Published online Aug 24, 2025. doi: 10.5306/wjco.v16.i8.109217
Revised: June 8, 2025
Accepted: July 25, 2025
Published online: August 24, 2025
Processing time: 105 Days and 18.5 Hours
Tracheoesophageal fistula (TEF) is a life-threatening complication of advanced esophageal squamous cell carcinoma (ESCC). Cervical ESCC is rare and fre
Here, we present a 59-year-old male patient with a 5-month history of CEC and difficulty eating for over 20 days, who developed TEF secondary to recurrent ESCC after chemoradiotherapy. He underwent total pharyngolaryngoesophage
This study highlights the efficacy of gastric pull-up and SCAIF reconstruction in managing TEF secondary to recurrent ESCC.
Core Tip: This article presents a unique approach to managing tracheoesophageal fistula (TEF) secondary to recurrent advanced-stage cervical esophageal cancer after chemoradiotherapy. There have been few reports regarding radical surgery for TEF. This is the first report of supraclavicular artery island flap (SCAIF) reconstruction for TEF repair and salvage surgery using gastric pull-up. The SCAIF was used to connect the tracheal stump located in the mediastinum, creating a permanent tracheostomy. The multidisciplinary team was pivotal in handling severe postoperative complications, including gastric conduit necrosis in the neck, managed with a U-shaped pectoralis major myocutaneous flap. We achieved optimal cure and significant improvement in the patient’s quality of life.
- Citation: Waheed HZ, Huang CQ, Bao YY, Chen Z, Chen HC, Cao ZZ, Zhong JT, Ye P, Fu SQ, Zhou SH. Successful cure of a patient with tracheoesophageal fistula in cervical esophageal cancer: A case report and review of literature. World J Clin Oncol 2025; 16(8): 109217
- URL: https://www.wjgnet.com/2218-4333/full/v16/i8/109217.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i8.109217
The cervical esophagus refers to the segment of the esophagus extending from the cricoid cartilage (16 cm from the incisors) to the thoracic inlet at the sternal notch (23 cm from the incisors)[1-3]. Cervical esophageal cancer (CEC) is relatively rare, accounting for only 2%-10% of all esophageal cancers. However, it is often diagnosed at an advanced stage during endoscopic assessment, with approximately 55% of cases classified as TNM stage T3 or T4 and 27% as T2, and it has a 5-year survival rate of around 30%[2]. Due to its advanced stage and anatomical complexity, CEC is typically managed with surgery or chemoradiotherapy (CRT), as recommended by the National Comprehensive Cancer Network, and supported by a multidisciplinary team (MDT)[3,4]. However, definitive CRT (dCRT) can lead to adverse effects, including dysphagia, stricture, and tracheoesophageal fistula (TEF) formation[2].
TEF in recurrent CEC is a rare and life-threatening complication that often requires innovative surgical management, and acquired TEF is malignant in 50% of cases in adult patients[5,6]. TEF is defined as an abnormal connection between the tracheobronchial tree and the esophagus, occurring in 5%-15% of esophageal cancers and 1% of tracheobronchial cancers[7]. Tumor invasion, facilitated by the thin tissue layer between the trachea and esophagus, can cause ulceration of the tracheal membrane or esophageal lumen, leading to TEF formation[7]. Esophageal squamous cell carcinoma (ESCC) has a higher incidence of TEF in patients undergoing CRT with variations among tumors depending on the region[8-11]. TEF is primarily diagnosed through esophagoscopy, fluoroscopic swallow studies, and radiological imaging[7]. Ma
A review of the literature from the past 5 years revealed limited reports of TEF secondary to esophageal cancer after treatment. To our knowledge, there have been few reports of radical surgery for TEF[8,14]. Other methods involve stenting[15,16]. However, there have been no reports regarding radical surgical resection of TEF caused by recurrent CEC after CRT, specifically using gastric tube replacement of the esophageal pharynx and a supraclavicular artery island flap (SCAIF) for the lower trachea.
Here, we report a case of advanced-stage CEC with the development of TEF after dCRT in which MDT collaboration was essential to determine the optimal management. The patient was treated successfully with SCAIF reconstruction and gastric pull-up surgery. We hypothesize that our method will improve functional and structural outcomes in patients with recurrent TEF following CRT[17-19]. Despite serious postoperative complications, the patient survived, and his quality of life improved following treatment with MDT collaboration.
On January 4, 2023, a 59-year-old male patient with a 5-month history of CEC with eating difficulty for more than 20 days experienced progressive dysphagia.
He also reported productive cough, difficulty eating, dysphonia, and pyrexia, but no respiratory distress. Over the next 20 days, his dysphagia worsened significantly, and he developed recurrent aspiration pneumonia and nutritional deterioration preceding admission to our hospital. Upon admission to our hospital, the patient was referred from the de
The patient had a history of hypertension, along with a 40-year history of smoking and alcohol consumption.
No similar cases had been reported in the patient’s family.
