Published online Aug 24, 2025. doi: 10.5306/wjco.v16.i8.108768
Revised: May 21, 2025
Accepted: July 10, 2025
Published online: August 24, 2025
Processing time: 119 Days and 18.2 Hours
Histone deacetylase inhibitors (HDACis), such as trichostatin A (TSA), have been recognized as promising anti-cancer agents due to their capacity to restore epigenetic regulation and reactivate tumor suppressor genes. However, emerging evidence indicates that unintended pro-metastatic effects may offset the therapeutic benefits of HDACis. Chen et al elucidate this paradox, demonstrating that TSA-induced hyperacetylation activates the BRD4/c-Myc/ER-stress axis, thereby promoting epithelial-mesenchymal transition and metastasis in eso
Core Tip: Histone deacetylase inhibitors (HDACis) have been extensively researched for their potential anticancer effects. However, in addition to their role in inhibiting tumor growth, it has also surprisingly facilitated the metastasis of esophageal squamous cell carcinoma. Clinical studies indicate that the effectiveness of HDACis in treating solid tumors often falls short of expectations, and these inhibitors may inherently carry certain risks. In this article, we examine the potential dangers associated with the clinical use of HDACis, including severe adverse effects, off-target toxicities, multidrug resistance, and limited efficiency in solid tumors. Furthermore, future research should focus on combination therapies, the development of selective or dual-target inhibitors to mitigate the adverse effects of HDACis treatments.
- Citation: Xiao S, Xu XZ, Liao M, Song DD, Tang JF, Zhou CF. Potential risks of histone deacetylase inhibitors in cancer therapeutics and feasible combination therapeutic strategies. World J Clin Oncol 2025; 16(8): 108768
- URL: https://www.wjgnet.com/2218-4333/full/v16/i8/108768.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i8.108768
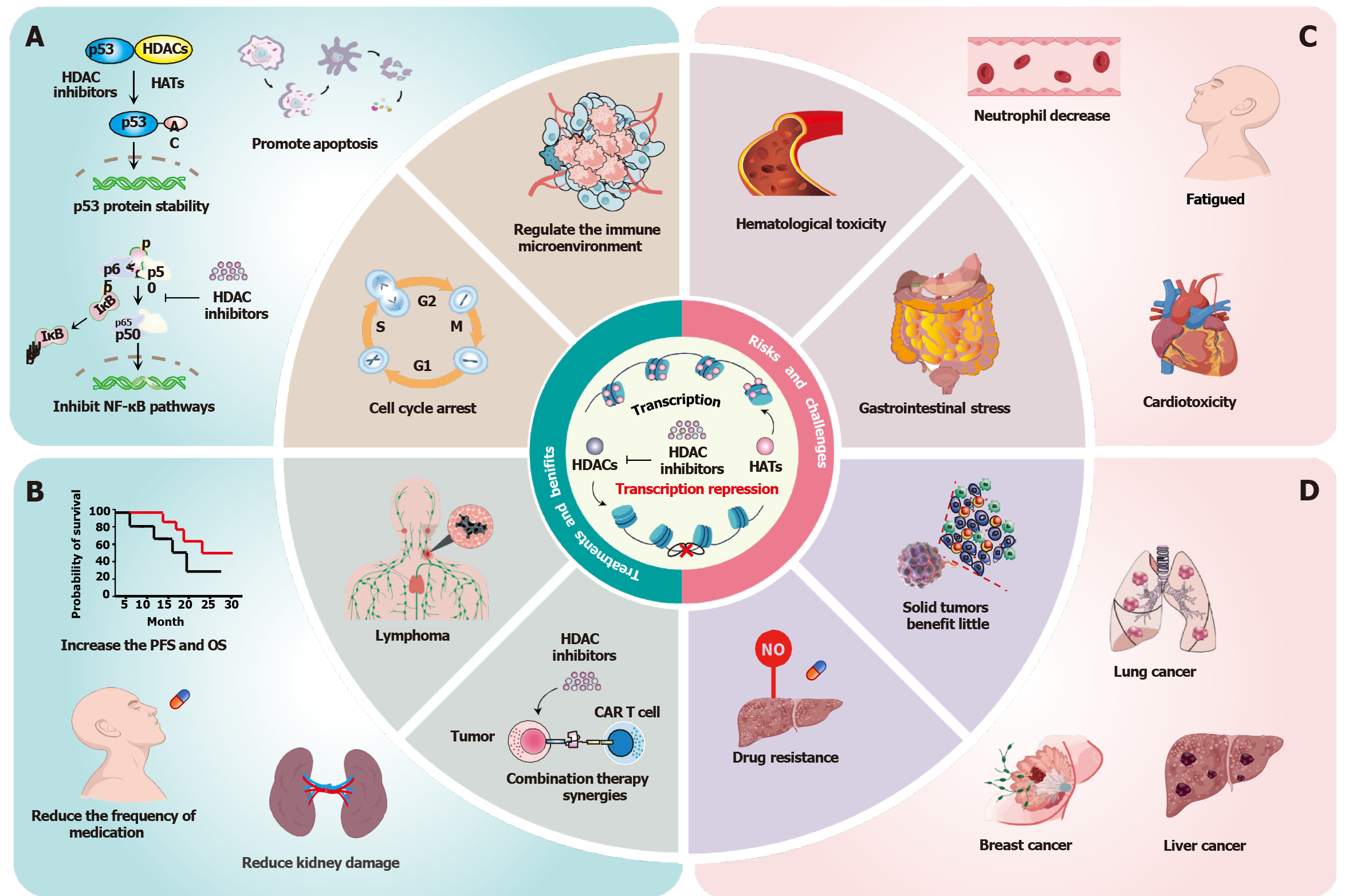
Esophageal cancer, particularly esophageal squamous cell carcinoma (ESCC), presents a significant global health challenge[1]. The poor prognosis associated with ESCC primarily stems from late diagnosis, aggressive tumor biology, and limited treatment efficacy[2]. Histone acetylation plays a critical role in gene expression by modifying chromatin structure, and its dysregulation is implicated in cancer