Published online Aug 24, 2025. doi: 10.5306/wjco.v16.i8.109206
Revised: May 19, 2025
Accepted: July 2, 2025
Published online: August 24, 2025
Processing time: 109 Days and 22.5 Hours
Cutaneous melanoma is an aggressive skin cancer with high metastatic potential. Accurate staging is critical to guide therapeutic strategies and improve prognosis. Whole-body magnetic resonance imaging (WB-MRI), particularly when combined with diffusion-weighted imaging (DWI), has emerged as promising tool for comprehensive, radiation-free assessment of metastatic spread.
To systematically review the diagnostic performance and clinical utility of WB-MRI in the staging and restaging of cutaneous melanoma, with comparison to conventional imaging modalities such as computed tomography (CT) and po
A systematic literature review was conducted using PubMed, Embase, Scopus and Web of Science databases for studies published in the last 10 years. Inclusion criteria focused on comparative diagnostic accuracy studies of WB-MRI vs CT and PET/CT for melanoma staging. The methodological quality of the studies was appraised using the QUADAS-2 tool.
Sixteen studies involving over 700 patients met the inclusion criteria. WB-MRI showed high sensitivity (73%-90%) and specificity (up to 98%) in detecting metastases, particularly in bone, liver and soft tissue. DWI enhanced lesion detection, and WB-MRI often influenced clinical management decisions. Ho
WB-MRI represents a robust imaging modality for staging cutaneous melanoma, offering superior soft-tissue contrast and functional imaging without ionizing radiation. Its strengths lie in detecting bone, liver and brain metastases. Challenges include limited lung lesion detection, cost, and availability. Advances in artificial intelligence, Hybrid PET/MRY systems, and radiomics are poised to expand WB-MRI’s role in personalized melanoma management.
Core Tip: Whole body magnetic resonance imaging (MRI) is reshaping melanoma staging by providing high diagnostic accuracy without ionizing radiation. This review outlines how recent advances in diffusion imaging have enhanced lesion detection, particularly in bone, liver and brain while highlighting current limitations in pulmonary assessment and future directions such as hybrid positron emission tomography/MRI and molecular imaging for personalized oncology.
- Citation: Russo A, Marinelli L, Patanè V, Alessandrella M, Pezzella MC, Troiani T, Brancaccio G, Scharf C, Argenziano G, Cappabianca S, Reginelli A. Whole-body magnetic resonance imaging for cutaneous melanoma staging: A scientific review. World J Clin Oncol 2025; 16(8): 109206
- URL: https://www.wjgnet.com/2218-4333/full/v16/i8/109206.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i8.109206
Cutaneous melanoma is an aggressive skin cancer arising from melanocytic cells, with a strong tendency for lymphatic and haematogenous dissemination[1-3]. Its incidence has been increasing globally, particularly in fair-skinned populations exposed to high ultraviolet radiation[4,5]. In 2023, the American Cancer Society projected nearly more than 90000 incident diagnoses nationwide[6,7], highlighting the growing need for improved diagnostic and staging tools. Accurate staging is fundamental to melanoma management, guiding treatment strategies and determining eligibility for systemic therapies[8,9]. The staging framework established by the American Joint Committee on Cancer stratifies disease progression according to Breslow depth, presence of ulceration, regional lymphatic involvement, and evidence of distant metastasis[10-12]. The progression from early-stage disease (I-II), typically addressed through surgical intervention, to advanced stages (III-IV) necessitates a therapeutic transition toward systemic treatments, such as immune checkpoint inhibitors and BRAF/MEK-targeted agents[12]. Crucial in disease status assessment, imaging serves as a fundamental component of the oncologic diagnostic workflow. Conventional modalities (computed tomography (CT), positron emission tomography/CT (PET/CT) commonly used in daily practice, provide rapid whole-body assessment; however, they are associated with certain limitations[13-15].
CT provides limited sensitivity in detecting micro-metastases, particularly in bone and liver, while PET/CT-although highly sensitive for metabolically active lesions-shows reduced utility in regions such as the brain owing to the naturally elevate avidity for glucose carriers (FDG)[16-19]. As an emerging imaging modality, whole-body magnetic resonance imaging (WB-MRI) offers high soft-tissue contrast and the significant benefit of being free from ionizing radiation, positioning it as a compelling alternative to conventional techniques[20-23]. When combined with diffusion-weighted imaging (DWI), WB-MRI enables functional assessment based on water molecule diffusivity, enhancing detection of high-cellularity metastatic lesions, especially in liver and bone. Contrast-enhanced sequences can further improve lesion delineation[24-27].
Clinically, WB-MRI is particularly suited for young patients, pregnant women, and individuals requiring repeated imaging. Despite its advantages, challenges remain-namely, longer acquisition times, higher cost, and the need for specialized interpretation skills[28-32]. Nevertheless, emerging techniques such as artificial intelligence (AI), radiomics, and hybrid PET/MRI platforms are rapidly expanding its capabilities[33-36].
This systematic review synthesizes current evidence on WB-MRI’s performance in staging and restaging cutaneous melanoma, with a focus on diagnostic accuracy, comparative efficacy vs CT and PET/CT, and discuss emerging innovations that may further enhance its clinical utility. By delineating the role of WB-MRI in the modern oncologic armamentarium, we seek to elucidate its potential in refining the precision of melanoma management.
To consolidate current knowledge, a structured analysis of the published literature was undertaken following systematic review methodology using studies published in peer-reviewed journals, focusing on the role of WB-MRI in the staging and restaging of Metastaic Melanoma. Databases such as PubMed, Scopus, Embase, WoS (Web of Science) were searched focusing on relevant keywords, including "whole-body MRI" and their abbreviations, “WB-MRI” AND "melanoma staging", AND "Whole body diffusion-weighted imaging", and their abbreviations, “WB-DWI MRI” AND "PET-CT vs MRI", “cancer staging” AND “cancer restaging” AND "oncological imaging". The systematic search of the literature was conducted between February and the first week of March 2025, targeting peer-reviewed studies published between 2014 and 2024.
Eligible studies were analysed at first by 2 independent reviewers (Marinelli L and Patanè V) and then collectively by the entire research group before proceeding and in case of disagreement between the 2 reviewers. A total amount of 131 studies were identified and screened according to these criteria.
The inclusion criteria for studies were: (1) Peer reviewed studies, with body of evidence derived from fresh published literature original studies, systematic revisions of the current literature, and pooled (meta analyses) published within the last 10 years; (2) Studies that directly compared WB-MRI with other imaging modalities for staging or restaging melanoma; and (3) Research that included patient outcomes and clinical impact assessments related to imaging findings.
The exclusion criteria included: (1) Studies focusing solely on preclinical or animal models; (2) Case reports and small sample-sized studies with limited statistical power; and (3) Research that did not differentiate between melanoma and other malignancies in imaging comparisons.
The final pool consists of 16 studies[23,37-50]. Data selection is summarized by the Prisma 2020 flowchart. Data extraction was performed using a standardized approach, and relevant information were categorized based on diagnostic accuracy, anatomical site detection, patient outcomes, and recommendations for clinical practice.
The methodological quality of the selected studies was appraised through the QUADAS-2 instrument, a standardized framework for evaluating the risk of bias in diagnostic accuracy research (Figure 1; Tables 1 and 2)[51]. The main sources of bias observed across the included studies were related to patient selection (e.g., retrospective designs or highly selected populations), variability in the index test application [e.g., heterogeneous magnetic resonance imaging (MRI) protocols or incomplete reporting of DWI sequence parameters], and inconsistencies in the reference standard (e.g., lack of uniform confirmatory imaging or histological validation). Flow and timing issues were less frequently encountered but present in studies where WB-MRI and comparator imaging were not performed within a clinically acceptable timeframe. These factors were considered during data interpretation. A summary of key sources of bias, including patient selection and reference standard variability, is provided in the narrative. Full risk-of-bias assessments are detailed in Tables 2 and 3.
| Study | Risk of bias | Applicability concerns | |||||
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Study 1 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Study 2 | High risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Study 3 | Low risk | Unclear risk | Low risk | Low risk | High risk | Low risk | Low risk |
| Study 4 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Study 5 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Study 6 | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear risk | Low risk |
| Study 7 | Low Risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Study 8 | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear risk | Low risk |
| Study 9 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Study 10 | Low risk | Low risk | Low risk | High risk | Low risk | Low risk | Low risk |
| Study 11 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Study 12 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Study 13 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Study 14 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Study 15 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Study 16 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Study | Patient selection (risk of bias) | Patient selection (applicability) | Index test (risk of bias) | Index test (applicability) | Reference standard (risk of bias) | Reference standard (applicability) | Flow and timing (risk of bias) |
| Study 1 | Low | Low | Low | Low | Low | Low | Low |
| Study 2 | High | Low | Low | High | Low | Low | Low |
| Study 3 | Low | High | Unclear | Low | Low | High | Low |
| Study 4 | Low | Unclear | High | Low | Low | Low | High |
| Study 5 | Low | Low | Low | Unclear | Low | Low | Low |
| Study 6 | Low | High | Low | Unclear | High | High | Low |
| Study 7 | Low | Low | High | Low | Low | Low | Low |
| Study 8 | Low | High | Unclear | Low | High | Low | Low |
| Study 9 | Low | Low | Low | High | Unclear | Unclear | Low |
| Study 10 | Low | Unclear | Low | Unclear | Low | Low | Low |
| Study 11 | Low | Low | High | Low | Low | Low | Low |
| Study 12 | Low | Low | Low | High | Low | Low | Low |
| Study 13 | Low | High | High | Low | Low | Low | Low |
| Study 14 | Low | Low | Low | High | Low | Low | Low |
| Study 15 | Low | High | Low | Low | High | High | Low |
| Study 16 | Low | Unclear | Unclear | Low | Low | Unclear | Low |
| Metastatic site | Sensitivity (%) | Specificity (%) |
| Bone | 85-95 | 90-98 |
| Liver | 80-90 | 92-97 |
| Brain | 90-95 | > 95 |
| Lung | 60-70 | 85-90 |
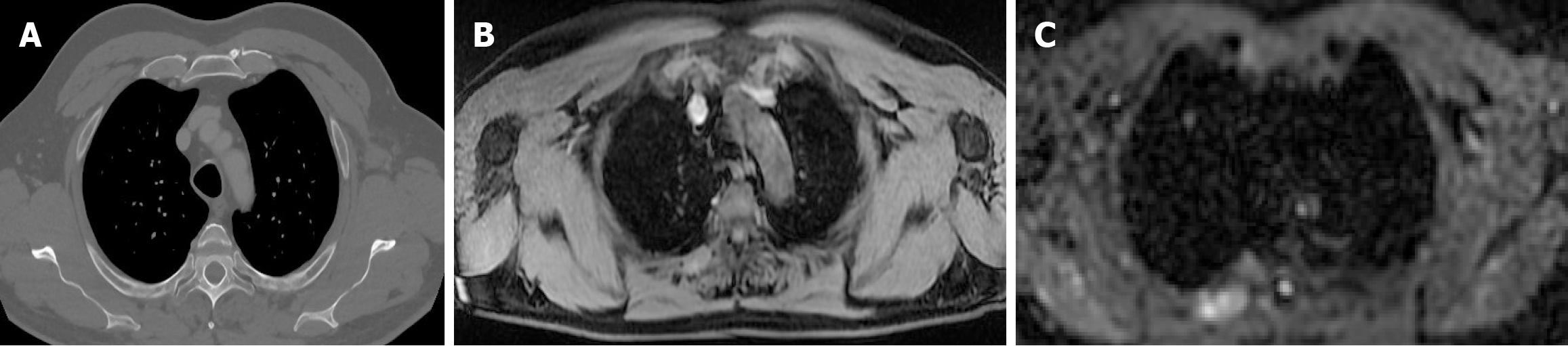
Imaging protocols were also reviewed, with attention to technical variables such as field strength (1.5T vs 3T), with studies, like Tunariu et al[26], suggesting that 3T scanners offer higher spatial resolution and improved signal-to-noise ratio-especially valuable for DWI-based assessments. However, 3T systems are more susceptible to artifacts, particularly in areas affected by magnetic field inhomogeneity such as the lungs. Sequence parameters (e.g., DWI, STIR, T1-weighted contrast-enhanced imaging), and scan duration also contribute significantly to diagnostic precision.
A total amount of publications were retrieved during the preliminary search phase, so the initial screening yielded 131 articles.
Fifty records were excluded for reasons including lack of a comparator imaging technique, focus not specific to melanoma, or non-comparative design. Following eligibility assessment, the final pool consists of 16 studies[23,37-50]. The selected studies were published between 2006 and 2024 and involved over 700 patients with advanced melanoma. Most studies compared WB-MRI (often with DWI) to CT or PET-CT.
Across the 16 studies, WB-MRI demonstrated high sensitivity (73%-90%) and specificity (up to 98%) for detecting melanoma metastases, particularly in bone, liver, and soft tissue. DWI significantly improved lesion conspicuity, outperforming CT and PET/CT in many extracranial regions. However, WB-MRI showed lower sensitivity for small pulmonary nodules, where CT remains superior. AI-based image interpretation and the use of contrast-enhanced sequences further improved diagnostic confidence. Some studies indicated that WB-MRI findings led to changes in clinical management in up to 24% of cases.
Although recent technological advances such as AI and hybrid PET/MRI platforms hold significant promise, the evidence supporting their use in melanoma imaging remains preliminary. A limited number of included studies specifically addressed the diagnostic contribution of AI-assisted interpretation or the clinical impact of PET/MRI. As such, these innovations are discussed as emerging trends rather than established standards. We have tempered the original language and now emphasize that further prospective studies are needed to validate their diagnostic and clinical utility in staging cutaneous melanoma.
AI applications in WB-MRI are currently being explored to enhance lesion detection, reduce interpretation variability, and streamline workflow; however, most published evidence derives from experimental or early-phase validation studies. Similarly, hybrid PET/MRI, while theoretically advantageous in combining metabolic and high-resolution anatomical data, has yet to be widely adopted in melanoma-specific protocols. These tools represent promising areas for future clinical integration but should be interpreted cautiously until more robust data are available.
The application of WB-MRI in melanoma staging has been the subject of extensive investigation. Numerous researchers demonstrated its capability to provide high sensitivity and specificity in detecting distant metastases. Comparative analyses with PET/CT have indicated that WB-MRI, particularly with DWI, offers superior performance in detecting bone, liver, and soft-tissue metastases, while PET/CT remains superior for pulmonary metastases. A meta-analysis encompassing multiple studies found that WB-MRI achieved a sensitivity of approximately 90% for extracranial metastases, whereas PET/CT demonstrated a slightly higher sensitivity for lung involvement. The ability of WB-MRI to detect metastases is further enhanced when combined with contrast-enhanced sequences and machine learning-assisted interpretations. Recent progress in AI integration within radiology has led to the emergence of deep learning-based algorithms aimed at enhancing lesion detection while minimizing false-positive findings. These models leverage large datasets to refine image interpretation, ultimately improving diagnostic accuracy and reliability. One of the key aspects of melanoma staging is the ability to differentiate between imaging modalities. CT is widely used due to its rapid acquisition time and accessibility; however, it lacks the functional imaging capability that WB-MRI provides. PET/CT, leveraging 18F-FDG uptake, has been the gold standard for detecting metabolically active tumors, yet it suffers from limitations in anatomical resolution, particularly in brain and hepatic metastases. WB-MRI, on the other hand, provides excellent soft-tissue contrast, allowing for improved lesion detection and characterization, especially in the liver, brain, and musculoskeletal system. Multiple investigations have assessed the relative diagnostic performance of WB-MRI vs PET/CT for the detection of metastases in distinct anatomical regions.
While PET/CT remains superior in detecting small lung nodules, WB-MRI has demonstrated higher accuracy in detecting bone and liver metastases. Additionally, PET/CT has been associated with false positives due to inflammatory processes, while WB-MRI provides superior specificity in distinguishing malignant from benign lesions. Different anatomical sites pose unique challenges for imaging. WB-MRI has shown superior performance in:
Bone metastases: Studies indicate that DWI-enhanced WB-MRI can detect osseous metastases with higher sensitivity than CT, particularly for early-stage bone involvement. The improved contrast resolution of MRI enables better delineation of cortical and medullary bone lesions compared to conventional CT scans.
Liver metastases: Contrast-enhanced WB-MRI outperforms PET/CT by providing a more detailed assessment of intrahepatic lesions. Given the high prevalence of hepatic metastases in advanced melanoma, enhanced soft-tissue differentiation and the integration of diffusion-weighted sequences make MRI particularly valuable in diagnostic settings.
PET/CT has limited utility in the brain due to physiological glucose uptake, whereas MRI remains the gold standard for detecting intracranial melanoma lesions. Standard MRI sequences such as T1-weighted contrast-enhanced imaging and FLAIR imaging remain indispensable for neuro-oncologic assessments.
Despite WB-MRI’s capabilities, CT remains the preferred modality for detecting small pulmonary nodules due to its higher spatial resolution. Nevertheless, ongoing research into motion-corrected MRI sequences is aiming to enhance WB-MRI’s diagnostic performance for pulmonary lesions.
To address site-specific diagnostic variability, the sensitivity and specificity of WB-MRI were stratified by metastatic location based on the included studies (Table 3).
WB-MRI, particularly when DWI and contrast-enhanced sequences were used, demonstrated improved lesion detection in bone and liver compared to CT and PET/CT. CT maintained higher performance in detecting small pulmonary metastases.
The implementation of WB-MRI in routine care is supported by its numerous clinical advantages. Unlike conventional imaging modalities [CT, positron emission tomography (PET), WB-MRI avoids the use of rays with ionizing properties, making it particularly suitable for repeated imaging in contexts such as long-term follow-up, pediatric oncology, and pregnancy. Moreover, WB-MRI provides superior visualization of soft-tissue structures compared to CT, enabling more accurate delineation of metastatic lesions and reducing diagnostic uncertainty. The inclusion of DWI enhances the ability to detect metastases based on differences in cellular density, improving the overall diagnostic yield. Unlike traditional MRI scans that focus on a single region, WB-MRI enables the assessment of multiple metastatic sites in a single examination, improving efficiency in staging assessments. PET/CT relies on metabolic activity for lesion detection, whereas WB-MRI is capable of identifying non-FDG-avid metastases, which is particularly important in cases where tumors exhibit metabolic heterogeneity. But WB-MRI has also some limitations. The spatial resolution of WB-MRI is lower than that of CT for detecting small lung lesions, necessitating multimodal imaging in cases where pulmonary involvement is suspected. Compared to CT and PET/CT, WB-MRI requires a longer scan duration, which may be challenging for some patients, particularly those with claustrophobia or difficulty remaining still for extended periods. The availability of WB-MRI is restricted due to higher equipment and operational costs compared to CT. Additionally, access to WB-MRI may be limited in community healthcare settings due to the need for specialized radiological expertise. Interpretation of WB-MRI, particularly with DWI, requires specialized radiological expertise, which may not be universally available. Training programs and AI-assisted tools are being developed to mitigate this issue and improve standardization in WB-MRI assessments.
The role of WB-MRI in oncology continues to expand, driven by advances in technology and a growing understanding of its diagnostic potential. As a non-invasive imaging modality, WB-MRI offers significant clinical benefits for melanoma staging, treatment monitoring, and disease surveillance. The incorporation of radiomics, deep learning driven lesion detection techniques, and functional imaging biomarkers has fundamentally transformed the clinical application of WB-MRI, marking a significant shift in diagnostic and prognostic approaches. One of the most compelling clinical impacts of WB-MRI is its ability to assess tumor burden and treatment response more accurately than conventional imaging. Melanoma patients undergoing systemic therapies, such including immunotherapeutic agents and precision-targeted drugs, such as BRAF and MEK inhibitors, require ongoing surveillance to monitor treatment efficacy. WB-MRI, particularly when enhanced with DWI and perfusion imaging, enables precise detection of subtle changes in tumor cellularity, vascularity, and metabolic activity, facilitating earlier identification of non-responders and guiding therapy modifications[49].
The rise of AI-driven diagnostic support has further enhanced the accuracy and efficiency of WB-MRI interpretation. AI models trained on large datasets have demonstrated the ability to improve lesion detection rates, reduce interobserver variability, and streamline workflow for radiologists. These models leverage advanced image segmentation, deep learning algorithms, and automated anomaly detection, making WB-MRI a more accessible and standardized imaging tool. In particular, AI has been instrumental in overcoming one of WB-MRI’s longstanding limitations: Long acquisition times and complex image interpretation. By automating key aspects of the image analysis process, AI integration has enabled real-time clinical decision support, significantly improving the speed and accuracy of melanoma staging.
Additionally, the emergence of radiomics and texture analysis has provided new avenues for quantitative assessment of tumor characteristics. Radiomic analysis extracts high-dimensional features from WB-MRI scans, capturing subtle variations in tumor morphology, heterogeneity, and microenvironmental properties. These features, when combined with machine learning models, can serve as predictive biomarkers for disease progression, oncologic relapse and evaluation of treatment outcomes.
Evidences suggest that radiomics-enhanced WB-MRI is capable of distinguishing aggressive melanoma subtypes from indolent disease, improving risk stratification and personalized treatment planning. Functional imaging approaches assessing tissue perfusion and contrast kinetics have significantly enhanced the oncologic utility of whole-body MRI. These techniques enable real-time assessment of tumor vascularity, angiogenesis, and perfusion characteristics, providing valuable insights into tumor behavior and response to anti-angiogenic therapies. Perfusion weighted imaging (PWI) has shown particular promise in differentiating viable tumor tissue from necrotic or fibrotic regions post-treatment, enabling refined evaluation of therapeutic outcomes.
A notable innovation in the field is the emergence of PET/MRI combined imaging, with comprehensive anatomical coverage, merging the functional avidity data from PET with the detailed anatomical resolution afforded by MRI. This hybrid approach enhances lesion detectability, particularly in challenging anatomical organs with high FDG avidity. Hybrid machines have demonstrated improved sensitivity in detecting early metastatic disease while reducing radiation exposure, making it an attractive alternative for long-term melanoma surveillance[44].
Furthermore, novel contrast agents and molecular imaging techniques are being developed to enhance the specificity of WB-MRI for melanoma detection. Ultra-small superparamagnetic iron oxide nanoparticles and targeted molecular probes have shown potential in improving lymph node metastasis detection, addressing a critical need in melanoma staging. These contrast agents enable highly detailed visualization of lymphatic drainage patterns and micro-metastatic disease, offering a more comprehensive assessment of disease spread. The integration of WB-MRI into multi-disciplinary oncologic management has profound implications for patient care. By providing a holistic view of disease burden, WB-MRI contributes to more precise tumor board discussions, improved treatment planning, and enhanced patient stratification for clinical trials. The adoption of standardized WB-MRI protocols across institutions is essential to maximize its clinical utility, ensuring consistent image acquisition, interpretation, and reporting guidelines[50].
Despite its numerous advantages, WB-MRI faces ongoing challenges, including accessibility, cost, and variability in image interpretation. However, ongoing research efforts, technological advancements, and AI-driven automation are poised to overcome these limitations, making WB-MRI an indispensable tool in modern oncology. Despite the clear diagnostic advantages of WB-MRI, widespread clinical adoption remains limited due to factors such as higher equipment costs, longer scan durations, and the need for experienced radiological interpretation. These challenges vary significantly by geographic region. For instance, WB-MRI is more commonly available in tertiary care or academic centers in Europe and North America, whereas its accessibility in community hospitals and low-to-middle income countries is more restricted. A recent Italian survey Albano et al[48] highlighted that WB-MRI is currently used in only a minority of oncology centers, and often reserved for select patient populations such as pediatric or pregnant patients.
Training programs for radiologists and ongoing development of AI-based interpretation tools are being pursued to mitigate expertise-related limitations. Furthermore, cost-effectiveness studies are needed to determine the long-term financial impact of integrating WB-MRI into routine melanoma surveillance, particularly in terms of reducing cumulative radiation exposure and enabling earlier detection of metastases.
Future studies should focus on refining AI-powered lesion classification, optimizing functional imaging protocols, and evaluating the long-term cost-effectiveness of WB-MRI in melanoma management. As precision medicine continues to evolve, WB-MRI is well-positioned to play a central role in personalized oncology, offering unparalleled insights into tumor biology, treatment response, and patient prognosis.
The emergence of radiomics-which involves extracting quantitative imaging biomarkers from MRI scans-has further propelled WB-MRI’s role in oncology. Through the extraction of voxel-level quantitative features, radiomics offers valuable information on intratumoral heterogeneity, therapeutic responsiveness, and the likelihood of disease pro
The comprehensive evaluation of cutaneous melanoma increasingly relies on WB-MRI as a robust diagnostic technique. As the incidence of melanoma continues to rise, the need for accurate, reliable, and non-invasive staging and surveillance strategies has never been more pressing. WB-MRI, particularly when enhanced with tissue diffusion (DWI) and contrast-kinetics sequences, offers a superior alternative to conventional imaging techniques, providing unparalleled soft-tissue contrast and functional imaging insights without the risks associated with ionizing radiation. One of the most significant advantages of WB-MRI is its ability to detect metastatic disease with high sensitivity and specificity across multiple anatomical regions. In particular, its superior performance in identifying bone, liver, and brain metastases has reshaped the standard imaging protocols for melanoma staging. Incorporating physiologic imaging parameters, like diffusion-based measurements from specialized MRI protocols (ADC), enables more accurate differentiation between benign and malignant lesions, ultimately enhancing diagnostic confidence and guiding clinical management. However, despite its growing acceptance in oncologic imaging, WB-MRI is not without limitations. Challenges such as longer acquisition times, higher costs, and limited availability in certain regions have restricted its widespread clinical adoption. Given its lower sensitivity for detecting small lung metastases compared to CT, WB-MRI often requires integration with other imaging techniques in cases of suspected thoracic spread. Overcoming these barriers requires further investment in technological advancements, infrastructure expansion, and training initiatives to enhance expertise in MRI interpretation. The evolution of WB-MRI is likely to be driven by its ongoing convergence with emerging innovations such as AI-based analytics, machine learning frameworks, radiomic profiling, and integrative imaging techniques. AI-driven image analysis has already demonstrated its potential in enhancing lesion detection accuracy, reducing interobserver variability, and optimizing scan protocols to minimize acquisition times. Radiomics, an emerging field that extracts high-dimensional data from imaging studies, offers exciting possibilities for improving melanoma characterization, enabling a shift toward personalized oncology where treatment strategies are tailored based on quantitative imaging biomarkers. Moreover, the advent of PET/MRI hybrid imaging represents a promising frontier in melanoma assessment. By combining the functional data from PET with the higher anatomical tissue resolution of MRI, PET/MRI could provide a more comprehensive evaluation of disease burden while reducing radiation exposure, particularly beneficial for patients requiring long-term surveillance. This hybrid approach has already shown promise in early-stage trials, and additional studies are needed to define its precise role within standard clinical workflows. Another critical area for future investigation is the drug related response assessment derived from WB-MRI. As immunotherapies and targeted treatments revolutionize melanoma management, imaging modalities must evolve to capture subtle treatment-induced changes in tumor biology. Functional imaging techniques, (like PWI), a yield meaningful data on initial response to therapy, identify pseudoprogression, and elucidate resistance mechanisms, supporting timely intervention strategies to optimize patient ma
| 1. | Reginelli A, Russo A, Berritto D, Patane V, Cantisani C, Grassi R. Ultra-High-Frequency Ultrasound: A Modern Diagnostic Technique for Studying Melanoma. Ultraschall Med. 2023;44:360-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Papadakis M, Paschos A, Papazoglou AS, Manios A, Zirngibl H, Manios G, Koumaki D. Computer-aided clinical image analysis as a predictor of sentinel lymph node positivity in cutaneous melanoma. World J Clin Oncol. 2022;13:702-711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Koumaki D, Papadakis M, Kouloumvakou S, Krasagakis K. Awareness, knowledge, and attitudes towards sun protection among patients with melanoma and atypical mole syndrome. World J Clin Oncol. 2022;13:587-598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Conforti C, Zalaudek I. Epidemiology and Risk Factors of Melanoma: A Review. Dermatol Pract Concept. 2021;11:e2021161S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 5. | Lv Q, Zhang Y, Yang R, Dai Y, Lin Y, Sun K, Xu H, Tao K. Photoacoustic Imaging Endometriosis Lesions with Nanoparticulate Polydopamine as a Contrast Agent. Adv Healthc Mater. 2024;13:e2302175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 9930] [Article Influence: 4965.0] [Reference Citation Analysis (2)] |
| 7. | Haider MB, Al Sbihi A, Reddy SN, Green P. Prevalence of malignant neoplasms in celiac disease patients - a nationwide United States population-based study. World J Clin Oncol. 2024;15:1048-1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 8. | Reginelli A, Patanè V, Urraro F, Russo A, De Chiara M, Clemente A, Atripaldi U, Balestrucci G, Buono M, D'ippolito E, Grassi R, D'onofrio I, Napolitano S, Troiani T, De Vita F, Ciardiello F, Nardone V, Cappabianca S. Magnetic Resonance Imaging Evaluation of Bone Metastases Treated with Radiotherapy in Palliative Intent: A Multicenter Prospective Study on Clinical and Instrumental Evaluation Assessment Concordance (MARTE Study). Diagnostics (Basel). 2023;13:2334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Duan JL, Yang J, Zhang YL, Huang WT. Amelanotic primary cervical malignant melanoma: A case report and review of literature. World J Clin Oncol. 2024;15:953-960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (3)] |
| 10. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4400] [Article Influence: 550.0] [Reference Citation Analysis (4)] |
| 11. | Zane KE, Cloyd JM, Mumtaz KS, Wadhwa V, Makary MS. Metastatic disease to the liver: Locoregional therapy strategies and outcomes. World J Clin Oncol. 2021;12:725-745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 12. | Boutros A, Croce E, Ferrari M, Gili R, Massaro G, Marconcini R, Arecco L, Tanda ET, Spagnolo F. The treatment of advanced melanoma: Current approaches and new challenges. Crit Rev Oncol Hematol. 2024;196:104276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 13. | Ehling J, Lammers T, Kiessling F. Non-invasive imaging for studying anti-angiogenic therapy effects. Thromb Haemost. 2013;109:375-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Ebner R, Sheikh GT, Brendel M, Ricke J, Cyran CC. ESR Essentials: staging and restaging with FDG-PET/CT in oncology-practice recommendations by the European Society for Hybrid, Molecular and Translational Imaging. Eur Radiol. 2025;35:1894-1902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Pavlidis ET, Galanis IN, Pavlidis TE. Management of obstructed colorectal carcinoma in an emergency setting: An update. World J Gastrointest Oncol. 2024;16:598-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
| 16. | Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S-150S. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2916] [Cited by in RCA: 2799] [Article Influence: 174.9] [Reference Citation Analysis (0)] |
| 17. | Geng DY, Chen QS, Chen WX, Zhou LS, Han XS, Xie QH, Guo GH, Chen XF, Chen JS, Zhong XP. Molecular targets and mechanisms of different aberrant alternative splicing in metastatic liver cancer. World J Clin Oncol. 2024;15:531-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Bhanderi H, Khalid F, Bodla ZH, Muhammad T, Du D, Meghal T. Autoimmune diabetes from pembrolizumab: A case report and review of literature. World J Clin Oncol. 2023;14:535-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 19. | Rogasch JMM, Hofheinz F, van Heek L, Voltin CA, Boellaard R, Kobe C. Influences on PET Quantification and Interpretation. Diagnostics (Basel). 2022;12:451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Maccioni F, Alfieri G, Assanto GM, Mattone M, Gentiloni Silveri G, Viola F, De Maio A, Frantellizzi V, Di Rocco A, De Vincentis G, Pulsoni A, Martelli M, Catalano C. Whole body MRI with Diffusion Weighted Imaging versus 18F-fluorodeoxyglucose-PET/CT in the staging of lymphomas. Radiol Med. 2023;128:556-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Rossi A, Prochowski Iamurri A, Diano D, Oboldi D, Sintuzzi E, Maurizio L, Andalò A, Cavallucci M, Ferroni F, Amadori E, Barone D, Petralia G. Patient centered radiology: investigating 3 Tesla whole body MRI acceptance in cancer patients. Radiol Med. 2023;128:960-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Van Nieuwenhove S, Van Damme J, Padhani AR, Vandecaveye V, Tombal B, Wuts J, Pasoglou V, Lecouvet FE. Whole-body magnetic resonance imaging for prostate cancer assessment: Current status and future directions. J Magn Reson Imaging. 2022;55:653-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Stecco A, Trisoglio A, Soligo E, Berardo S, Sukhovei L, Carriero A. Whole-Body MRI with Diffusion-Weighted Imaging in Bone Metastases: A Narrative Review. Diagnostics (Basel). 2018;8:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Taylor SA, Mallett S, Beare S, Bhatnagar G, Blunt D, Boavida P, Bridgewater J, Clarke CS, Duggan M, Ellis S, Glynne-Jones R, Goh V, Groves AM, Hameeduddin A, Janes SM, Johnston EW, Koh DM, Miles A, Morris S, Morton A, Navani N, O'Donohue J, Oliver A, Padhani AR, Pardoe H, Patel U, Punwani S, Quinn L, Rafiee H, Reczko K, Rockall AG, Shahabuddin K, Sidhu HS, Teague J, Thaha MA, Train M, van Ree K, Wijeyekoon S, Halligan S; Streamline investigators. Diagnostic accuracy of whole-body MRI versus standard imaging pathways for metastatic disease in newly diagnosed colorectal cancer: the prospective Streamline C trial. Lancet Gastroenterol Hepatol. 2019;4:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 25. | Zugni F, Padhani AR, Koh DM, Summers PE, Bellomi M, Petralia G. Whole-body magnetic resonance imaging (WB-MRI) for cancer screening in asymptomatic subjects of the general population: review and recommendations. Cancer Imaging. 2020;20:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Tunariu N, Blackledge M, Messiou C, Petralia G, Padhani A, Curcean S, Curcean A, Koh DM. What's New for Clinical Whole-body MRI (WB-MRI) in the 21st Century. Br J Radiol. 2020;93:20200562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Tsao SY. Potential of mRNA vaccines to become versatile cancer vaccines. World J Clin Oncol. 2022;13:663-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Bonaffini PA, Ippolito D, Casiraghi A, Besostri V, Franzesi CT, Sironi S. Apparent diffusion coefficient maps integrated in whole-body MRI examination for the evaluation of tumor response to chemotherapy in patients with multiple myeloma. Acad Radiol. 2015;22:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Siegel MJ, Jokerst CE, Rajderkar D, Hildebolt CF, Goyal S, Dehdashti F, Wagner Johnston N, Siegel BA. Diffusion-weighted MRI for staging and evaluation of response in diffuse large B-cell lymphoma: a pilot study. NMR Biomed. 2014;27:681-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Hagtvedt T, Smith HJ, Aaløkken TM. Response to "usefulness of diffusion-weighted magnetic resonance imaging for assessing early treatment response in lymphoma patients". Acta Radiol. 2015;56:NP12-NP13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Wu X, Kellokumpu-Lehtinen PL, Pertovaara H, Korkola P, Soimakallio S, Eskola H, Dastidar P. Diffusion-weighted MRI in early chemotherapy response evaluation of patients with diffuse large B-cell lymphoma--a pilot study: comparison with 2-deoxy-2-fluoro- D-glucose-positron emission tomography/computed tomography. NMR Biomed. 2011;24:1181-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Sorrentino FS, De Rosa F, Di Terlizzi P, Toneatto G, Gabai A, Finocchio L, Salati C, Spadea L, Zeppieri M. Uveal melanoma: Recent advances in immunotherapy. World J Clin Oncol. 2024;15:23-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Papadimitroulas P, Brocki L, Christopher Chung N, Marchadour W, Vermet F, Gaubert L, Eleftheriadis V, Plachouris D, Visvikis D, Kagadis GC, Hatt M. Artificial intelligence: Deep learning in oncological radiomics and challenges of interpretability and data harmonization. Phys Med. 2021;83:108-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 34. | Najjar R. Clinical applications, safety profiles, and future developments of contrast agents in modern radiology: A comprehensive review. iRADIOLOGY. 2024;2:430-468. [RCA] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 35. | Oey O, Liu YY, Sunjaya AF, Simadibrata DM, Khattak MA, Gray E. Gut microbiota diversity and composition in predicting immunotherapy response and immunotherapy-related colitis in melanoma patients: A systematic review. World J Clin Oncol. 2022;13:929-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Russo A, Patanè V, Gagliardi F, Urraro F, Ronchi A, Vitiello P, Sica A, Argenziano G, Nardone V, Reginelli A. Preliminary Experience in Ultra-High Frequency Ultrasound Assessment of Cutaneous Primary Lymphomas: An Innovative Classification. Cancers (Basel). 2024;16:2456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Rasmussen JH, Nørgaard M, Hansen AE, Vogelius IR, Aznar MC, Johannesen HH, Costa J, Engberg AM, Kjær A, Specht L, Fischer BM. Feasibility of Multiparametric Imaging with PET/MR in Head and Neck Squamous Cell Carcinoma. J Nucl Med. 2017;58:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Laurent V, Trausch G, Bruot O, Olivier P, Felblinger J, Régent D. Comparative study of two whole-body imaging techniques in the case of melanoma metastases: advantages of multi-contrast MRI examination including a diffusion-weighted sequence in comparison with PET-CT. Eur J Radiol. 2010;75:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Jouvet JC, Thomas L, Thomson V, Yanes M, Journe C, Morelec I, Bracoud L, Durupt F, Giammarile F, Berthezene Y. Whole-body MRI with diffusion-weighted sequences compared with 18 FDG PET-CT, CT and superficial lymph node ultrasonography in the staging of advanced cutaneous melanoma: a prospective study. J Eur Acad Dermatol Venereol. 2014;28:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Summers P, Saia G, Colombo A, Pricolo P, Zugni F, Alessi S, Marvaso G, Jereczek-Fossa BA, Bellomi M, Petralia G. Whole-body magnetic resonance imaging: technique, guidelines and key applications. Ecancermedicalscience. 2021;15:1164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 41. | Jansen YJL, Willekens I, Seremet T, Awada G, Schwarze JK, De Mey J, Brussaard C, Neyns B. Whole-Body MRI for the Detection of Recurrence in Melanoma Patients at High Risk of Relapse. Cancers (Basel). 2021;13:442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Reinert CP, Liang C, Weissinger M, Vogel J, Forschner A, Nikolaou K, la Fougère C, Seith F. Whole-Body Magnetic Resonance Imaging (MRI) for Staging Melanoma Patients in Direct Comparison to Computed Tomography (CT): Results from a Prospective Positron Emission Tomography (PET)/CT and PET/MRI Study. Diagnostics (Basel). 2023;13:1963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 43. | Hausmann D, Jochum S, Utikal J, Hoffmann RC, Zechmann C, Neff KW, Goerdt S, Schoenberg SO, Dinter DJ. Comparison of the diagnostic accuracy of whole-body MRI and whole-body CT in stage III/IV malignant melanoma. J Dtsch Dermatol Ges. 2011;9:212-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Brussaard C, Faggioni L, Ramirez-Barbosa FE, Vervoort M, Jansen Y, Neyns B, de Mey J, Willekens I, Cioni D, Neri E. Differentiation between normal and metastatic lymph nodes in patients with skin melanoma: Preliminary findings using a DIXON-based whole-body MRI approach. Eur J Radiol Open. 2024;12:100560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Pfannenberg C, Schwenzer N. [Whole-body staging of malignant melanoma: advantages, limitations and current importance of PET-CT, whole-body MRI and PET-MRI]. Radiologe. 2015;55:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Petralia G, Padhani A, Summers P, Alessi S, Raimondi S, Testori A, Bellomi M. Whole-body diffusion-weighted imaging: is it all we need for detecting metastases in melanoma patients? Eur Radiol. 2013;23:3466-3476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Petralia G, Padhani AR, Pricolo P, Zugni F, Martinetti M, Summers PE, Grazioli L, Colagrande S, Giovagnoni A, Bellomi M; Italian Working Group on Magnetic Resonance. Whole-body magnetic resonance imaging (WB-MRI) in oncology: recommendations and key uses. Radiol Med. 2019;124:218-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 48. | Albano D, Stecco A, Micci G, Sconfienza LM, Colagrande S, Reginelli A, Grassi R, Carriero A, Midiri M, Lagalla R, Galia M. Whole-body magnetic resonance imaging (WB-MRI) in oncology: an Italian survey. Radiol Med. 2021;126:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Mosavi F, Ullenhag G, Ahlström H. Whole-body MRI including diffusion-weighted imaging compared to CT for staging of malignant melanoma. Ups J Med Sci. 2013;118:91-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Johnston L, Starkey S, Mukovozov I, Robertson L, Petrella T, Alhusayen R. Surveillance After a Previous Cutaneous Melanoma Diagnosis: A Scoping Review of Melanoma Follow-Up Guidelines. J Cutan Med Surg. 2023;27:516-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 51. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 9572] [Article Influence: 683.7] [Reference Citation Analysis (0)] |