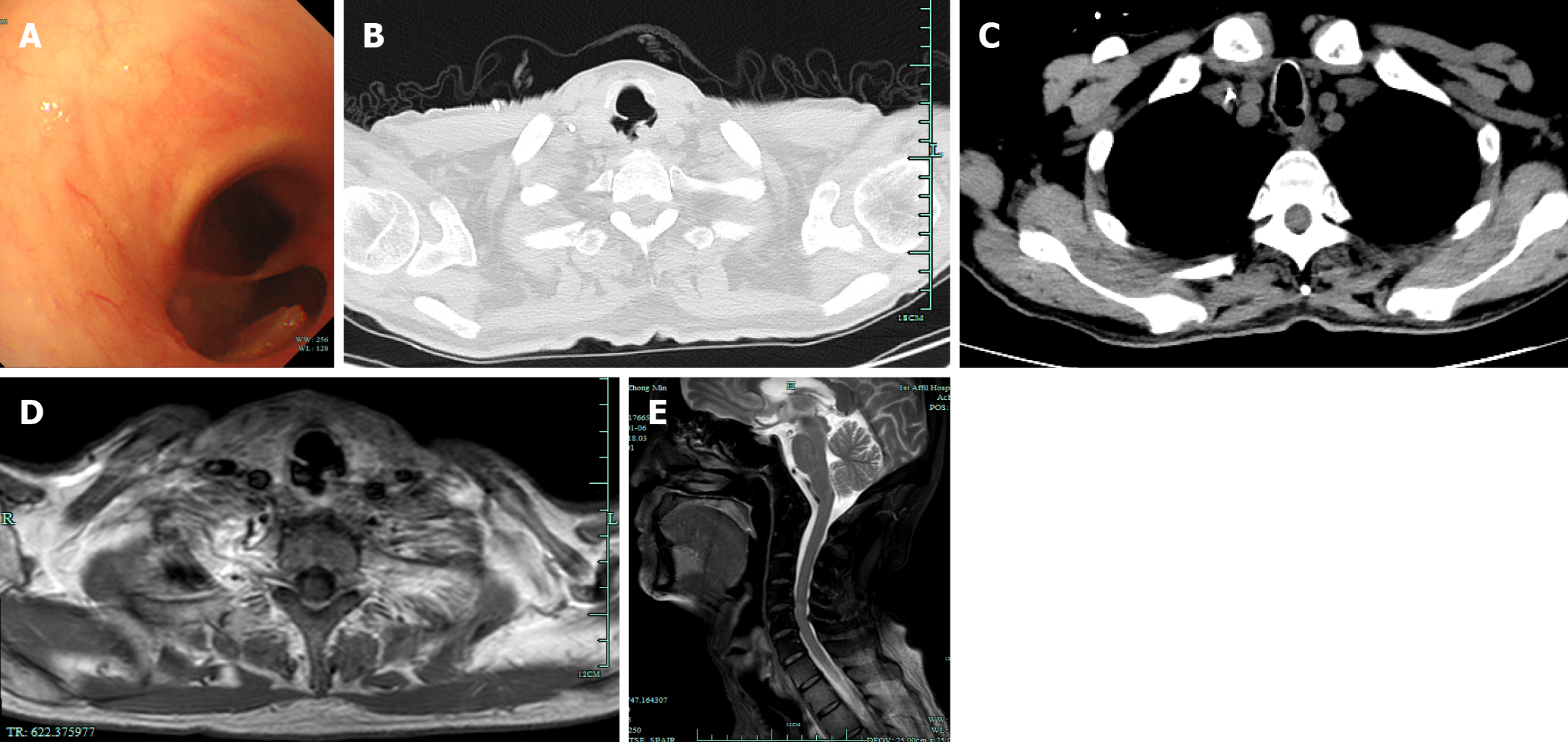
Physical examination showed a mass in the right neck region (level III). Indirect laryngoscopy revealed no mass, and the bilateral vocal cords remained mobile. Flexible bronchoscopy examination revealed a TEF 0.8 cm from the glottis and about 1.5 cm in length (Figure 1A).
Blood tests showed elevated WBC count (1.2 × 1.010/L) and tumor marker levels: Carcinoembryonic antigen, 7.2 × 1.0-6 g/L (normal range: 0–5 × 1.0-6 g/L); SCC antigen, 3.5 × 1.0-6 g/L (normal range: < 1.5 × 1.0-6 g/L; CYFRA 21-1, 5.8 × 1.0-6 g/L (normal range: < 3.3 × 1.0-6 g/L).
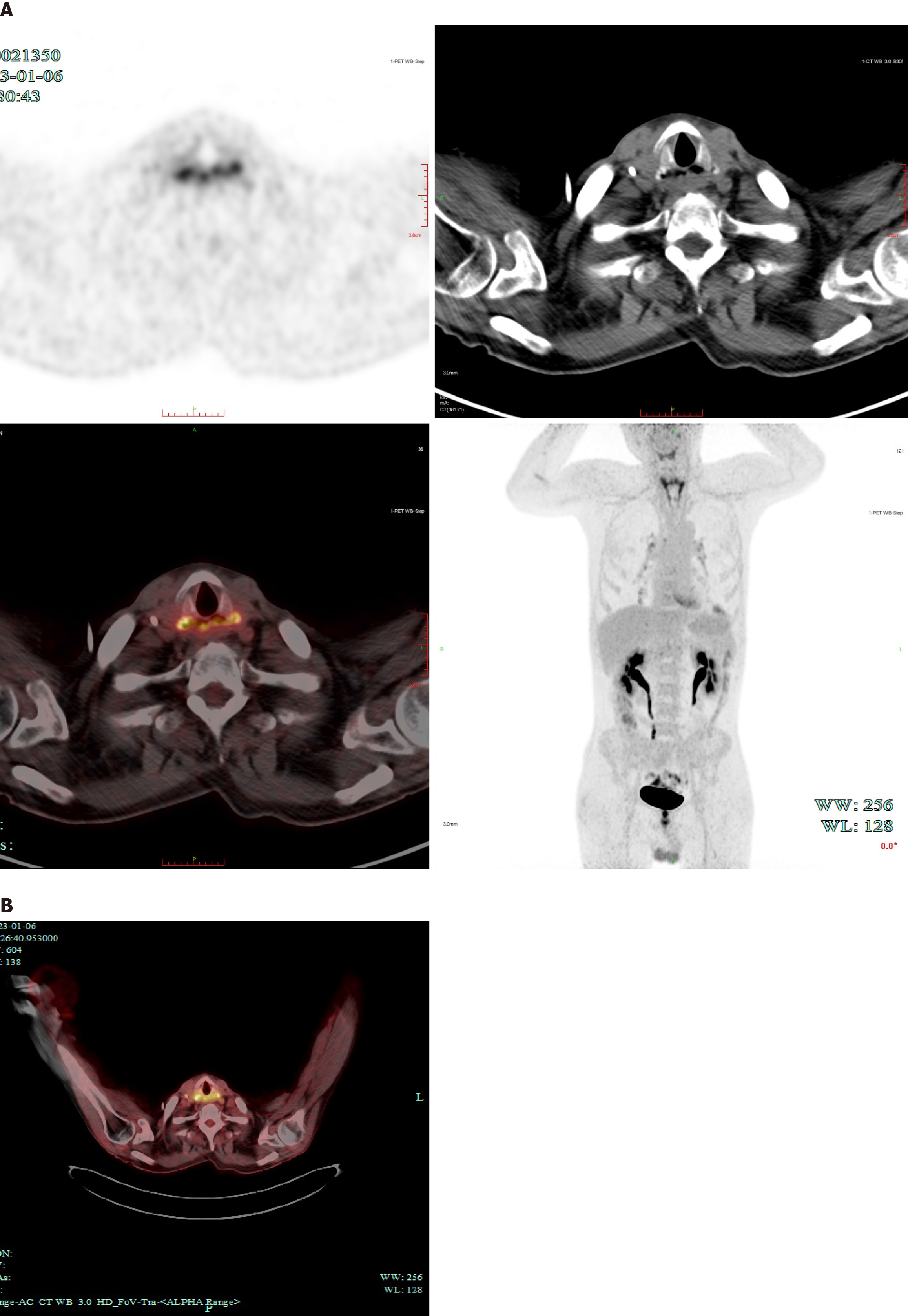
Computed tomography (CT) and magnetic resonance imaging of the chest and abdomen revealed a discontinuity between the anterior wall of the upper esophagus and the posterior wall of the trachea, indicative of TEF (Figure 1B-E). Positron emission tomography CT indicated thinning of the anterior esophageal wall and increased uptake, suggesting potential tumor recurrence (Figure 2).
A diagnosis of TEF in recurrent CEC (rT4bN2M0) was made.
After admission and preoperative assessment, the MDT determined that surgical intervention was the optimal course of action in this case. Preoperative management of the patient’s condition involved infection control by intravenous levofloxacin and supportive care, encompassing nebulization, expectoration, fluid supplementation, and intravenous nutritional support.
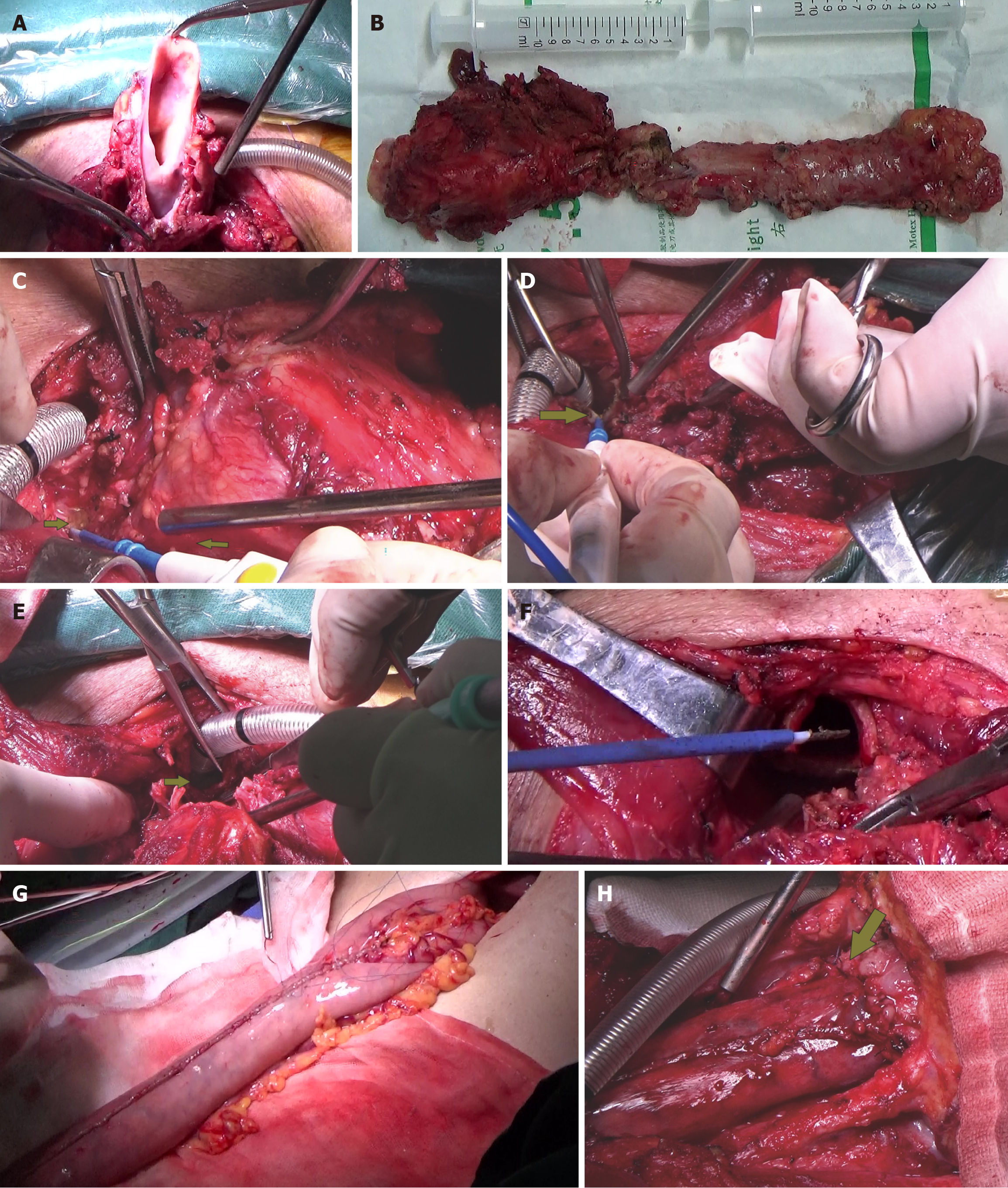
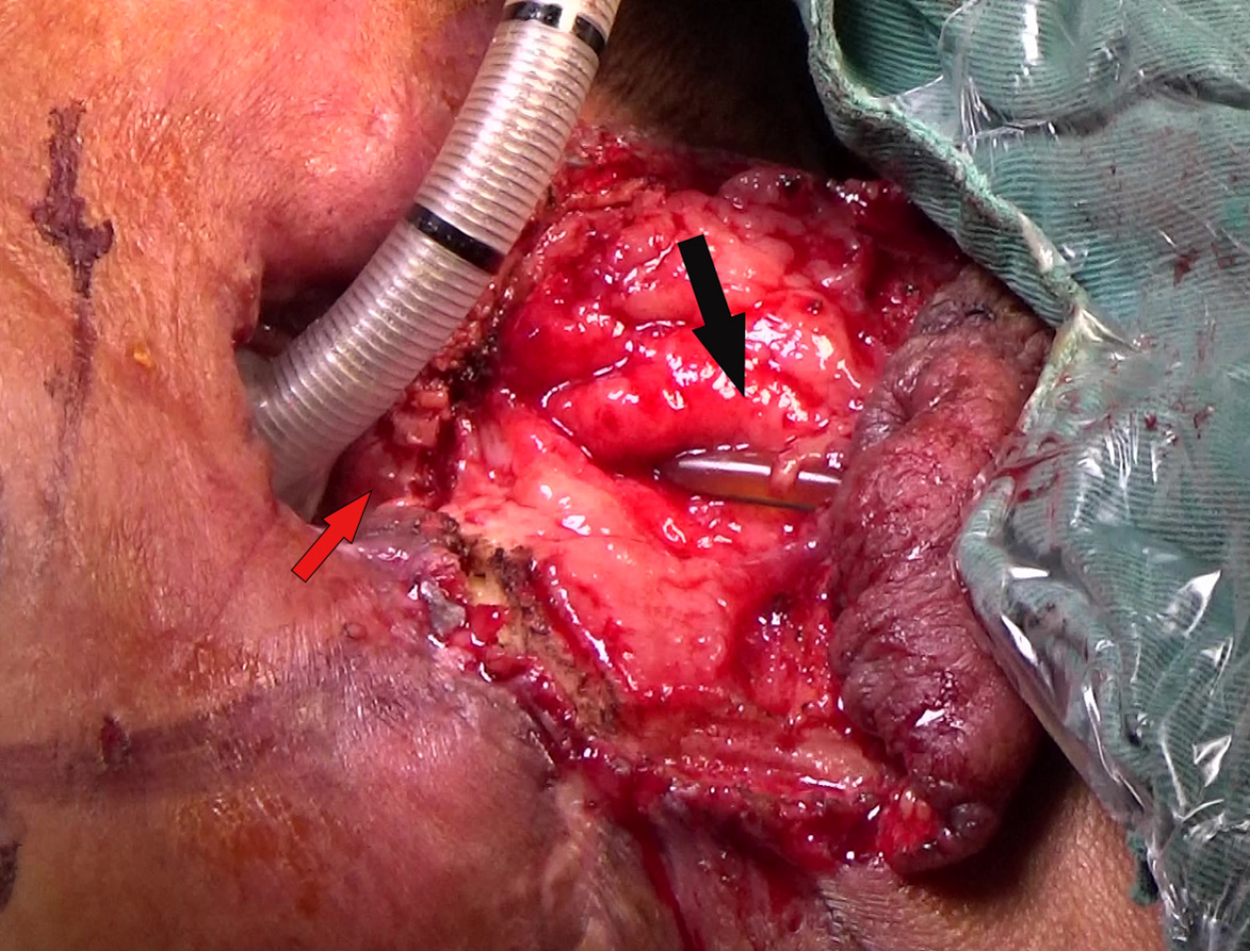
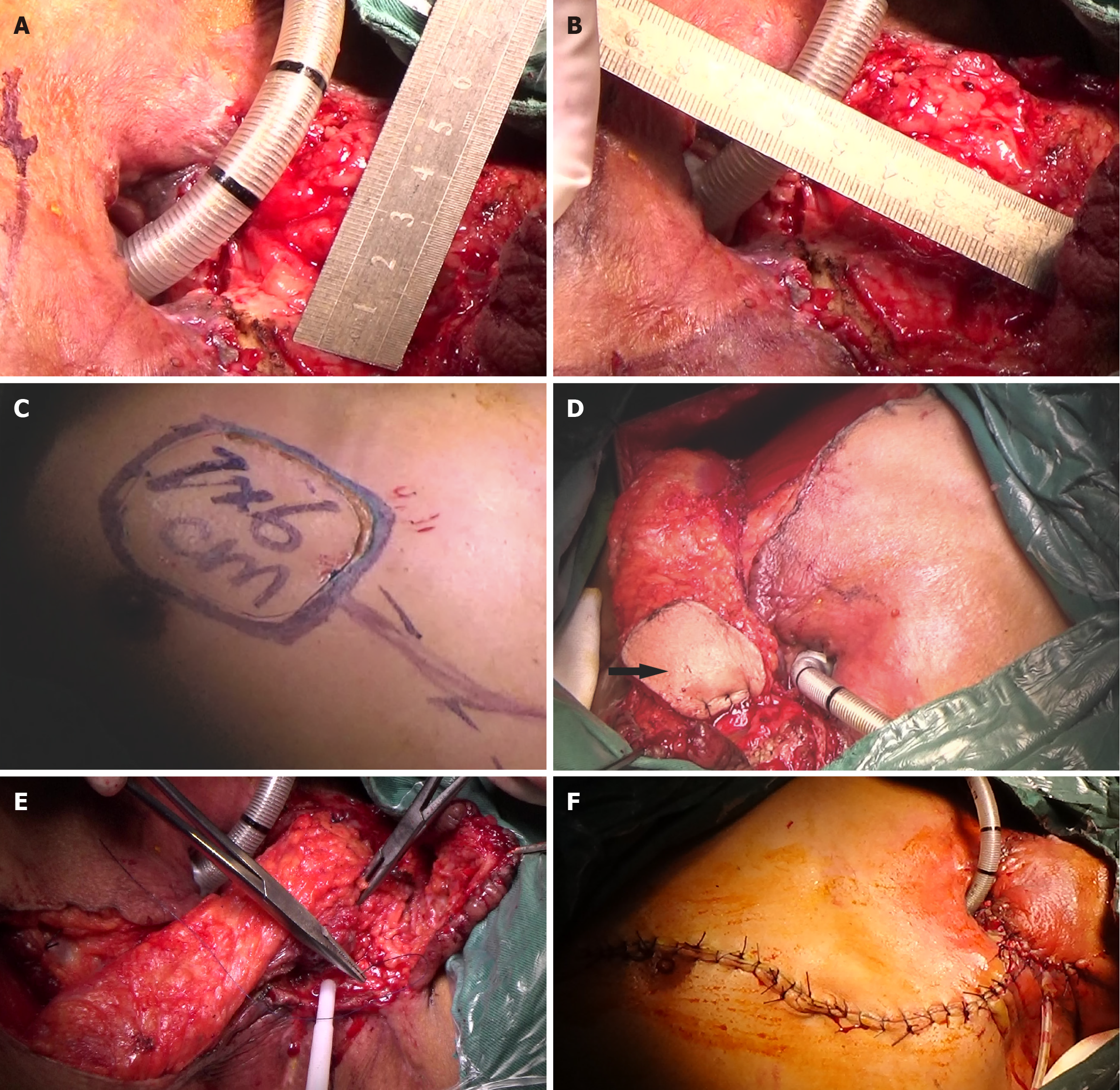
The results of intraoperative biopsy and frozen pathology with the patient under general anesthesia revealed moderate dysplasia in the squamous epithelium, with small regions for cancer invasion. In addition, small pieces of tracheal wall tissue and granulation tissue were also observed. Total pharyngolaryngoesophagectomy (PLE) (Figure 3A and B), left thyroidectomy (Figure 3C), bilateral cervical lymphadenectomy, and three-field lymphadenectomy were performed. During surgery, a TEF about 2 cm in length was confirmed. The cervical esophagus was closely adherent to the tracheal membrane and the surrounding tissues (Figure 3C). About 5 cm (at the second thoracic spine level) of the tracheal membrane were removed (Figure 3D-F), and the esophagus was carefully separated until it communicated with the mediastinum. The tubular stomach was then anastomosed to the posterior pharyngeal wall and the base of the tongue (Figure 3G and H), with lateral reinforcement using a pedicled strap muscle flap. A 7 cm × 5 cm SCAIF was harvested from the left shoulder to repair the tracheal membrane defect, and a permanent tracheostomy was performed (Figure 4). Postoperative routine pathological results confirmed squamous cell carcinoma in the cervical esophagus.
Acute myocardial infarction and management: After surgery, the patient was transferred to the intensive care unit (ICU) and returned to the ward on postoperative day 3. The patient experienced cardiovascular complications (60%-70% stenosis), which were managed with cardiovascular interventions and medication. Intensive care was necessary for respiratory failure, circulatory stability, and nutritional needs.
On postoperative day 3, the patient was returned to the ward with a consistently high heart rate (averaging 120 beats/min, peaking at 140 beats/min) and chest discomfort. Laboratory tests showed elevated liver enzymes and cardiac markers. ECG revealed sinus tachycardia with ST segment and T wave changes, raising concerns about acute myocardial infarction. Despite high-flow oxygen therapy, his oxygen saturation remained low (around 70%), and he was moved back to the surgical ICU for critical care.
The patient initially refused coronary artery intervention (CAI) but consented after 6 days of anticoagulant therapy and platelet aggregation inhibitors. CAI revealed severe stenosis in the right coronary artery. Percutaneous coronary intervention (PCI) of the right coronary artery was performed, with a 2.5 mm × 15 mm balloon and a 2.75 mm × 33 mm stent. Post-procedure angiography showed full stent expansion with TIMI grade 3 blood flow. The patient became stable and transferred to our department.
Blood circulation instability and management: After coronary stent implantation, the patient showed a significant drop in hemoglobin level. Continuous blood transfusions were administered, and hemoglobin increased gradually from
Respiratory circulation instability and management: The patient’s minimum oxygen saturation was around 70% after the second ICU transfer. He remained on a ventilator with sedation, with maintenance of 100% oxygen saturation. After 27 days of ventilator support, he was weaned off the ventilator with stable oxygen saturation and respiratory function, and transferred back to our ENT department after 28 days of treatment in the ICU. Two days later, however, he presented with headache, urinary incontinence, and drowsiness. Although his oxygen saturation remained unstable, he was readmitted to the ICU after discussion among the MDT. After 5 days of treatment in the ICU, he was transferred back to our ENT department.
Serious infection and management: The patient tested positive for severe acute respiratory syndrome coronavirus 2 RNA upon admission but was symptom-free after 7 days of treatment. During the ICU stay, the patient had fever and elevated inflammatory markers. Initial sputum culture showed the presence of Pseudomonas cepacia. After 5 days of antibiotic therapy with imipenem and cilastatin sodium, mixed infection with P. cepacia, Acinetobacter baumannii, and carbapenem-resistant Klebsiella pneumoniae (CRKP) was found. Blood cultures also showed CRKP. A combination of antibiotics (polymyxin, sulfamethoxazole, and linezolid) was administered, which led to normalization of temperature and inflammatory markers after 10 days. The antibiotic regimen was adjusted to colistin sulfate and tigecycline, which was maintained until discharge. Neck pus cultures consistently showed no bacterial growth.
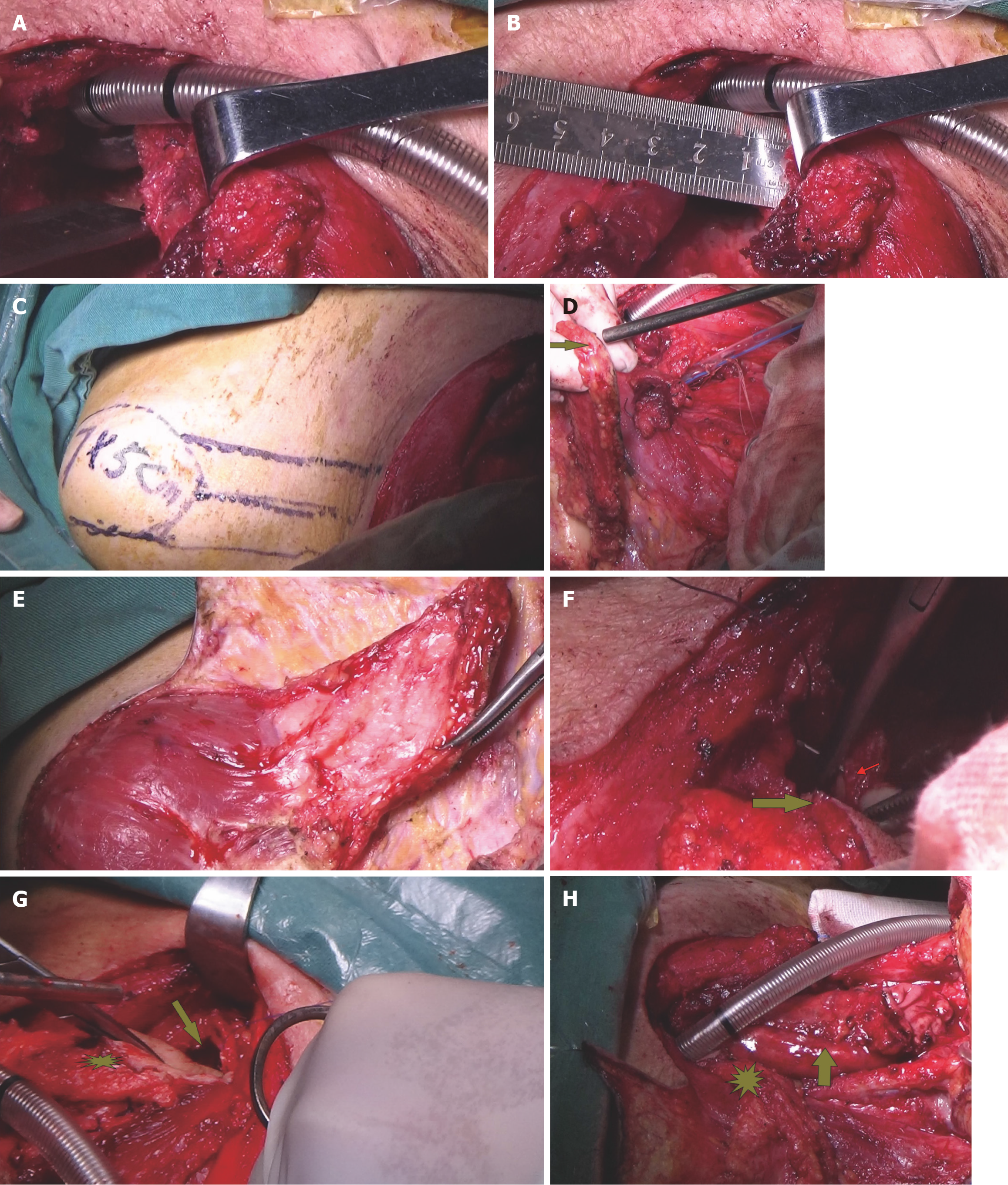
Partial gastric conduit necrosis and management: On postoperative day 12, infection and purulent secretions were found in the left neck. After clearing the purulent secretions, partial gastric conduit necrosis in the neck was found and necrotic tissue was cut out resulting in a 3 cm × 5 cm circumferential defect in the neck (Figure 5). Three days later, a MDT discussion was held, which included the departments of general thoracic surgery, infectious disease, cardiovascular medicine, the cardiovascular ultrasound center, and our ENT department. The patient’s general condition was considered to be poor. He had myocardial infarction just after hospitalization, underwent right coronary artery PCI, and a stent was implanted. Treatment of coronary artery thrombosis with double anticoagulation therapy resulted in a concomitant significant decrease in hemoglobin and multiple organ failure, which made it impossible to perform surgery. It was recommended to strengthen organ support therapy and anti-infection therapy.
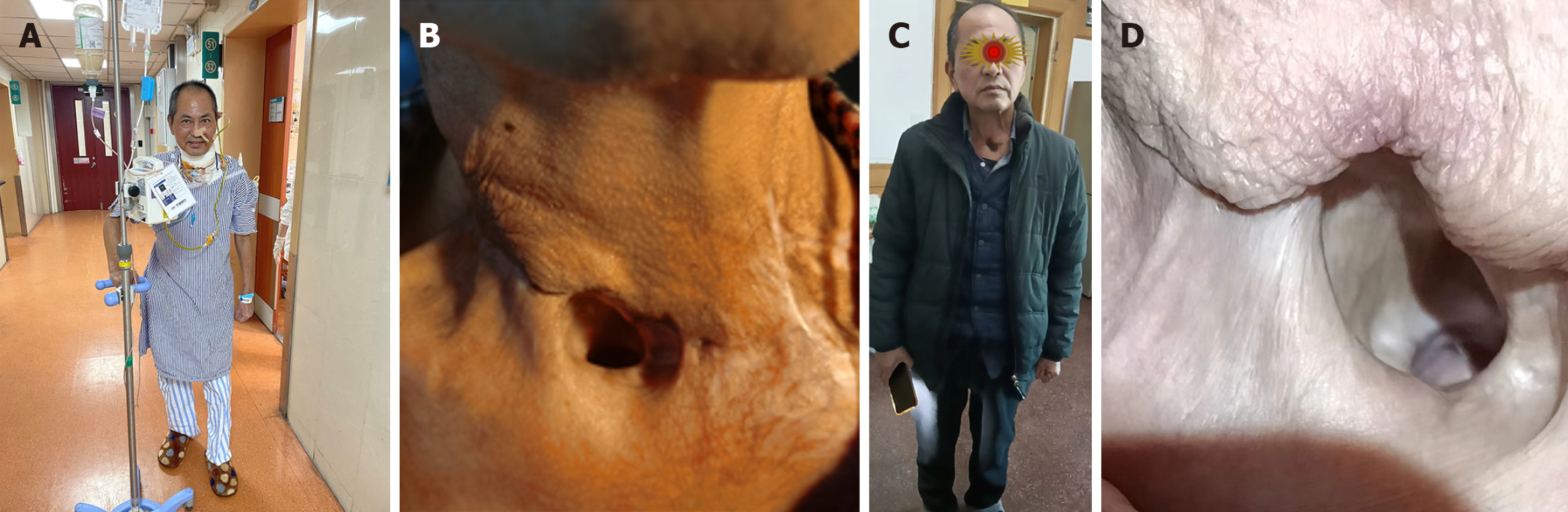
After treatment, the patient’s body temperature, inflammatory indicators, vital signs, and general condition improved. A MDT discussion was held again on March 8, 2023, and further surgery was considered. On March 9, 2023, a 7 cm × 6 cm pectoralis major myocutaneous flap (PMMF) was used to repair the defect caused by partial gastric conduit necrosis in the neck. The U-shaped PMMF was anastomosed inward with the broken end of the esophagus, the side wall of the pharynx, and the base of the tongue to close the pharyngeal cavity (Figure 6). Three days later, a fistula measuring approximately 1 cm was found at the left neck. There were no bacteria in cultures of the secretions. The pharyngeal fistula healed 1 month after PMMF, and the patient was able to consume fluids. The patient finally recovered after 112 days in hospital (Figure 7A and B).
Due to dysphagia, the patient was only able to consume a semi-fluid diet 4 months after discharge after anastomotic dilation five times under gastroscopy at the department of gastroenterology of our hospital. At follow-up on March 20, 2025 (follow-up period, 27 months), the patient had no recurrence or metastasis, could consume a semi-fluid diet, and had gained significant weight (Figure 7C and D).
TEF often arises in patients with advanced esophageal cancer managed with dCRT. ESCC usually involves the trachea, and the percentages of patients developing tracheal invasion and TEF, which are considered life-threatening, are 20–36% and 5–10%, respectively[8]. Managing TEF during dCRT is challenging due to recurrent aspiration pneumonia, dy
Reconstructive surgery for TEF depends on various factors, including the surgeon’s discretion, the type of reconstruction, the area and dimensions of the defect, the likelihood of achieving complete tumor removal, donor site availability, and vascular accessibility[20]. The selection of flap type is influenced by the patient’s capacity to endure prolonged anesthesia and the condition of neck vessels after previous treatment, such as surgery or CRT. Aesthetic considerations, such as color matching for cutaneous skin defects and the demand for complex tissue folding and bulk, are also important[21]. Management of TEF involves stent insertion and surgical interventions, including resection, bypass surgery, and feeding gastrostomy[13]. Other methods, including reconstruction, are dependent on the patient’s condition[20,22].
In patients managed with CRT, TEF is best treated with esophageal stenting, taking the tumor region into consideration[7,23]. However, in patients with aspiration pneumonia, surgical resection is the primary option after managing pneumonia[7]. Patients with dysphagia and aspiration after CRT are suboptimal candidates for reconstructive surgery. For recurrent CEC after CRT, stent implantation can alleviate dyspnea and dysphagia, thus improving the patient’s quality of life. However, it can contribute significantly to poor healing of the fistula[23], and tumor progression leads to poor prognosis, with an average survival of only 6 weeks[23].
Here, we reported a patient with recurrent CEC after dCRT who developed TEF and was managed by total PLE and gastric pull-up with SCAIF reconstruction after MDT discussion. After 24 months, the patient had no recurrence or metastasis, could consume a semi-fluid diet, and had gained significant weight. To our knowledge, only two other cases of radical surgery for TEF caused by CEC after chemotherapy or dCRT have been reported. The first case was caused by hypopharyngeal and CEC after one course of chemotherapy[8]. reported using a permanent tracheostomy and induction chemotherapy instead of CRT combined with nutritional supplements via a nasogastric tube for hypopharyngeal cancer and CEC. In their case, cervical TEF was identified due to marked shrinkage of the tumor with chemotherapy, and chemotherapy was continued despite the complications. They then performed PLE and subtotal stomach reconstruction to treat the TEF. The therapeutic outcome was satisfactory and their patient remained alive with no recurrence at 9 years postoperatively[8]. In the second case, Bolger et al[14] reported recurrent CEC after 10 years of dCRT. The patient also received PLE with gastric pull-up. The patient’s health improved significantly, with good recovery, and was discharged home on day 14, achieving adequate oral nutrition with supplemental jejunal tube feeding[14]. These cases show that surgery is an effective salvage procedure for local recurrence or TEF after dCRT.
Stenting is also effective for TEF caused by CEC sensitive to CRT. One case involved stent insertion for TEF after 1 week of induction CRT, with the patient remaining cancer-free after 2 years[15]. Another involved dual stenting for TEF of CEC and thoracic esophageal cancer following definitive radiotherapy[16].
Repairing defects in the lower trachea presents significant challenges, especially when involving membranous defects of the intrathoracic trachea. The limited mediastinal space complicates surgical procedures. If the TEF resulting from CEC is situated below the upper margin of the sternum, tracheal intubation may be insufficient[8]. Other approaches include distal tracheostomy or even sternotracheal stoma creation or placement of a stent, which can lead to a substantial decline in the patient’s quality of life.
In our case, we introduced SCAIF to repair tracheal defects with a permanent tracheostomy above the manubrium of the sternum. To our knowledge, this is the first report in the English literature of use of a SCAIF to connect the tracheal stump located in the mediastinum to create a permanent tracheostomy. This repair may improve the patient’s pos
Reconstructive options include the deltopectoral flap, which was introduced for permanent tracheostomy recon
In contrast, SCAIF is an alternative option for reconstruction with minimal complications compared to other approaches. It has been noted that SCAIF does not require microsurgical techniques, decreasing operating time and minimizing the requirement for postoperative care, which is beneficial in complex cases involving fistulas due to radiotherapy damage. It has been reported that the surgical duration for SCAIF flaps is shorter than for flaps such as RFFF and ALTF. Microsurgery requires highly specialized surgical techniques and advanced settings, which are not always available, making SCAIF a reliable option[21,25].
Donor site morbidity remains a critical consideration, as it may significantly influence both clinical outcomes and quality of life. In our case, harvesting a SCAIF (up to 8 cm in width, such as the 5-cm flap used) allowed primary closure of the donor site without significant deformity. Although suture tension can lead to widened scars, patients generally report positive outcomes and satisfaction during follow-up care[25]. Recent studies have emphasized the significance of flap vascularity and tension-free inset techniques, demonstrating that careful design and appropriate flap size selection are crucial for minimizing complications and ensuring flap viability in head and neck reconstruction[26,27].
Gastric pull-up also plays a significant role in this surgery salvage treatment. Ebeling et al[28] showed that a myo
Total esophagectomy with gastric tube reconstruction remains the most widely used form of surgical management for esophageal cancer. However, it is not always effective or safe due to its potential complications, including leakage, stenosis, and fistula formation, which can occur due to a decrease in blood flow at the distal end of the tube. Posto
Unfortunately, on postoperative day 12, our patient was found to have partial gastric conduit necrosis in the neck, further complicated by a history of COVID-19 prior to the operation and an acute myocardial infarction on postoperative day 9.
Coronary angiography revealed significant stenosis (60%-70%) and occlusions in multiple coronary arteries. The right coronary artery was nearly completely occluded, and a minimally invasive drug-eluting stent was placed at the right coronary lesion leading to successful placement. The patient underwent anticoagulation and antiplatelet therapy, with a significant drop in hemoglobin and unstable blood pressure (maintained with norepinephrine). The neck had multidrug-resistant bacteria (CRKP, P. cepacia), respiratory circulation instability, and a blood culture positive for CRKP. The patient’s poor general condition made surgical repair of partial gastric conduit necrosis a significant challenge. After continued supportive treatment, the patient’s condition stabilized, and fresh granulation tissue grew at the neck wound.
Following another MDT discussion, it was deemed feasible to perform surgical treatment for the neck defect resulting from partial gastric necrosis. Reconstructive options, such as a muscle flap, were considered[33]. We selected a PMMF for reconstruction of the necrotic conduit based on several considerations. First, the patient’s physical condition had just recovered and was not suitable for a long-duration surgery. Second, the patient had undergone preoperative CRT, and the neck vessels may not have been suitable for transplantation of a free flap. Third, PMMF is close to the neck, has a clear blood supply and drainage vessels, and its postoperative failure rate is lower than that of a free flap.
In this case, we chose to perform reconstruction using a U-shaped PMMF as described by Morshed et al[34] rather than the tubular PMMF used by Barter et al[9]. The U-shaped PMMF offers several advantages, as described by Chu et al[35]; for example, it can reduce the rate of stenosis of the neopharynx by increasing the diameter of the neopharynx. It is particularly suitable for patients with multiple severe postoperative complications, as in our case, because of its shorter operative time, ease of manipulation, lack of need for microsurgical techniques, and lower risk of postoperative complications. The U-shaped PMMF is also particularly suitable for large circumferential defects in the neck after radiotherapy[36]. Although the patient had a small pharyngeal fistula, it healed with conservative treatment through dressing within 1 month after PMMF repair surgery.
This case showed that SCAIF and gastric pull-up are suitable for effective management of TEF in post-CRT patients with CEC. The assembly of a MDT was crucial for management of the post-surgical challenges, ultimately achieving optimal results, effective treatment, and improved quality of life. Adopting this approach will enable head and neck surgeons to implement these techniques more broadly in similar clinical settings.
| 1. | Mendenhall WM, Sombeck MD, Parsons JT, Kasper ME, Stringer SP, Vogel SB. Management of Cervical Esophageal Carcinoma. Semin Radiat Oncol. 1994;4:179-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 2. | Hoeben A, Polak J, Van De Voorde L, Hoebers F, Grabsch HI, de Vos-Geelen J. Cervical esophageal cancer: a gap in cancer knowledge. Ann Oncol. 2016;27:1664-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Grass GD, Cooper SL, Armeson K, Garrett-Mayer E, Sharma A. Cervical esophageal cancer: a population-based study. Head Neck. 2015;37:808-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Ajani JA, D'Amico TA, Bentrem DJ, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Farjah F, Gerdes H, Gibson M, Grierson P, Hofstetter WL, Ilson DH, Jalal S, Keswani RN, Kim S, Kleinberg LR, Klempner S, Lacy J, Licciardi F, Ly QP, Matkowskyj KA, McNamara M, Miller A, Mukherjee S, Mulcahy MF, Outlaw D, Perry KA, Pimiento J, Poultsides GA, Reznik S, Roses RE, Strong VE, Su S, Wang HL, Wiesner G, Willett CG, Yakoub D, Yoon H, McMillian NR, Pluchino LA. Esophageal and Esophagogastric Junction Cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21:393-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 229] [Reference Citation Analysis (0)] |
| 5. | Fu Y, Yang T, Liang P, Deng X. A Case of Asymptomatic Tracheoesophageal Fistula. Ear Nose Throat J. 2023;1455613231200810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Buchanan ME, Fishman EK, Azadi JR. CT Evaluation of the Esophagus: The Role of CT Imaging and CT Imaging Findings in Diagnosing Esophageal Abnormalities. Curr Probl Diagn Radiol. 2023;52:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Kakuturu J, Dhamija A, Toker A. Malignant tracheoesophageal fistula: diagnosis and management. Curr Chall Thorac Surg. 2022;4:29-29. [DOI] [Full Text] |
| 8. | Ohno S, Tanaka Y, Sato Y, Endo M, Asai R, Fukada M, Yasufuku I, Okumura N, Takahashi T, Matsuhashi N. A case of advanced hypopharyngeal cervical esophageal cancer treated by curative resection with management of tracheoesophageal fistula. Clin J Gastroenterol. 2023;16:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Barter L, Forner D, French DG, Ednie A, Darling GE, Rigby MH. Role of the Pectoralis Major Muscle Flap in the Multidisciplinary Treatment of Esophageal Cancer. Plast Reconstr Surg Glob Open. 2024;12:e6290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Zhang Y, Liu Y, Sun Y, Xu M, Wang XL. Local random flaps for cervical circumferential defect or tracheoesophageal fistula reconstruction after failed gastric pull-up: Two case reports. World J Clin Cases. 2021;9:10328-10336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Ramella V, Ferrari A, Novati FC, Arnež ZM, Marchi G, Rodda A, Bottosso S, Papa G. Secondary Microsurgical Reconstruction of the Cervical Esophagus: Safer Flaps and Practical Tips in a Challenging Situation. J Clin Med. 2024;13:2726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Mullan D, Shepherd D, Li A, Laasch H, Najran P. Minimally Invasive Treatment Strategies for Tracheoesophageal Fistulae. Dig Dis Interv. 2018;02:011-017. [DOI] [Full Text] |
| 13. | Chen YH, Li SH, Chiu YC, Lu HI, Huang CH, Rau KM, Liu CT. Comparative study of esophageal stent and feeding gastrostomy/jejunostomy for tracheoesophageal fistula caused by esophageal squamous cell carcinoma. PLoS One. 2012;7:e42766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Bolger JC, Gilbert R, Yeung JC. Complex Resections: Management of Cervical Squamous Cell Carcinoma. Foregut: The Journal of the American Foregut Society. 2024;4:158-163. [DOI] [Full Text] |
| 15. | Lee CC, Yeo CM, Ng WK, Verma A, Tey JC. T4 cervical esophageal cancer cured with modern chemoradiotherapy: A case report. World J Clin Cases. 2020;8:1950-1957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Reading D, Burmeister B. Malignant Broncho-Oesophageal Fistula following Radiotherapy for Noninvasive T3 Oesophageal Squamous Cell Carcinoma. Case Rep Gastroenterol. 2022;16:333-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Liu J, Deng T, Li C, Peng L, Li Q, Zhu G, Wang W, Cai Y, Lan X, He Y, Wang Z, Wang S. Reconstruction of Hypopharyngeal and Esophageal Defects Using a Gastric Tube after Total Esophagectomy and Pharyngolaryngectomy. ORL J Otorhinolaryngol Relat Spec. 2016;78:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Spiegel JL, Pilavakis Y, Weiss BG, Canis M, Welz C. Quality of life in patients after reconstruction with the supraclavicular artery island flap (SCAIF) versus the radial free forearm flap (RFFF). Eur Arch Otorhinolaryngol. 2019;276:2311-2318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Granzow JW, Li AI, Boyd JB, Van Natta TL. Use of Dual, Tubularized Supraclavicular Artery Island Flaps in Series to Restore Gastrointestinal Continuity After a Failed Ileocolic Esophageal Reconstruction. Ann Plast Surg. 2015;75:306-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Kremer T, Gazyakan E, Maurer JT, Ott K, Gerken A, Schmittner M, Ronellenfitsch U, Kneser U, Nowak K. Intra- and Extrathoracic Malignant Tracheoesophageal Fistula-A Differentiated Reconstructive Algorithm. Cancers (Basel). 2021;13:4329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Kokot N, Kim JH, West JD, Zhang P. Supraclavicular Artery Island Flap: Critical Appraisal and Comparison to Alternate Reconstruction. Laryngoscope. 2022;132 Suppl 3:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Dewey EH, Castro JR, Mojica J, Lazarus CL, Su HK, Alpert EH, Dos Reis LL, Urken ML. Reconstruction of expanding tracheoesophageal fistulae in post-radiation therapy patients who undergo total laryngectomy with a bipaddled radial forearm free flap: Report of 8 cases. Head Neck. 2016;38 Suppl 1:E172-E178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Kim HS, Khemasuwan D, Diaz-Mendoza J, Mehta AC. Management of tracheo-oesophageal fistula in adults. Eur Respir Rev. 2020;29:200094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 24. | Escalante D, Vincent AG, Wang W, Shokri T, Ducic Y. Reconstructive Options During Nonfunctional Laryngectomy. Laryngoscope. 2021;131:E1510-E1513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Nikolaidou E, Pantazi G, Sovatzidis A, Vakouli S, Vardaxi C, Evangelopoulos I, Gougousis S. The Supraclavicular Artery Island Flap for Pharynx Reconstruction. J Clin Med. 2022;11:3126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Kadota H, Imai Y. Safe and reliable use of supraclavicular flaps for head and neck reconstruction. J Craniofac Surg. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Ruffin W, Gal TJ. Impact of Flap Size and Comorbidities on Supraclavicular Artery Island Flap Outcomes. OTO Open. 2024;8:e175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Ebeling O, Albicker S, Sutter T, Panidis T, Lindemann W. Interdisciplinary cooperation using the example of gastric pull up for pharyngeal replacement. Laryngo-Rhino-Otologie. 2023;. [DOI] [Full Text] |
| 29. | Kawai KI, Kakibuchi M, Sakagami M, Fujimoto J, Toyosaka A, Nakai K. Supercharged gastric tube pull-up procedure for total esophageal reconstruction. Ann Plast Surg. 2001;47:390-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Fujioka M, Taniguchi K, Yoneda A, Fukui K, Yoshino K, Idemitsu M. Additional superdrainage reduces anastomotic fistula and stenosis after gastric tube reconstruction with cervical anastomosis for esophageal cancer. JTCVS Tech. 2023;19:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 31. | Wormuth JK, Heitmiller RF. Esophageal conduit necrosis. Thorac Surg Clin. 2006;16:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Nusrath S, Raju KVVN, Nekkanti SS, Basudhe M, Thammineedi SR. Successful Salvage of Partial Gastric Conduit Necrosis by Primary Anastomosis in a Post-Esophagectomy Patient. Indian J Surg Oncol. 2024;15:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Athanasiou A, Hennessy M, Spartalis E, Tan BHL, Griffiths EA. Conduit necrosis following esophagectomy: An up-to-date literature review. World J Gastrointest Surg. 2019;11:155-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Morshed K, Szymański M, Gołabek W. Reconstruction of the hypopharynx with U-shaped pectoralis major myocutaneous flap after total pharyngo-laryngectomy. Eur Arch Otorhinolaryngol. 2005;262:259-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Chu YH, Lai WS, Lin YY, Liu SC, Lee JC. Pharyngeal reconstruction using a U-shaped pectoralis major myocutaneous flap: an effective technique that should not be forgotten. Eur Arch Otorhinolaryngol. 2020;277:217-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Saussez S, Cuno A, Urbain F, Chantrain G, Lequeux T. Reconstruction of circumferential oro- and hypopharyngeal defects with U-shaped pectoralis major myocutaneous flap. Otolaryngol Head Neck Surg. 2006;134:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |