. |
| 13. | Panzuto F, Nasoni S, Falconi M, Corleto VD, Capurso G, Cassetta S, Di Fonzo M, Tornatore V, Milione M, Angeletti S. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer. 2005;12:1083-1092. |
| 14. | Mezzetti M, Raveglia F, Panigalli T, Giuliani L, Lo Giudice F, Meda S, Conforti S. Assessment of outcomes in typical and atypical carcinoids according to latest WHO classification. Ann Thorac Surg. 2003;76:1838-1842. |
| 15. | Oberg K, Jelic S. Neuroendocrine gastroenteropancreatic tumors: ESMO clinical recommendation for diagnosis, treatment and follow-up. Ann Oncol. 2009;20 Suppl 4:150-153. |
| 16. | Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M, Komminoth P. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395-401. |
| 17. | Rindi G, Klöppel G, Couvelard A, Komminoth P, Körner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2007;451:757-762. |
| 18. | Pape UF, Jann H, Müller-Nordhorn J, Bockelbrink A, Berndt U, Willich SN, Koch M, Röcken C, Rindi G, Wiedenmann B. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer. 2008;113:256-265. |
| 19. | Rockall AG, Reznek RH. Imaging of neuroendocrine tumours (CT/MR/US). Best Pract Res Clin Endocrinol Metab. 2007;21:43-68. |
| 20. | McAuley G, Delaney H, Colville J, Lyburn I, Worsley D, Govender P, Torreggiani WC. Multimodality preoperative imaging of pancreatic insulinomas. Clin Radiol. 2005;60:1039-1050. |
| 21. | Rösch T, Lightdale CJ, Botet JF, Boyce GA, Sivak MV Jr, Yasuda K, Heyder N, Palazzo L, Dancygier H, Schusdziarra V. Localization of pancreatic endocrine tumors by endoscopic ultrasonography. N Engl J Med. 1992;326:1721-1726. |
| 22. | Noone TC, Hosey J, Firat Z, Semelka RC. Imaging and localization of islet-cell tumours of the pancreas on CT and MRI. Best Pract Res Clin Endocrinol Metab. 2005;19:195-211. |
| 23. | Sheth S, Hruban RK, Fishman EK. Helical CT of islet cell tumors of the pancreas: typical and atypical manifestations. AJR Am J Roentgenol. 2002;179:725-730. |
| 25. | Balci NC, Semelka RC. Radiologic features of cystic, endocrine and other pancreatic neoplasms. Eur J Radiol. 2001;38:113-119. |
| 26. | Binstock AJ, Johnson CD, Stephens DH, Lloyd RV, Fletcher JG. Carcinoid tumors of the stomach: a clinical and radiographic study. AJR Am J Roentgenol. 2001;176:947-951. |
| 27. | Woodard PK, Feldman JM, Paine SS, Baker ME. Midgut carcinoid tumors: CT findings and biochemical profiles. J Comput Assist Tomogr. 1995;19:400-405. |
| 28. | Gouya H, Vignaux O, Augui J, Dousset B, Palazzo L, Louvel A, Chaussade S, Legmann P. CT, endoscopic sonography, and a combined protocol for preoperative evaluation of pancreatic insulinomas. AJR Am J Roentgenol. 2003;181:987-992. |
| 29. | Paulson EK, McDermott VG, Keogan MT, DeLong DM, Frederick MG, Nelson RC. Carcinoid metastases to the liver: role of triple-phase helical CT. Radiology. 1998;206:143-150. |
| 30. | Gibril F, Doppman JL, Reynolds JC, Chen CC, Sutliff VE, Yu F, Serrano J, Venzon DJ, Jensen RT. Bone metastases in patients with gastrinomas: a prospective study of bone scanning, somatostatin receptor scanning, and magnetic resonance image in their detection, frequency, location, and effect of their detection on management. J Clin Oncol. 1998;16:1040-1053. |
| 31. | Pisegna JR, Doppman JL, Norton JA, Metz DC, Jensen RT. Prospective comparative study of ability of MR imaging and other imaging modalities to localize tumors in patients with Zollinger-Ellison syndrome. Dig Dis Sci. 1993;38:1318-1328. |
| 32. | Ichikawa T, Peterson MS, Federle MP, Baron RL, Haradome H, Kawamori Y, Nawano S, Araki T. Islet cell tumor of the pancreas: biphasic CT versus MR imaging in tumor detection. Radiology. 2000;216:163-171. |
| 33. | Herwick S, Miller FH, Keppke AL. MRI of islet cell tumors of the pancreas. AJR Am J Roentgenol. 2006;187:W472-W480. |
| 34. | Owen NJ, Sohaib SA, Peppercorn PD, Monson JP, Grossman AB, Besser GM, Reznek RH. MRI of pancreatic neuroendocrine tumours. Br J Radiol. 2001;74:968-973. |
| 35. | Semelka RC, Custodio CM, Cem Balci N, Woosley JT. Neuroendocrine tumors of the pancreas: spectrum of appearances on MRI. J Magn Reson Imaging. 2000;11:141-148. |
| 36. | Bader TR, Semelka RC, Chiu VC, Armao DM, Woosley JT. MRI of carcinoid tumors: spectrum of appearances in the gastrointestinal tract and liver. J Magn Reson Imaging. 2001;14:261-269. |
| 37. | Debray MP, Geoffroy O, Laissy JP, Lebtahi R, Silbermann-Hoffman O, Henry-Feugeas MC, Cadiot G, Mignon M, Schouman-Claeys E. Imaging appearances of metastases from neuroendocrine tumours of the pancreas. Br J Radiol. 2001;74:1065-1070. |
| 38. | Dromain C, de Baere T, Baudin E, Galline J, Ducreux M, Boige V, Duvillard P, Laplanche A, Caillet H, Lasser P. MR imaging of hepatic metastases caused by neuroendocrine tumors: comparing four techniques. AJR Am J Roentgenol. 2003;180:121-128. |
| 39. | Vossen JA, Buijs M, Liapi E, Eng J, Bluemke DA, Kamel IR. Receiver operating characteristic analysis of diffusion-weighted magnetic resonance imaging in differentiating hepatic hemangioma from other hypervascular liver lesions. J Comput Assist Tomogr. 2008;32:750-756. |
| 40. | Liapi E, Geschwind JF, Vossen JA, Buijs M, Georgiades CS, Bluemke DA, Kamel IR. Functional MRI evaluation of tumor response in patients with neuroendocrine hepatic metastasis treated with transcatheter arterial chemoembolization. AJR Am J Roentgenol. 2008;190:67-73. |
| 41. | Lee SS, Byun JH, Park BJ, Park SH, Kim N, Park B, Kim JK, Lee MG. Quantitative analysis of diffusion-weighted magnetic resonance imaging of the pancreas: usefulness in characterizing solid pancreatic masses. J Magn Reson Imaging. 2008;28:928-936. |
| 42. | Anaye A, Mathieu A, Closset J, Bali MA, Metens T, Matos C. Successful preoperative localization of a small pancreatic insulinoma by diffusion-weighted MRI. JOP. 2009;10:528-531. |
| 43. | Katzenellenbogen BS, Fang H, Ince BA, Pakdel F, Reese JC, Wooge CH, Wrenn CK. William L. McGuire Memorial Symposium. Estrogen receptors: ligand discrimination and antiestrogen action. Breast Cancer Res Treat. 1993;27:17-26. |
| 44. | Mankoff DA, Link JM, Linden HM, Sundararajan L, Krohn KA. Tumor receptor imaging. J Nucl Med. 2008;49 Suppl 2:149S-163S. |
| 45. | Ricke J, Klose KJ, Mignon M, Oberg K, Wiedenmann B. Standardisation of imaging in neuroendocrine tumours: results of a European delphi process. Eur J Radiol. 2001;37:8-17. |
| 46. | Mundschenk J, Unger N, Schulz S, Höllt V, Schulz S, Steinke R, Lehnert H. Somatostatin receptor subtypes in human pheochromocytoma: subcellular expression pattern and functional relevance for octreotide scintigraphy. J Clin Endocrinol Metab. 2003;88:5150-5157. |
| 47. | Taniyama Y, Suzuki T, Mikami Y, Moriya T, Satomi S, Sasano H. Systemic distribution of somatostatin receptor subtypes in human: an immunohistochemical study. Endocr J. 2005;52:605-611. |
| 48. | Bakker WH, Krenning EP, Reubi JC, Breeman WA, Setyono-Han B, de Jong M, Kooij PP, Bruns C, van Hagen PM, Marbach P. In vivo application of [111In-DTPA-D-Phe1]-octreotide for detection of somatostatin receptor-positive tumors in rats. Life Sci. 1991;49:1593-601. |
| 49. | Bakker WH, Albert R, Bruns C, Breeman WA, Hofland LJ, Marbach P, Pless J, Pralet D, Stolz B, Koper JW. [111In-DTPA-D-Phe1]-octreotide, a potential radiopharmaceutical for imaging of somatostatin receptor-positive tumors: synthesis, radiolabeling and in vitro validation. Life Sci. 1991;49:1583-1591. |
| 50. | Krenning EP, Bakker WH, Kooij PP, Breeman WA, Oei HY, de Jong M, Reubi JC, Visser TJ, Bruns C, Kwekkeboom DJ. Somatostatin receptor scintigraphy with indium-111-DTPA-D-Phe-1-octreotide in man: metabolism, dosimetry and comparison with iodine-123-Tyr-3-octreotide. J Nucl Med. 1992;33:652-658. |
| 51. | Carcinoid tumors, carcinoid syndrome, and related disorders. Williams textbook of endocrinology. 10th ed. Philadelphia, Pa: Saunders 2003; 661-690. |
| 52. | Kälkner KM, Janson ET, Nilsson S, Carlsson S, Oberg K, Westlin JE. Somatostatin receptor scintigraphy in patients with carcinoid tumors: comparison between radioligand uptake and tumor markers. Cancer Res. 1995;55:5801s-5804s. |
| 53. | Kwekkeboom DJ, Krenning EP, Bakker WH, Oei HY, Kooij PP, Lamberts SW. Somatostatin analogue scintigraphy in carcinoid tumours. Eur J Nucl Med. 1993;20:283-292. |
| 54. | Westlin JE, Janson ET, Arnberg H, Ahlström H, Oberg K, Nilsson S. Somatostatin receptor scintigraphy of carcinoid tumours using the [111In-DTPA-D-Phe1]-octreotide. Acta Oncol. 1993;32:783-786. |
| 55. | Gibril F, Reynolds JC, Chen CC, Yu F, Goebel SU, Serrano J, Doppman JL, Jensen RT. Specificity of somatostatin receptor scintigraphy: a prospective study and effects of false-positive localizations on management in patients with gastrinomas. J Nucl Med. 1999;40:539-553. |
| 56. | Hofmann M, Maecke H, Börner R, Weckesser E, Schöffski P, Oei L, Schumacher J, Henze M, Heppeler A, Meyer J. Biokinetics and imaging with the somatostatin receptor PET radioligand (68)Ga-DOTATOC: preliminary data. Eur J Nucl Med. 2001;28:1751-1757. |
| 57. | Kowalski J, Henze M, Schuhmacher J, Mäcke HR, Hofmann M, Haberkorn U. Evaluation of Positron Emission Tomography Imaging Using [68Ga]-DOTA-D Phe1-Tyr3-Octreotide in Comparison to [111In]-DTPAOC SPECT. First Results in Patients with Neuroendocrine Tumors. Mol Imaging Biol. 2003;5:42-48. |
| 58. | Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, Kovacs P, Von Guggenberg E, Bale R, Virgolini IJ. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508-518. |
| 59. | Putzer D, Gabriel M, Henninger B, Kendler D, Uprimny C, Dobrozemsky G, Decristoforo C, Bale RJ, Jaschke W, Virgolini IJ. Bone metastases in patients with neuroendocrine tumor: 68Ga-DOTA-Tyr3-octreotide PET in comparison to CT and bone scintigraphy. J Nucl Med. 2009;50:1214-1221. |
| 60. | Ambrosini V, Campana D, Bodei L, Nanni C, Castellucci P, Allegri V, Montini GC, Tomassetti P, Paganelli G, Fanti S. 68Ga-DOTANOC PET/CT clinical impact in patients with neuroendocrine tumors. J Nucl Med. 2010;51:669-673. |
| 61. | Gabriel M, Oberauer A, Dobrozemsky G, Decristoforo C, Putzer D, Kendler D, Uprimny C, Kovacs P, Bale R, Virgolini IJ. 68Ga-DOTA-Tyr3-octreotide PET for assessing response to somatostatin-receptor-mediated radionuclide therapy. J Nucl Med. 2009;50:1427-1434. |
| 62. | Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61-72. |
| 63. | Gambhir SS, Czernin J, Schwimmer J, Silverman DH, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med. 2001;42:1S-93S. |
| 64. | Pauwels EK, Sturm EJ, Bombardieri E, Cleton FJ, Stokkel MP. Positron-emission tomography with [18F]fluorodeoxyglucose. Part I. Biochemical uptake mechanism and its implication for clinical studies. J Cancer Res Clin Oncol. 2000;126:549-559. |
| 65. | Zhao S, Kuge Y, Mochizuki T, Takahashi T, Nakada K, Sato M, Takei T, Tamaki N. Biologic correlates of intratumoral heterogeneity in 18F-FDG distribution with regional expression of glucose transporters and hexokinase-II in experimental tumor. J Nucl Med. 2005;46:675-682. |
| 66. | Bos R, van Der Hoeven JJ, van Der Wall E, van Der Groep P, van Diest PJ, Comans EF, Joshi U, Semenza GL, Hoekstra OS, Lammertsma AA. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol. 2002;20:379-387. |
| 67. | Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49 Suppl 2:24S-42S. |
| 68. | Adams S, Baum R, Rink T, Schumm-Dräger PM, Usadel KH, Hör G. Limited value of fluorine-18 fluorodeoxyglucose positron emission tomography for the imaging of neuroendocrine tumours. Eur J Nucl Med. 1998;25:79-83. |
| 69. | Pasquali C, Rubello D, Sperti C, Gasparoni P, Liessi G, Chierichetti F, Ferlin G, Pedrazzoli S. Neuroendocrine tumor imaging: can 18F-fluorodeoxyglucose positron emission tomography detect tumors with poor prognosis and aggressive behavior? World J Surg. 1998;22:588-592. |
| 70. | Garin E, Le Jeune F, Devillers A, Cuggia M, de Lajarte-Thirouard AS, Bouriel C, Boucher E, Raoul JL. Predictive value of 18F-FDG PET and somatostatin receptor scintigraphy in patients with metastatic endocrine tumors. J Nucl Med. 2009;50:858-864. |
| 71. | Krenning EP, Valkema R, Kwekkeboom DJ, de Herder WW, van Eijck CH, de Jong M, Pauwels S, Reubi JC. Molecular imaging as in vivo molecular pathology for gastroenteropancreatic neuroendocrine tumors: implications for follow-up after therapy. J Nucl Med. 2005;46 Suppl 1:76S-82S. |
| 72. | Pearse AG. The cytochemistry and ultrastructural of polypeptide hormone producing cells of the APUD series and the embryogenic, physiologic and pathologic implications of the concept. J Histochem Cytochem. 1969;17:303-313. |
| 73. | Becherer A, Szabó M, Karanikas G, Wunderbaldinger P, Angelberger P, Raderer M, Kurtaran A, Dudczak R, Kletter K. Imaging of advanced neuroendocrine tumors with (18)F-FDOPA PET. J Nucl Med. 2004;45:1161-1167. |
| 74. | Koopmans KP, de Vries EG, Kema IP, Elsinga PH, Neels OC, Sluiter WJ, van der Horst-Schrivers AN, Jager PL. Staging of carcinoid tumours with 18F-DOPA PET: a prospective, diagnostic accuracy study. Lancet Oncol. 2006;7:728-734. |
| 75. | Kauhanen S, Seppänen M, Ovaska J, Minn H, Bergman J, Korsoff P, Salmela P, Saltevo J, Sane T, Välimäki M. The clinical value of [18F]fluoro-dihydroxyphenylalanine positron emission tomography in primary diagnosis, staging, and restaging of neuroendocrine tumors. Endocr Relat Cancer. 2009;16:255-265. |
| 76. | Jager PL, Chirakal R, Marriott CJ, Brouwers AH, Koopmans KP, Gulenchyn KY. 6-L-18F-fluorodihydroxyphenylalanine PET in neuroendocrine tumors: basic aspects and emerging clinical applications. J Nucl Med. 2008;49:573-586. |
| 77. | Haug A, Auernhammer CJ, Wängler B, Tiling R, Schmidt G, Göke B, Bartenstein P, Pöpperl G. Intraindividual comparison of 68Ga-DOTA-TATE and 18F-DOPA PET in patients with well-differentiated metastatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2009;36:765-770. |
| 78. | Barakat MT, Meeran K, Bloom SR. Neuroendocrine tumours. Endocr Relat Cancer. 2004;11:1-18. |
| 79. | Mignon M. Natural history of neuroendocrine enteropancreatic tumors. Digestion. 2000;62 Suppl 1:51-58. |
| 80. | de Herder WW, Niederle B, Scoazec JY, Pauwels S, Kloppel G, Falconi M, Kwekkeboom DJ, Oberg K, Eriksson B, Wiedenmann B. Well-differentiated pancreatic tumor/carcinoma: insulinoma. Neuroendocrinology. 2006;84:183-188. |
| 81. | Falconi M, Plockinger U, Kwekkeboom DJ, Manfredi R, Korner M, Kvols L, Pape UF, Ricke J, Goretzki PE, Wildi S. Well-differentiated pancreatic nonfunctioning tumors/carcinoma. Neuroendocrinology. 2006;84:196-211. |
| 82. | Pape UF, Böhmig M, Berndt U, Tiling N, Wiedenmann B, Plöckinger U. Survival and clinical outcome of patients with neuroendocrine tumors of the gastroenteropancreatic tract in a german referral center. Ann N Y Acad Sci. 2004;1014:222-233. |
| 83. | Corleto VD, Panzuto F, Falconi M, Cannizzaro R, Angeletti S, Moretti A, Delle Fave G, Farinati F. Digestive neuroendocrine tumours: diagnosis and treatment in Italy. A survey by the Oncology Study Section of the Italian Society of Gastroenterology (SIGE). Dig Liver Dis. 2001;33:217-221. |
| 84. | Virgolini I, Traub-Weidinger T, Decristoforo C. Nuclear medicine in the detection and management of pancreatic islet-cell tumours. Best Pract Res Clin Endocrinol Metab. 2005;19:213-227. |
| 85. | Proye C, Malvaux P, Pattou F, Filoche B, Godchaux JM, Maunoury V, Palazzo L, Huglo D, Lefebvre J, Paris JC. Noninvasive imaging of insulinomas and gastrinomas with endoscopic ultrasonography and somatostatin receptor scintigraphy. Surgery. 1998;124:1134-1143; discussion 1143-1144. |
| 86. | Lebtahi R, Cadiot G, Sarda L, Daou D, Faraggi M, Petegnief Y, Mignon M, le Guludec D. Clinical impact of somatostatin receptor scintigraphy in the management of patients with neuroendocrine gastroenteropancreatic tumors. J Nucl Med. 1997;38:853-858. |
| 87. | Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WA, Kooij PP, Oei HY, van Hagen M, Postema PT, de Jong M, Reubi JC. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20:716-731. |
| 88. | Henze M, Schuhmacher J, Dimitrakopoulou-Strauss A, Strauss LG, Mäcke HR, Eisenhut M, Haberkorn U. Exceptional increase in somatostatin receptor expression in pancreatic neuroendocrine tumour, visualised with (68)Ga-DOTATOC PET. Eur J Nucl Med Mol Imaging. 2004;31:466. |
| 89. | Froidevaux S, Eberle AN, Christe M, Sumanovski L, Heppeler A, Schmitt JS, Eisenwiener K, Beglinger C, Mäcke HR. Neuroendocrine tumor targeting: study of novel gallium-labeled somatostatin radiopeptides in a rat pancreatic tumor model. Int J Cancer. 2002;98:930-937. |
| 90. | Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, Kovacs P, Von Guggenberg E, Bale R, Virgolini IJ. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508-518. |
| 91. | Nakamoto Y, Higashi T, Sakahara H, Tamaki N, Itoh K, Imamura M, Konishi J. Evaluation of pancreatic islet cell tumors by fluorine-18 fluorodeoxyglucose positron emission tomography: comparison with other modalities. Clin Nucl Med. 2000;25:115-119. |
| 92. | Pasquali C, Rubello D, Sperti C, Gasparoni P, Liessi G, Chierichetti F, Ferlin G, Pedrazzoli S. Neuroendocrine tumor imaging: can 18F-fluorodeoxyglucose positron emission tomography detect tumors with poor prognosis and aggressive behavior? World J Surg. 1998;22:588-592. |
| 93. | Eriksson B, Orlefors H, Oberg K, Sundin A, Bergström M, Långström B. Developments in PET for the detection of endocrine tumours. Best Pract Res Clin Endocrinol Metab. 2005;19:311-324. |
| 94. | Koopmans KP, Neels OC, Kema IP, Elsinga PH, Sluiter WJ, Vanghillewe K, Brouwers AH, Jager PL, de Vries EG. Improved staging of patients with carcinoid and islet cell tumors with 18F-dihydroxy-phenyl-alanine and 11C-5-hydroxy-tryptophan positron emission tomography. J Clin Oncol. 2008;26:1489-1495. |
| 95. | Orlefors H, Sundin A, Garske U, Juhlin C, Oberg K, Skogseid B, Langstrom B, Bergstrom M, Eriksson B. Whole-body (11)C-5-hydroxytryptophan positron emission tomography as a universal imaging technique for neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and computed tomography. J Clin Endocrinol Metab. 2005;90:3392-400. |