development and progression[3,4]. The overactivity of histone deacetylases (HDACs) can result in the silencing of tumor suppressor genes, thereby facilitating uncontrolled cell growth[4,5]. HDAC inhibitors (HDACis) have shown promise in treating various cancers and neurological disorders, particularly in hematological malignancies[6,7]. They play a crucial role in regulating protein acetylation, disrupting epigenetic silencing, restoring the expression of tumor suppressor genes, and inducing apoptosis or autophagy in cancer cells (Figure 1A and B)[8,9]. In certain tumors, increased acetylation inhibits tumor proliferation; however, it may also facilitate epithelial-mesenchymal transition (EMT) and metastasis, causing the failure of HDACis[5,10]. This research highlights the complexity of epigenetic therapies and emphasizes the necessity of optimizing the application of HDACis in ESCC and potentially other cancers.
Chen et al[11] reveal a novel mechanism by which trichostatin A (TSA) promotes the migration of ESCC cells. They demonstrate that TSA upregulates BRD4, which triggers downstream c-Myc overexpression and endoplasmic reticulum (ER) stress[11]. The ER stress signaling pathway is linked to the regulation of EMT-related transcription factors and signaling pathways, thereby contributing to cancer metastasis[12]. This finding suggests that TSA promotes the ESCC metastasis by activating ER stress-induced EMT. Indeed, the regulatory effect of HDACis on EMT is complex, with specific effects varying depending on the type of inhibitor, cancer model, or molecular pathway[13]. Increasing evidence suggests that certain HDACis promote tumor migration and invasion by inducing transcription factors[13,14]. For instance, HDACis downregulate the expression of E-cadherin by modulating the Claudin-1 mRNA stability, thereby facilitating EMT and invasiveness in multiple colon cancer cell lines[15]. Furthermore, Chen et al[11] found that high levels of histone acetylation in clinical ESCC samples were associated with poor prognosis of patients, suggesting that HDACis monotherapy could promote aggressive, metastatic phenotypes in subsets of patients. These findings are consistent with previous reports that HDACis enhance tumor plasticity in other cancers, but also provide unprecedented mechanistic insights into TSA promotes ESCC metastasis.
The dual role of TSA -both tumor suppression and metastasis promotion- is a critical challenge in the field of epigenetic therapy. Given that TSA promotes ESCC metastasis by the BRD4/c-Myc/ER-stress axis in Chen et al's work[11]. Therefore, the combination of BET inhibitors and TSA is a possible therapy strategy in ESCC. In a preclinical study, the combination therapy of TSA and JQ1 (a BET inhibitor) effectively inhibits the progression of non-small cell lung cancer (NSCLC) by suppressing BET and c-Myc expression in cellular and xenograft mouse models[16]. Moreover, directly targeting c-Myc with small-molecule inhibitors or RNA interference can neutralize this central oncogenic node. For example, MYC inhibitor 361 can effectively inhibit tumor growth in mice by enhancing tumor immune cell infiltration and the expression of PD-L1[17]. In addition, ER stress is an adaptive response that promotes cancer cell survival in previous studies[18]. Preclinical studies have shown that the IRE1α inhibitor 4μ8c directly targets IRE1α/XBP1 signaling and promotes survival in mice with ovarian tumors in situ[19]. The authors also found that TSA promotes ESCC metastasis by activating ER stress. Therefore, the combination of ER stress inhibitors with TSA may inhibit ESCC metastasis by mitigating ER stress.
The clinical application of HDACis is associated with potential risks and challenges, including severe adverse effects, off-target toxicities, and limited efficacy against solid tumors (Figure 1C and D)[5]. The primary challenge of HDACis therapy is the occurrence of severe adverse effects and off-target toxicities, which can impact multiple organ systems[20]. Clinical trials report frequent gastrointestinal disturbances, hematological cytopenias (thrombocytopenia, neutropenia), and potential cardiotoxicity and hepatotoxicity, significantly limiting clinical utility[21,22]. Traditional HDACis are almost all pan-HDACis, which exacerbate the occurrence of adverse events in clinical trials[23]. Mechanistically, broad-spectrum HDAC inhibition disrupts both oncogenic and tumor-suppressive pathways. Consequently, insufficient selectivity may be the main reason for the adverse effects and off-target toxicity of HDACis in cancer treatment. Furthermore, the limited efficiency of monotherapy for solid tumors is a critical challenge for HDACi therapy. Studies have shown that HDAC expression varies greatly in different tumor types. For instance, clinical trials have shown that class I HDAC (HDAC1/2/3) is overexpressed in 71% of gastric cancers and 58% of triple-negative breast cancers (TNBC), whereas class IIb subtype (HDAC6/10) shows an environment-dependent tumor suppressor function[24]. Those results suggest that stratifying patients based on HDAC isoforms and the expression levels provides critical insights into their responsiveness to HDACis therapy. Moreover, HDAC isoform expression patterns combined with c-Myc amplification status improve the prediction of therapeutic response[25]. Furthermore, multidrug resistance (MDR) is another critical challenge. Preclinical studies further identify HDACis-mediated MDR by upregulating the expression of ABCB1. For instance, HDACis upregulates P-glycoprotein (P-gp) by increasing the expression of STAT3 in colorectal cancer cells[26]. This upregulation enhances drug efflux from cancer cells and reduces intracellular drug concentration, thereby inducing MDR in cancer cells.
The combination of HDACis with chemotherapy, radiotherapy, targeted therapy, and immunotherapy is expected to improve the therapeutic effect of the drugs on tumors while reducing their toxicity[7]. Therapeutic synergism is manifested through four main molecular mechanisms. First, HDACis enhance chemotherapy sensitization by impairing DNA damage repair (DDR). Preclinical studies have demonstrated that HDACis compromise DDR to potentiate the effects of chemotherapeutic agents. For instance, the HDAC6 inhibitor ACY1215 synergizes with gemcitabine/oxaliplatin in gallbladder cancer by downregulating BRCA1/2[27], while domatinostat overcomes resistance to gemcitabine and taxol in pancreatic cancer stem cells through the induction of oxidative stress[28]. Clinically, a phase II trial showed superior response rates for chidamide combined with chemotherapy compared to monotherapy (51.18% vs 39.06%) in peripheral T-cell lymphoma[29]. The second mechanism involves kinase inhibitor synergism. Ruxolitinib (a JAK inhibitor) with SAHA (an HDACi) co-treatment inhibits the progression of TNBC[30], and HDACi-DNMT conjugates inhibit the proliferation of gastric cancer[31]. The third mechanism is immunomodulation through microenvironment remodeling. The HDAC6 inhibitor ACY241 enhances anti-PD-L1 efficacy in multiple myeloma and solid tumors by activating the AKT/mTOR/p65 pathways[32]. Furthermore, chidamide combined with PD-L1 blockade demonstrates synergistic activity in NSCLC and TNBC[33]. Finally, radiosensitization is another important mechanism. Chidamide amplifies radiotherapy-induced oxidative stress, promoting apoptosis and reducing stemness in squamous lung cancer cell models[34].
To mitigate off-target toxicity, the development of isomer-selective HDACis or dual inhibitors presents a promising strategy for minimizing adverse reactions in future therapeutic approaches[35]. Notably, the dual HDAC1/3 inhibitor showed a significant inhibitory effect on HCT116 cell xenografts[36]. Similarly, HDAC3/6 dual-selective inhibitors demonstrate low micromolar antiproliferative activity against MDA-MB-231 cells[37]. Additionally, personalized medical approaches, such as pharmacogenomic testing to identify patients at elevated risk for adverse reactions, can facilitate the development of tailored treatment protocols[13]. To address the challenge of MDR, combining HDACis with inhibitors of P-gp may enhance the intracellular accumulation of HDACis within cancer cells. Additionally, molecular targeting strategies designed to modulate the expression of ABC transporters can improve the efficacy of HDACis and help overcome resistance mechanisms[38]. For instance, employing small-molecule inhibitors or RNA interference techniques to downregulate P-gp may significantly enhance the therapeutic effects of HDACis in cancer types exhibiting resistance[39]. Collectively, while HDACis therapy presents potential risks due to severe adverse effects and off-target toxicities, employing strategic combination therapies, developing isomer-selective HDACis or dual-target inhibitors, and im
In summary, Chen et al[11] reveal a novel mechanism through which TSA enhances the migration of ESCC cells by activating ER stress-induced EMT. The pro-metastatic effects of TSA in ESCC highlight that epigenetic therapies are not universally benign. This study emphasizes the importance of mechanism-based drug design and combination treatment approaches. Furthermore, traditional HDACis exhibit severe adverse effects in clinical applications, including off-target toxicities and limited therapeutic efficacy against solid tumors. This letter offers new insights into the potential risks associated with HDACis and suggests future strategies to enhance their clinical utility, such as the implementation of diverse combination therapies and the development of selective and dual-target inhibitors. Additionally, considering the differential expression levels of specific HDACs across various malignancies, patient stratification is critical for successfully applying HDACis therapies, ultimately improving therapeutic efficacy and minimizing adverse effects.
We thank the reviewers for their comments that helped to improve the manuscript.
| 1. | Dinh HQ, Pan F, Wang G, Huang QF, Olingy CE, Wu ZY, Wang SH, Xu X, Xu XE, He JZ, Yang Q, Orsulic S, Haro M, Li LY, Huang GW, Breunig JJ, Koeffler HP, Hedrick CC, Xu LY, Lin DC, Li EM. Integrated single-cell transcriptome analysis reveals heterogeneity of esophageal squamous cell carcinoma microenvironment. Nat Commun. 2021;12:7335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 121] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 2. | Wang Z, Zhao Y, Wo Y, Peng Y, Hu W, Wu Z, Liu P, Shang Y, Liu C, Chen X, Huang K, Chen Y, Hong H, Li F, Sun Y. The single cell immunogenomic landscape after neoadjuvant immunotherapy combined chemotherapy in esophageal squamous cell carcinoma. Cancer Lett. 2024;593:216951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 3. | Neganova ME, Klochkov SG, Aleksandrova YR, Aliev G. Histone modifications in epigenetic regulation of cancer: Perspectives and achieved progress. Semin Cancer Biol. 2022;83:452-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 4. | Miziak P, Baran M, Borkiewicz L, Trombik T, Stepulak A. Acetylation of Histone H3 in Cancer Progression and Prognosis. Int J Mol Sci. 2024;25:10982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 5. | Liang T, Wang F, Elhassan RM, Cheng Y, Tang X, Chen W, Fang H, Hou X. Targeting histone deacetylases for cancer therapy: Trends and challenges. Acta Pharm Sin B. 2023;13:2425-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 88] [Reference Citation Analysis (0)] |
| 6. | Ceccacci E, Minucci S. Inhibition of histone deacetylases in cancer therapy: lessons from leukaemia. Br J Cancer. 2016;114:605-611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 205] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 7. | Ho TCS, Chan AHY, Ganesan A. Thirty Years of HDAC Inhibitors: 2020 Insight and Hindsight. J Med Chem. 2020;63:12460-12484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 507] [Article Influence: 101.4] [Reference Citation Analysis (0)] |
| 8. | Biersack B, Polat S, Höpfner M. Anticancer properties of chimeric HDAC and kinase inhibitors. Semin Cancer Biol. 2022;83:472-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Dang F, Wei W. Targeting the acetylation signaling pathway in cancer therapy. Semin Cancer Biol. 2022;85:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 10. | Han JH, Kim YK, Kim H, Lee J, Oh MJ, Kim SB, Kim M, Kim KH, Yoon HJ, Lee MS, Minna JD, White MA, Kim HS. Snail acetylation by autophagy-derived acetyl-coenzyme A promotes invasion and metastasis of KRAS-LKB1 co-mutated lung cancer cells. Cancer Commun (Lond). 2022;42:716-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 11. | Chen YM, Yang WQ, Fan YY, Chen Z, Liu YZ, Zhao BS. Trichostatin A augments cell migration and epithelial-mesenchymal transition in esophageal squamous cell carcinoma through BRD4/c-Myc endoplasmic reticulum-stress pathway. World J Gastroenterol. 2025;31:103449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (2)] |
| 12. | Zhang W, Shi Y, Oyang L, Cui S, Li S, Li J, Liu L, Li Y, Peng M, Tan S, Xia L, Lin J, Xu X, Wu N, Peng Q, Tang Y, Luo X, Liao Q, Jiang X, Zhou Y. Endoplasmic reticulum stress-a key guardian in cancer. Cell Death Discov. 2024;10:343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 13. | Oliveira T, Hermann E, Lin D, Chowanadisai W, Hull E, Montgomery M. HDAC inhibition induces EMT and alterations in cellular iron homeostasis to augment ferroptosis sensitivity in SW13 cells. Redox Biol. 2021;47:102149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 14. | Wu N, Sun Q, Yang L, Sun H, Zhou Z, Hu Q, Li C, Wang D, Zhang L, Hu Y, Cong X. HDAC3 and Snail2 complex promotes melanoma metastasis by epigenetic repression of IGFBP3. Int J Biol Macromol. 2025;300:140310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Krishnan M, Singh AB, Smith JJ, Sharma A, Chen X, Eschrich S, Yeatman TJ, Beauchamp RD, Dhawan P. HDAC inhibitors regulate claudin-1 expression in colon cancer cells through modulation of mRNA stability. Oncogene. 2010;29:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Meng Y, Qian X, Zhao L, Li N, Wu S, Chen B, Sun T, Wang X. Trichostatin A downregulates bromodomain and extra-terminal proteins to suppress osimertinib resistant non-small cell lung carcinoma. Cancer Cell Int. 2021;21:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Han H, Jain AD, Truica MI, Izquierdo-Ferrer J, Anker JF, Lysy B, Sagar V, Luan Y, Chalmers ZR, Unno K, Mok H, Vatapalli R, Yoo YA, Rodriguez Y, Kandela I, Parker JB, Chakravarti D, Mishra RK, Schiltz GE, Abdulkadir SA. Small-Molecule MYC Inhibitors Suppress Tumor Growth and Enhance Immunotherapy. Cancer Cell. 2019;36:483-497.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 315] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 18. | Liu Y, Tan Z, Yang Y. Negative feedback and modern anti-cancer strategies targeting the ER stress response. FEBS Lett. 2020;594:4247-4265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, Zhang S, Bettigole SE, Gupta D, Holcomb K, Ellenson LH, Caputo T, Lee AH, Conejo-Garcia JR, Glimcher LH. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell. 2015;161:1527-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 675] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 20. | Kopljar I, Gallacher DJ, De Bondt A, Cougnaud L, Vlaminckx E, Van den Wyngaert I, Lu HR. Functional and Transcriptional Characterization of Histone Deacetylase Inhibitor-Mediated Cardiac Adverse Effects in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Stem Cells Transl Med. 2016;5:602-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Subramanian S, Bates SE, Wright JJ, Espinoza-Delgado I, Piekarz RL. Clinical Toxicities of Histone Deacetylase Inhibitors. Pharmaceuticals (Basel). 2010;3:2751-2767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 270] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 22. | Shah RR. Safety and Tolerability of Histone Deacetylase (HDAC) Inhibitors in Oncology. Drug Saf. 2019;42:235-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 23. | Li Y, Seto E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb Perspect Med. 2016;6:a026831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 884] [Article Influence: 98.2] [Reference Citation Analysis (0)] |
| 24. | Sun Y, Hong JH, Ning Z, Pan D, Fu X, Lu X, Tan J. Therapeutic potential of tucidinostat, a subtype-selective HDAC inhibitor, in cancer treatment. Front Pharmacol. 2022;13:932914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 25. | Damaskos C, Psilopatis I, Garmpi A, Dimitroulis D, Nikolettos K, Vrettou K, Sarantis P, Koustas E, Kouraklis G, Antoniou EA, Karamouzis MV, Nikolettos N, Tsikouras P, Marinos G, Kontomanolis E, Kontzoglou K, Garmpis N. Evaluation of the Histone Deacetylase 2 (HDAC-2) Expression in Human Breast Cancer. Cancers (Basel). 2024;16:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Wang H, Huang C, Zhao L, Zhang H, Yang JM, Luo P, Zhan BX, Pan Q, Li J, Wang BL. Histone deacetylase inhibitors regulate P-gp expression in colorectal cancer via transcriptional activation and mRNA stabilization. Oncotarget. 2016;7:49848-49858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Yan B, Chen Q, Shimada K, Tang M, Li H, Gurumurthy A, Khoury JD, Xu B, Huang S, Qiu Y. Histone deacetylase inhibitor targets CD123/CD47-positive cells and reverse chemoresistance phenotype in acute myeloid leukemia. Leukemia. 2019;33:931-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Roca MS, Moccia T, Iannelli F, Testa C, Vitagliano C, Minopoli M, Camerlingo R, De Riso G, De Cecio R, Bruzzese F, Conte M, Altucci L, Di Gennaro E, Avallone A, Leone A, Budillon A. Correction to: HDAC class I inhibitor domatinostat sensitizes pancreatic cancer to chemotherapy by targeting cancer stem cell compartment via FOXM1 modulation. J Exp Clin Cancer Res. 2022;41:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Shi Y, Jia B, Xu W, Li W, Liu T, Liu P, Zhao W, Zhang H, Sun X, Yang H, Zhang X, Jin J, Jin Z, Li Z, Qiu L, Dong M, Huang X, Luo Y, Wang X, Wang X, Wu J, Xu J, Yi P, Zhou J, He H, Liu L, Shen J, Tang X, Wang J, Yang J, Zeng Q, Zhang Z, Cai Z, Chen X, Ding K, Hou M, Huang H, Li X, Liang R, Liu Q, Song Y, Su H, Gao Y, Liu L, Luo J, Su L, Sun Z, Tan H, Wang H, Wang J, Wang S, Zhang H, Zhang X, Zhou D, Bai O, Wu G, Zhang L, Zhang Y. Chidamide in relapsed or refractory peripheral T cell lymphoma: a multicenter real-world study in China. J Hematol Oncol. 2017;10:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 187] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 30. | Liang X, Zang J, Li X, Tang S, Huang M, Geng M, Chou CJ, Li C, Cao Y, Xu W, Liu H, Zhang Y. Discovery of Novel Janus Kinase (JAK) and Histone Deacetylase (HDAC) Dual Inhibitors for the Treatment of Hematological Malignancies. J Med Chem. 2019;62:3898-3923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 31. | Wang L, Zhang C, Hong Y, Li X, Li T, Gao A, Pan S, Liu B, Jin H, Cui D. Integrating Epigenetic Modulators in Nanofibers for Synergistic Gastric Cancer Therapy via Epigenetic Reprogramming. Nano Lett. 2021;21:298-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Ray A, Das DS, Song Y, Hideshima T, Tai YT, Chauhan D, Anderson KC. Combination of a novel HDAC6 inhibitor ACY-241 and anti-PD-L1 antibody enhances anti-tumor immunity and cytotoxicity in multiple myeloma. Leukemia. 2018;32:843-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Tu K, Yu Y, Wang Y, Yang T, Hu Q, Qin X, Tu J, Yang C, Kong L, Zhang Z. Combination of Chidamide-Mediated Epigenetic Modulation with Immunotherapy: Boosting Tumor Immunogenicity and Response to PD-1/PD-L1 Blockade. ACS Appl Mater Interfaces. 2021;13:39003-39017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 34. | Huang X, Bi N, Wang J, Ren H, Pan D, Lu X, Wang L. Chidamide and Radiotherapy Synergistically Induce Cell Apoptosis and Suppress Tumor Growth and Cancer Stemness by Regulating the MiR-375-EIF4G3 Axis in Lung Squamous Cell Carcinomas. J Oncol. 2021;2021:4936207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Ganai SA. Novel Approaches Towards Designing of Isoform-Selective Inhibitors Against Class II Histone Deacetylases: The Acute Requirement for Targetted Anticancer Therapy. Curr Top Med Chem. 2016;16:2441-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Li X, Zhang Y, Jiang Y, Wu J, Inks ES, Chou CJ, Gao S, Hou J, Ding Q, Li J, Wang X, Huang Y, Xu W. Selective HDAC inhibitors with potent oral activity against leukemia and colorectal cancer: Design, structure-activity relationship and anti-tumor activity study. Eur J Med Chem. 2017;134:185-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Soumyanarayanan U, Ramanujulu PM, Mustafa N, Haider S, Fang Nee AH, Tong JX, Tan KSW, Chng WJ, Dymock BW. Discovery of a potent histone deacetylase (HDAC) 3/6 selective dual inhibitor. Eur J Med Chem. 2019;184:111755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Ni X, Li L, Pan G. HDAC inhibitor-induced drug resistance involving ATP-binding cassette transporters (Review). Oncol Lett. 2015;9:515-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Kim YK, Kim NH, Hwang JW, Song YJ, Park YS, Seo DW, Lee HY, Choi WS, Han JW, Kim SN. Histone deacetylase inhibitor apicidin-mediated drug resistance: involvement of P-glycoprotein. Biochem Biophys Res Commun. 2008;368:959-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |