Published online Aug 24, 2025. doi: 10.5306/wjco.v16.i8.107596
Revised: April 21, 2025
Accepted: June 26, 2025
Published online: August 24, 2025
Processing time: 147 Days and 1 Hours
Low rectal cancer poses a significant surgical challenge because of its close proximity to the anal sphincter, often requiring radical resection with permanent colostomy to achieve oncological safety. Revisited rectal anatomy, advances in surgical techniques and neoadjuvant therapies have enabled the possibility of sphincter-preserving procedures, however, it is uniformly not applicable. Se
To identify predictive factors that determine the feasibility of SPS in patients with low rectal cancer.
A comprehensive literature search was conducted using PubMed/MEDLINE databases. The search focused on various factors influencing the feasibility of SPS in low rectal cancer. These included patient-related factors, anatomical considerations, findings from different imaging modalities, advancements in diagnostic tools and techniques, and the role of neoadjuvant chemoradiotherapy. The re
Multiple studies have identified a range of predictive factors influencing the feasibility of SPS in low rectal cancer. Patient-related factors include age, sex, preoperative continence status, comorbidities, and body mass index. Anatomical considerations, such as tumor distance from the anal verge, involvement of the external anal sphincter, and levator ani muscles, also play a critical role. Additionally, a favourable response to neoadjuvant chemoradiotherapy has been associated with improved suitability for sphincter preservation. Several biomarkers, such as inflammatory markers like interleukins and C-reactive protein, as well as tumor markers like carcinoembryonic antigen, are important. Molecular markers, including BRAF and KRAS mutations and mi
SPS is feasible in low rectal cancer and depends on patient factors, tumor anatomy and biology, preoperative treatment response, and biomarkers. In addition, tools and technology including AI can further help in selecting an ideal patient for long term optimal outcome.
Core Tip: Managing lower rectal cancer is challenging due to its anatomical complexity, often resulting in the need for a permanent stoma. Sphincter preservation significantly improves patients' quality of life. It is crucial to identify key factors that influence the feasibility of sphincter-sparing surgery while ensuring favourable long-term oncological and functional outcomes. Keeping the predictive factors in mind, accurate identification of the ideal subset of patients for sphincter preservation is possible, which may obviate unnecessary sphincter sacrifice.
- Citation: Sarangi Y, Kumar A. Predictive model for sphincter preservation in lower rectal cancer. World J Clin Oncol 2025; 16(8): 107596
- URL: https://www.wjgnet.com/2218-4333/full/v16/i8/107596.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i8.107596
Lower rectal cancer refers to a tumor located within 5 cm from the anal verge. Management of low rectal cancers is challenging. Sir Ernest Miles[1] was the first to describe the abdomino-perineal resection (APR) in 1908. For nearly a century, this procedure remained the gold standard for rectal cancer. APR was associated with high morbidity and a high recurrence rate[1]. The need for a permanent colostomy following APR prompted surgeons to explore sphincter-preserving procedures that adhere to sound oncological principles. Until 1948, all rectal cancers, regardless of tumor height, were managed using APR. In 1948, Dixon[2] introduced anterior resection (AR) as a surgical technique for rectal cancer aimed at preserving bowel continuity. He published his outcomes on 400 patients, following which AR gradually replaced APR for cancers located in the middle and upper third of the rectum. In 1982, Heald et al[3] introduced the concept of total mesorectal excision (TME), challenging Miles' concept of cylindrical tumor spread, which proposed that lymphatic dissemination occurs in upward, lateral, and downward directions. Heald et al[3] emphasised that the primary pathway for lymphatic spread is upward and confined within the mesorectal envelope. Fain et al[4] and Ravitch[5] popularised the use of stapling devices, which significantly advanced sphincter-preserving surgery (SPS) and reduced the rate of APR. A deeper understanding of margin status, recognizing that a distal resection margin (DRM) of less than 2 cm can be adequate, along with the increased emphasis on the circumferential resection margin (CRM), has enabled surgeons to achieve sphincter preservation more effectively[6,7]. The oncological safety of sphincter preservation surgery is based on the principle that low rectal cancers primarily spread lymphatically in the oral direction within the mesorectum, with distal spread occurring only within a few millimetres. Similarly, newer surgical techniques also resulted in better sphincter preservation[8]. SPS are defined as resections with clear CRM, DRM, and complete TME without performing an APR. Several techniques are now available for sphincter preservation, including low AR (LAR), ultra-low AR (ULAR), intersphincteric resection (ISR), and local excision methods such as transanal endoscopic microsurgery (TEM), transanal minimally invasive surgery (TAMIS), and transanal excision (TAE). Additionally, transanal TME (TaTME) has emerged as an advanced approach for select cases. Advancements in stapling devices, a better understanding of tumor spread, and the recognition of the CRM as a key prognostic factor (over the distal margin) have significantly contributed to an increased rate of sphincter preservation. Additionally, the growing use of neoadjuvant chemoradiotherapy for locally advanced rectal tumors has further enhanced this trend. The current local recurrence rate after SPS ranges from 0%-23%[9]. The local recurrence rate compared to APR for following SPS is not worse compared to APR, as well documented by many studies[10-13]. However, deciding whose sphincter can be preserved without hampering oncological safety is challenging. The decision to proceed with SPS rather than an APR is based on many factors, including response to chemoradiation, adequate margins, the patient’s functional status and comorbidities, sphincter function, and the patient's desire to preserve their sphincter. Early identification of these factors enables surgeons to determine whether sphincter preservation is feasible. This review explores the factors that may predict sphincter-preserving resection without compromising oncological outcomes and quality of life.
This review studied the various factors predicting the SPS for low rectal cancer. Literature on sphincter preservation surgery for low rectal cancer was reviewed between 2000 and 2024 using PubMed and MEDLINE. We searched for terms like ‘low rectal cancer’, ‘resection’, ‘surgery’, ‘inter-sphincter’, ‘sphincter-saving’, ‘predictive factor’, ‘factors associated with’ and Boolean operators like AND, OR, and NOT. Secondary sources from these publications were manually assessed for relevance. Only English language articles which were relevant to the factors associated with sphincter-sparing surgery in low rectal cancer were included. Case reports were excluded. Study selection was conducted by both the authors (Yajnadatta Sarangi, Ashok Kumar). Any disagreement regarding study selection was resolved by Ashok Kumar. Publications were categorised as patient-related factors, tumor-related factors, imaging, biochemical and molecular markers, tools and technologies, and the preoperative neoadjuvant chemoradiotherapy. The results are discussed in detail.
We have divided the various predictive factors influencing sphincter-sparing surgery into patient-related factors, tumor related factors, surgeon-related and others [the role of neoadjuvant chemoradiotherapy (NACRT), the role of magnetic resonance imaging (MRI), biochemical markers], tools and techniques (Figure 1).
Various studies have found that a young age at presentation is associated with a higher rate of sphincter preservation compared to older age[14,15]. The probable cause of this is the old age patient associated with poor sphincter function compared to younger age group patients. Similarly, some studies suggest that females may have a higher sphincter preservation rate than males, possibly due to the greater ease of creating a working space in the female pelvis compared to the male pelvis[16]. While there is no definitive supporting data, it is technically more challenging to operate on the pelvis of an obese male compared to that of a female[17]. There are some studies indicating that a high body mass index (BMI) correlates with a lower rate of sphincter preservation compared to a lower BMI, likely due to the challenges of navigating a narrow pelvis in patients with higher BMI[18,19]. Diefenhardt et al[20] in a post hoc analysis of the CAO/ARO/AIO-04 randomized phase III trial found that obese patients are associated with less SPS compared to non-obese. Further sub analysis found that female patients underwent more SPS compared to males. Meyerhardt et al[21] analysed the results of intergroup trial 0114 and found that obese males are associated with an increased rate of APR. Higher BMI favours intersphincteric dissection rather than stapled anastomosis. In a study by Weiser et al[22], ISR was preferred in patients with high BMI because a narrow pelvis makes it difficult to staple.
Numerous studies have excluded patients with impaired anal function, as well as those with severe preoperative conditions such as cardiac failure, liver cirrhosis, renal dysfunction, respiratory dysfunction, or psychiatric disorders from ISR. Additionally, impaired preoperative sphincter function is linked to unfavourable outcomes following sphincter preservation surgery.
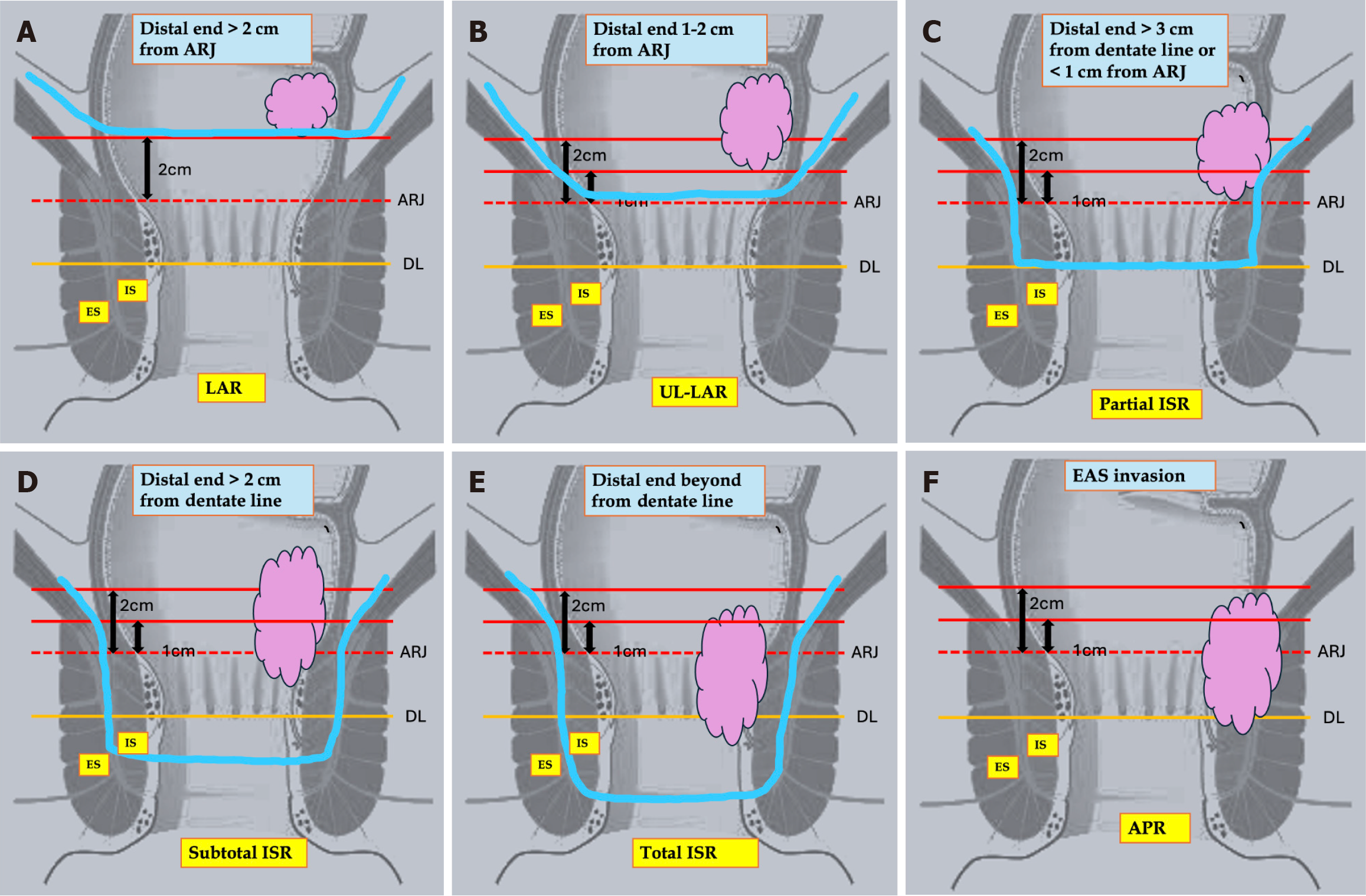
Ideal anatomical and tumor factors linked to improved outcomes after SPS include well- or moderately differentiated T1–3 tumors located at least 0.5 to 1.5 cm above the dentate line, regardless of internal anal sphincter (IAS) invasion. Mobile tumors are better predictors of sphincter preservation. Larger tumor size and T4 stage increase the risk of recurrence. Most studies have found that poorly differentiated T4 tumors, fixed tumors on digital rectal examination, external anal sphincter (EAS)/levator ani muscle (LAM) infiltration, and/or untreatable distant metastases are unsuitable for SPS. Rullier et al[23] have described the best guide to SPS based on MRI classification of the pelvis. Types of SPS depend on the distance of the distal end of the tumor from the anorectal junction (ARJ) and the dentate line (DL). The author divided tumors into 4 types. For type 1 tumors located more than 1 cm from the ARJ, ULAR is typically the preferred approach, and coloanal anastomosis can be performed using either a hand-sewn technique or a double-stapling method. For type II, III, and IV tumors, ISR is indicated. Japanese studies further modified the types of ISR into three categories: (1) Total ISR (complete removal of the IAS at the intersphincteric groove); (2) Subtotal ISR (the resection line lies between the DL and the intersphincteric groove); and (3) Partial ISR (the resection is at the level of the dentate[24]. The selection of the distal transection line should be based on the lower border of the tumor to achieve adequate distal clearance (DRM ≥ 1 cm). Table 1[9,22,25-55] summarises the anatomical and pathological tumor characteristics that favour SPS in low rectal cancer.
| Ref. | Sample size | Results |
| [9] | n = 130 | Distal end of tumor within 4 cm from anal verge. T1 to T3 tumor. Well-moderately differentiated adenocarcinoma, performance status ECOG 0-2. No pre operative faecal incontinence. Non fixed tumor. Treatable distant metastases |
| [22] | n = 148 | Distal end within 5 cm from anal verge. No metastasis. No EAS/LAM involvement. No incontinence |
| [25] | n = 27 | T2 and T3 rectal cancer. 0.5 to 1.5 cm above dentate line. Well differentiated. No involvement of EAS or pubo rectalis muscle. Node negative disease |
| [26] | n = 121 | T1-T3 rectal cancer. Well to moderately differentiated. No EAS/LAM involvement. Good sphincter function |
| [27] | n = 92 | Rectal carcinoma located at or below 4.5 cm from the anal verge. Lower edge of the tumor less than 2 cm from the anorectal ring. No EAS/LAM involvement. No metastasis. No pre operative faecal incontinence. Non fixed tumor. All T2 tumor and t3 tumor with IS involvement Underwent NACRT |
| [28] | n = 65 | Lower end of tumor lies within 0.5-2 cm from DL T1–2 tumor. T3 Tumor (above puborectalis sling). Well to moderately differentiated tumor. Patients with possibly distinct invasion of the pelvic floor musculature. Underwent prior NACRT. EAS and levator ani free from tumor. Pre operative incontinence |
| [29] | n = 90 | T1–3 tumor. Lower margin 2-5 cm from anal verge. Absent of EAS/LAM invasion. No pre operative faecal incontinence. T4 tumor only if distal margin adequate and sphincters are free |
| [30] | n = 278 | Distal margin at the DL or 1–2 mm distal to it. T2–3 tumor. Well/moderately differentiated adenocarcinoma. Distal end of tumor within 3 to 6 cm from anal verge. Absent of EAS/LAM invasion. No preoperative faecal incontinence |
| [31] | n = 122 | Medically fit for surgery. Normal sphincter function. Distance between the tumor and the anorectal junction < 2 cm. No EAS/LAM involvement. No signs of disseminated disease |
| [32] | n = 132 | Distal edge of tumor within 1-5 cm from anal verge. No involvement of inter sphincteric groove. No EAS/LA involvement. No metastasis |
| [33] | n = 40 | Within 2 cm from dentate line. T1-2 tumor. Diameter 1-5 cm. Well-differentiated or moderately differentiated tumor. Sufficient anal function demonstrated by digital palpation and sphincter manometry. Absence of distant metastases. Absence of intestinal obstruction. Absence of EAS/LAM involvement |
| [34] | n = 107 | No EAS/LAM involvement. Distal margin of at least 2 cm for T2 or T3 tumors or 1 cm for T1 tumors. Well to moderately differentiated tumor. No incontinence |
| [35] | n = 47 | No pre operative faecal incontinence. The distal tumor margin at the dentate line or, at most, 1 to 2 mm distal to the dentate line. No EAS/LAM involvement. Well-differentiated or moderately differentiated histology. No distant metastases (except for resectable liver metastases). All patients received neo adjuvant chemo radiotherapy therapy |
| [36] | n = 210 | Low rectal cancer. Distal margin should at least 1 cm. No pre operative faecal incontinence. T3/T4 and n+ tumor all received NACRT |
| [37] | n = 26 | Distal end of tumor 1-2 cm from ano-rectal ring. T1-T3 tumor. No Infiltration of EAS and LAM |
| [38] | n = 43 | Lower edge of tumor within 5 cm from anal verge. Well to moderately differentiated. Absence of EAS/LAM invasion. Absence of distant metastases |
| [39] | n = 1289 (systemic review) | Distal end < 1 cm from ano-rectal ring. No metastasis. No EAS /LAM involvements. Mobile tumor not fixed. No pre operative faecal incontinence |
| [40] | n = 175 | The lower edge of the tumor was less than 2 cm from the anorectal ring or involving anorectal ring. No EAS/LAM involvement. No metastasis. No pre operative faecal incontinence. Non fixed tumor. Underwent NACRT |
| [41] | n = 60 | The inferior border of the tumor located within 5 cm from the anal verge (or 2 cm from the anorectal junction). Absence of synchronous distant metastasis. No EAS/LAM involvement. Fully continent preoperatively |
| [42] | n = 149 | T1 to T3. Lower edge of tumor within 5 cm from anal verge. Well to moderately differentiated tumor. No EAS/LAM involvement. No incontinence pre operatively |
| [43] | n = 55 | Normal pre operative sphincter function; Distance between the tumor and the anorectal junction (upper edge of the surgical anal canal) of less than 2 cm. No involvement of the EAS/LAM. No signs of disseminated disease. Patients having T3, T4, and node positive rectal cancer Underwent NACRT |
| [44] | n = 77 | Lower end of tumor within 2 cm from anorectal ring. No EAS/LAM involvement |
| [45] | n = 134 | Underwent NACRT. No EAS/LAM involvement |
| [46] | n = 163 | Tumor height. Tumor size. Post NACRT T and N stage |
| [47] | n = 219 | Lower border of the tumor was clinically at or within 1 cm above the anorectal ring. Intersphincteric groove was preserved. No EAS/LAM involvement. Elderly patients, obese patients, and those with a poor anal sphincter tone |
| [48] | n = 60 | Low rectal cancer located up to 5 cm from the anal verge. No EAS/LAM involvement |
| [49] | n = 2125 | No EAS/LAM involvement. Well differentiated tumor. No pre-operative faecal incontinence |
| [50] | n = 18452 | Young age, early stage disease, female sex and well differentiated histology |
| [51] | n = 37 | Tumors located at 1.5 cm from the dentate line or 1 cm from the anorectal ring. Pre op Wexner score > 10. No EAS/LAM involvement |
| [52] | n = 147 | Low rectal cancer located up to 4 cm from the anal verge. No EAS/LAM involvement. T3/T4 and node positive tumor. Good response to NACRT |
| [53] | n = 161 | Within 5 cm from the anal verge. Minimally-invasive approach. No previous history of CRC. No evidence of synchronous CRC |
| [54] | n = 330 | Tumor distance 3.3 cm 1.9 cm from anal verge. No EAS/LAM involvement |
| [55] | n = 434 | Tumor distance from the anal verge before and after CRT, the occurrence of clinical T downstaging, post-CRT weight and clinical N stage |
Various preoperative factors that determine the SPS are preoperative MRI, Margin status, i.e., DRM and CRM, Bio
Role of MRI: MRI accurately predicts the DRM, CRM, sphincter involvement, and the exact distal end of the tumor in rectal cancer staging[56]. MRI accurately predicts the distance of the tumor from the anal verge, which is one of the most important factors to consider when evaluating sphincter preservation surgery. Additionally, MRI accurately predict the integrity of the plane between the mesorectum and the levator ani and the external sphincter below, and the integrity of this plane is another important factor predicting successful outcomes after SPS. The MERCURY II study demonstrated the usefulness of MRI in accurately assessing the low rectal cancer surgical plane or intersphincteric plane, thereby reducing the local recurrence rate[57]. MRI is essential for assessing and analysing the feasibility of ISR after NACRT. By predicting DRM and CRM after NACRT, MRI emerges as the best modality for aiding surgery planning. Post-NACRT, T stage and CRM status are crucial in deciding between ISR and APR. Additionally, MRI accurately identifies patients initially planned for APR who become eligible for ISR after being downstaged by NACRT. Diffusion-weighted imaging (DWI) and the apparent diffusion coefficient (ADC) in post-treatment MRI serve as valuable tools for evaluating tumor response and assisting in tumor prognostication. MERCURY trial demonstrated that both pretreatment and post-treatment MRI staging accurately correlated with disease free survival and recurrence rate. The MERCURY study group found that the MR-tumor regression grading (TRG) criteria accurately correlate with tumor response after NACRT[56]. Good response after NACRT, measured by the amount of fibrosis, can be accurately estimated by MRI and graded as TRG. A good response after NACRT is an important factor for good oncological outcomes after SPS. Krdzalic et al[58] found that post-NACRT MRI accurately predict the sphincter preservation in patients with low rectal cancer, with distance from ARJ playing the most important role in determining the sphincter preservation. Similarly, Kim et al[59] found that post-NACRT, MR CRM involvement accurately predicts the failure of SPS. Popita et al[60] found that diagnostic accuracy of MRI of anal sphincter invasion was 91.3%, with a negative predictive value of 100%. Gu et al[61] established a 3D pelvic model using MRI and spiral computed tomography (CT), finding that distance of > 5 cm from anal verge and a BMI < 25 kg/m² were linked to better SPS outcomes. Conversely, shorter upper pubis to sacrococcyx diameter, sacrococcyx distance, and excessive sacral curvature predicted sphincter preservation failure in some cases. Zhou et al[62] studied the CT pelvimetry and found that the diameter of the upper pubis to coccyx affects the success of SPS for mid-low rectal cancer patients. MRI also helps in deciding whether to go for local excision or TME for early-stage rectal cancer. Presence of mesorectal node, T3 tumor and presence of EMVI are absolute contraindications for local excisions, which are accurately identified by MRI staging. Many studies have shown that MRI sometimes over-stages the T stage of the tumor, artificial intelligence (AI) models have been used with MRI to prevent over-staging of the T stage[63]. Advances imaging techniques to predict sphincter preservation: (1) DWI: It is based on the principle of restricting the diffusion of water molecules in tissue, resulting in high signal intensity in highly cellular tissue and low signal intensity in fibrotic or acellular tissue. It helps in response assessment after NACRT. Response assessment after NACRT can be classified as TRG, where patients with a good response have better outcomes after SPS. ADC quantitative parameter derived from DWI, where low ADC results in high viable tumor cells and low response to NACRT; (2) Magnetisation transfer MRI: This is based on the principle of evaluating macromolecular protein content by using the differences in the magnetisation interaction of free water protons and macromolecularly bound protons[64]. Martens et al[65] found that magnetisation transfer imaging can accurately differentiate good and bad responders after NACRT; (3) Dynamic contrast-enhanced MRI: Dynamic contrast-enhanced imaging techniques based on the principle of tumor vessel function (perfusion, permeability) and extracellular-extravascular space composition. The poorer the vascularity of the tissue, the poorer the response to therapy. So various studies found that it can be used to asses pre-treatment prognostications and response assessment after NACRT. With this imaging, one can predict a good prognostic tumor and plan SPS accordingly[66]; and (4) multiparametric imaging: Multiple imaging techniques can be used to identify favourable prognostic tumors, where SPS can be planned, such as tumor perfusion assessed by perfusion CT and metabolism evaluated by positron emission tomography (PET), which can identify unfavourable biological tumors where SPS can be avoided. However, there is not much literature on this subject and its still evolving. Gu et al[67] studied the DC MRI and PET and found a positive correlation regarding tumor vascularity and metabolic activity. Fischer et al[68] Studied the integrated PET/perfusion CT and found that it correlates positively with tumor response.
Biochemical and molecular markers: As long-term oncological outcomes are the main objective of SPS, Various biomarkers have been studied in the past to predict outcomes of sphincter preservation surgery without local recurrence. Various biomarkers available are neutrophil–lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), plasma fibrinogen-NLR, monocyte–lymphocyte ratio and C-reactive protein (CRP). Partl et al[69] studied pre-treatment NLR, PLR, and CRP values on sphincter preservation in low-lying LARC and found that pre-treatment CRP value was an independent factor determining SPS in patients with low rectal cancer. The high amount of CD8+ T cells post-neoadjuvant therapy is associated with a favourable prognosis[70,71]. Tada et al[72] found that increases in post-NACRT interleukin (IL)-6 levels were linked to poor treatment response. They also observed that TNF-α and IL-6 levels were significantly higher post-treatment in non-responders compared to responders. Post-treatment high carcino-embryonic antigen (CEA) is also associated with poor results after sphincter preservation surgery[73]. Increased CRP expression is linked to cancer cell growth, proliferation, and migration. Additional markers for prognosis include various liquid biopsy techniques like the detection of circulating tumor cells (CTCs), circulating tumor DNA, microRNA, cell-free DNA (cfDNA), extracellular vesicles and exosomes. CTCs in the bloodstream indicate metastatic disease, so SPS should be avoided in these patients. KRAS and BRAF mutation in plasma cfDNA is strongly correlated with poor prognosis, and patients with KRAS and BRAF mutation SPS should be avoided[74]. PDL1 expression is also associated with poor treatment response for low rectal cancer after NACRT and preferably treated with immunotherapy before considering SPS[75]. Patients with deficient mismatch repair or microsatellite instability-high status poorly respond to chemoradiotherapy and should preferably be treated with immunotherapy for proper response[76]. Table 2[77,78] summarises various biochemical factors that predict poor long-term outcomes after sphincter preservation.
| Classification | Markers |
| Haematological markers[78] | Neutrophil–lymphocyte ratio, preoperative plasma fibrinogen and neutrophil–lymphocyte ratio, platelet-to-lymphocyte ratio, monocytes–lymphocyte ratio |
| Inflammatory markers[78] | Systemic immune-inflammation index, prognostic nutritional index, systemic inflammation response index, ∆pan-immune-inflammation value, CD8+ cell population, interleukin-6 population, TNF-alpha, CRP level |
| Molecular markers | MSI, BRAF, KRAS, EGFR status, PI3K AKT pathway, CEA level |
| Liquid biopsy | Circulating tumor cells, circulating tumor DNA, microRNA, cell-free DNA |
| Pathologic | Tumor budding, EMVI on MRI |
Role of NACRT: All patients with low rectal cancer who are candidates for SPS should preferably receive NACRT, as it helps to sterilize both the CRM and DRM while facilitating tumor downsizing. It also aids in reducing tumor deposits, budding, and micro metastases[79].
The most compelling evidence regarding the impact of preoperative chemoradiotherapy on sphincter preservation originates from the German rectal cancer study group[80]. Post-NACRT assessment is crucial in determining whether to proceed with ISR, LAR, or APR. Poor NACRT responders are more suitable for APR than SPS. The recurrence rate multiplied by a factor of 9 when there is a poor response to NACRT compared to when there is a response[81]. Numerous studies have demonstrated favourable oncologic outcomes following tumor downstaging[52]. Retrospective studies show that 30%–89% of patients initially thought to need an APR had SPS after preoperative chemoradiation[82,83]. NACRT is also associated with various post-operative problems like sexual, urinary and faecal disturbances[84-86]. Table 3[52,79,87-95] outlines studies showing the downstaging effects of NACRT.
| Ref. | Sample size | Results |
| [52] | n = 461 | Induction chemotherapy resulted incomplete pathological response |
| [79] | n = 256 | NACRT leads to downstaging of disease |
| [87] | n = 1861 | Short-term preoperative radiotherapy reduces the risk of local recurrence in patients with rectal cancer who undergo a standardized total mesorectal excision |
| [88] | n = 88 | Pre operative radiation therapy improves sphincter preservation rate |
| [89] | n = 1861 | Radiotherapy improves local control of tumours |
| [90] | n = 1861 | Preoperative radiotherapy leads to sphincter preservation and reduce recurrence rate |
| [91] | n = 88 | Pre operative radiation improves clinical response and sphincter preservations |
| [92] | n = 09 | Neoadjuvant therapy improve sphincter preservation rate |
| [93] | n = 251 | In low lying rectal cancer good response to Preoperative radiation therapy improves sphincter preservation |
| [94] | n = 32 | Consolidated total neo adjuvant therapy leads to sphincter preservations |
| [95] | n = 920 | Addition of chemotherapy to radiotherapy leads to complete pathological response |
Surgical margin: The key factors in preventing local recurrence after sphincter preservation surgeries are CRM and DRM. With a better understanding of embryological anatomy, it has become clear that the principal lymphatic spread is upward and located within the mesorectal sleeve. The traditional concept of a 5 cm margin was challenged, and it was evident from the works of Williams et al[96] and Pollett et al[7], who advocated a margin of 2 cm. The most important factor determining the possibility of sphincter preservation surgeries is DRM. Sphincter-sparing surgeries carry the potential risk of leaving microscopic tumor cells behind, DRM. Studies indicate that a sufficient DRM can be 1 cm, as opposed to the traditional 2 cm margin[27,97,98]. Many studies have shown that Patients with large bulky tumors or nodal disease without NACRT have a high risk of DRM involvement beyond 1 cm but a low risk beyond 2 cm[99]. The rates of local recurrence and recurrence-free survival were similar regardless of whether the distal margin was more or less than 1 cm. Further studies confirmed that DRM can be reduced to less than 1 cm without compromising oncological safety after NACRT[100-104]. Many studies have shown equivalent survival outcomes after NACRT even with DRM of less than 1 cm[105]. Kwak et al[106] found that even with a 5 mm margin and margin negative on frozen section, it did not hamper the oncological safety of patients after NACRT, and outcomes were noninferior to patients with DRM > 5 mm. Some authors have proposed a cautious approach in which they have advised a 1 cm margin for stage 1 and 2 tumors, and greater than 1 cm for stage 3 tumor[107]. A better understanding of the distal margin has facilitated an increasing number of SPS in patients who were previously managed with APR. Since pathological specimens tend to contract by 50%-60% following formalin fixation, a distal margin of less than 1 cm is considered acceptable, provided the margin remains negative for tumor involvement[8,108]. Similarly, current evidence suggests CRM is the most important predictor of the recurrence and long-term survival[109,110]. The local recurrence rate is 3 to 4 times higher when CRM > 1 cm compared to when CRM is < 1 cm[111-115]. Factors associated with the involvement of CRM are a poor response to NACRT, palpable tumor fixation, etc. Patients with an adequate distal margin of 1 cm after NACRT and a negative CRM can be considered for SPS.
Surgeon as a factor determining SPS: The anatomy of the lower rectum is complex, making the expertise of the treating surgeon crucial to the final outcomes. The ability of the surgeon to perform an oncologically safe surgery with good functional results is of utmost importance. Many studies have found that the rate of APR is significantly higher when compared to an expert colorectal centre[116-118]. Specialised rectal surgery training improved sphincter preservation rates[119-121]. Similarly, surgeons with larger case volume and hospital volume lead to a lower rate of APR[122-124]. Heldenberg et al[125] found that surgery in a specialised colo-rectal unit leads to an increase in sphincter preservation compared to a non-specialised center.
Similarly, ISR is a highly specialised surgery that requires the highest level of expertise in colorectal surgery. Kuo et al[126] in their study found that the learning curve was associated with significant differences in outcomes after ISR. They found that surgical expertise (as < 18 or ≥ 18 laparoscopic ISR) was associated with significantly less operating time and protective stoma creation. They found that the minimum number of experiences in laparoscopic ISR to perform laparoscopic ISR is 19. Due to the complex nature of the surgery involved in UL-LAR and ISR, highly specialised training and a dedicated colorectal surgeon in a high-volume center are necessary to achieve good long-term outcomes[127].
Endomechanical devices: The advent of double staple techniques is one of the important factors that caused drastic changes in SPR for low rectal cancer. Stapling has made the life of surgeons in the lower pelvis easier, where working in a narrow space is very difficult. Some studies comparing the rate of APR before and after stapled devices have made it evident that stapling devices improve sphincter preservation rates[128]. Similarly, in a population-based study in England over the period of 1996-2004, the author found a decline in APR rates from 29.4% in 1996-1997 to 21.2% in 2003-2004[129]. Similarly, a population-based study from the USA found that the APR rate declined from 31.8% in 1998 to 19.2% in 2011, with a significant trend change in 2004 at 21.6% (P < 0.001)[130]. From these studies, it is evident that surgical staplers have contributed to a decline in APR rates. Stapling devices also made progress from double-tine stapling to triple-line technology. Gradual development of powered circular staplers resulted in more controlled firing, ease of use, enhanced safety, and reduced incidence of misfire, thereby reducing anastomotic disruption compared to older circular staplers[131,132].
Similarly, multidisciplinary team discussion (MDT) based managements and improvements in the field of NACRT are also important factors that help in sphincter preservation, as described in the above paragraph.
Method of surgical intervention: There are no definitive data suggesting a higher rate of sphincter preservation in low rectal cancer with MIS compared to open surgery. However, robotic surgery is described as having an advantage in various steps of sphincter surgery, such as ISR and ULAR. Some studies found that robotic ISR is associated with a lower conversion rate and less blood loss compared to laparoscopic ISR[133,134]. Minimally invasive approaches can be particularly useful for patients with a narrow pelvis and obesity who have low rectal cancer. Both open and laparoscopic approaches involve a perineal phase after the completion of the abdominal procedure. In a study by Kim et al[135], a significant number of patients found that ISR could be completed using only the robotic approach without requiring a perineal phase. The robotic platform provides better ergonomics, fewer tremors, improved dexterity, greater degrees of freedom, and an enhanced, stable three-dimensional camera, which are especially crucial when operating in a narrow pelvis. Following NACRT, there is tissue edema and an obscure plane of dissection, which leads to a restricted view that results in straying into the incorrect plane in low rectal cancer. Especially in ISR, the robotic platform provides improved anatomical visualization, which facilitates correct plane identification and, as a result, improves the sphincter preservation rate. Although evidence is sparse regarding the superiority of robotic platforms in the rate of sphincter preservation, there are some retrospective studies that demonstrate the advantages of robotic surgery in sphincter preservation rates. Zhu et al[136] compared robotic and laparoscopic surgery for rectal cancer and found that the robotic platform was associated with a higher number of sphincter preservation compared to laparoscopic surgery. Petropoulou et al[137] in their study also found that a higher rate of SPS in the robotic approach compared to the laparoscopic and open groups. The REAL study group from China in their randomised controlled trial reported a higher number of APRs in the laparoscopic group compared to the robotic group[138]. Ansari et al[139] found that the robotic platform for low rectal cancer had a higher SPS rate compared to laparoscopic and open surgery. Rouanet et al[140] found that the number of patients who underwent coloanal anastomosis were higher compared to the laparoscopic groups after TME, indicating the efficacy of the robotic platform for very low rectal cancer. Gebhardt et al[141] also found similar findings that the robotic platform performed better in very low-lying rectal cancer.
TaTME was developed to overcome technical challenges in laparoscopic TME and is considered advantageous for patients with a narrow pelvis, visceral obesity, or large tumor diameter[142]. The primary reason surgeons adopt TaTME is its ability to clearly define the circumferential and DRM, achieved by placing a purse-string suture at the distal margin at the start of the procedure. TaTME allows precise distal margin assessment from the start, enhancing resection quality, improving outcomes, and reducing APR rates to promote sphincter preservation[143,144]. TaTME can be performed using the transanal approach or via TEM and TAMIS paltforms[145,146]. Additionally, some studies have shown the feasibility of performing TaTME using a robotic platform[147-149]. Several factors that favour the use of TaTME include male gender, locally advanced rectal cancer, tumors located in the distal third of the rectum, a narrow and/or deep pelvis, visceral obesity, prostatic hypertrophy, large tumor diameter, distorted tissue planes resulting from neoadjuvant radiotherapy, and tumors considered challenging to access via the conventional abdominal approach) is regarded as challenging through conventional abdominal approach[150].
Role of AI: AI has already made a deep road into medical sciences. AI can be used to predict SPS in patients with low rectal cancer in various ways. Combining AI models and MRI can accurately predict the possibility of SPS in low rectal cancer. Radiomics can accurately predict the response after NACRT and assist in planning SPS in favourable patients. Jia et al[151] in their meta-analysis found that the radiomic models can accurately predict the effect of NACRT, with sensitivity, specificity, and AUC of 0.82 (95%CI: 0.71-0.90), 0.86 (95%CI: 0.80-0.91), and 0.91 (95%CI: 0.88-0.93), respec
As described above, considering all favourable factors at present, the Rullier classification and Japanese modification, which is based on an MRI-derived and surgeon assessments of the distal margin, are currently being used to determine the planned resection. Figure 2 describes the surgical margins and various resection modalities for low-lying rectal cancer.
Factors affecting the local excision of tumor: Due to the increasing use of screening programs, it’s now possible to detect rectal cancer at an early stage. Early-stage rectal cancer, particularly T1 tumors with invasive adenocarcinoma limited to the submucosa and not extending into the muscularis propria (T2), can be treated with radical resection (RR) involving TME or local excision. In properly selected cases, local excision can achieve a cure. Local excision is linked to lower rates of perioperative complications, stoma construction, and mortality than RR[165]. However, some studies have found that local excision in early-stage cancer is associated with higher rates of local recurrence among patients compared to those treated with TME[166]. The GRECCAR 2 trial failed to show the benefit of local excision, as many patients in the local excision group required a completion TME[167]. In Phase 2, the ACOSOG Z6041 trial demonstrated that neoadjuvant chemoradiotherapy followed by local excision in carefully selected patients with clinically staged T2N0 tumors might achieve outcomes comparable to TME[168]. So it is imperative to identify the factors that predict local excision. Accurate determination of the T and N stages is essential for planning local excision prior to surgery. In this regard, T2-weighted high-resolution MRI and endorectal ultrasound (ERUS) complement each other to best select a patient for local excision; however, the ability to reliably distinguish rectal intramural tumor depth (T1 from T2), ERUS scores over MRI[169,170]. It is important to note that distinguishing between clinical T1 and T2 stages can be challenging, and preoperative as
| Factors |
| Smaller tumors (< 3cm) |
| Occupying < 30% of the rectal lumen circumference |
| < 8 cm from the anal verge |
| With invasion limited to the mucosa superficial submucosa (SM1) |
| Well-to-moderately differentiation |
| No lymphovascular invasion |
| No perineural invasion |
| Low tumor budding |
Only a few studies were conducted assessing the various factors that predict successful SPS in low-lying rectal cancer. Table 5[14,15,72,174-182] summarises studies that combine all relevant factors determining the feasibility of SPS in low-lying rectal cancers.
| Ref. | Types of study | Sample size | Predictive factors |
| [14] | Retrospective | 540 | Younger age. Good performance status. cT1-T2. Higher relative lymphocyte value. Lower CRP value. Longer interval between CRT and surgery |
| [15] | Retrospective | 268 | Younger age at diagnosis, proximal location in the rectum, nonfixed tumor, and institution experience |
| [62] | Retrospective | 42 | BMI, distance of tumor from anal verge, diameter of upper pubis to coccyx |
| [174] | Retrospective | 230 | Cancers within 4 cm of anal verge, surgeon procedure volume, neoadjuvant radiotherapy |
| [175] | Retrospective | 409 | CRM |
| [176] | Retrospective | 1020 | Preoperative CEA ≥ 10 ng/ml. T4 stage, N stage, low rectal tumor, advanced age, ASA ≥ III, and male sex |
| [176] | Retrospective | 330 | Age > 40. Female sex. Well differentiated tumor. cT1-T2. Distance of tumor from anal verge. Total infiltrated circumference. BMI |
| [177] | Retrospective | 47713 | Age < 60, female gender, and white race, high procedural volume |
| [178] | Retrospective | 541 | Tumor height prior to CRT higher than 4.5 cm, non mucinous or signet ring adenocarcinoma, pathological T stage higher than T3, preoperative CRT |
| [179] | Retrospective | 403 | Tumor location, tumor markers, ASA score, T4 stage, lymph node metastasis, distant metastasis |
| [180] | Metanalysis | 3026 | Older age especially > 65 years of age, male sex, ASA score ≥ 3, comorbidity, and distant metastasis |
| [181] | Retrospective | 331 | Neoadjuvant chemoradiotherapy, cT3 stage, distant metastasis |
| [182] | Retrospective | 179 | neoadjuvant chemotherapy, preoperative radiotherapy, mucinous adenocarcinoma, nerve invasion, and tumour height, CEA |
Several clinical biomarkers, radiological, and tumor-related factors have been described to select patients for sphincter preservation in low rectal cancer. However, many newer markers and techniques are evolving. Based on the literature, authors have compiled various factors (Table 6) that may be useful in decision-making for sphincter preservation. Future studies can develop nomograms to predict SPS in patients with low rectal cancer based on these factors. Currently, The Anus Preservation in Low Rectal Adenocarcinoma Based on mismatch repair/microsatellite instability Status study is being conducted to explore the best model for predicting anus preservation in rectal cancer, taking various factors and MMR status into account[183]. AI models can integrate these factors to identify patients for whom the sphincter can be preserved without compromising long-term oncological results.
| Predictive parameter | Factors |
| Patient related | Young age, low BMI, female sex, no psychological problem, no past history of colorectal cancer, no pre operative faecal incontinence (Wexner score > 10) |
| Tumor related (Clinical and pathological) | Preferably T1 to T3 tumor, no involvement of inter sphincteric groove, well to moderately differentiated tumor, EAS and levator ani free from tumor, DRM-1 cm, CRM-1 mm, no distant metastasis, node negative disease |
| Pre-operative (Biomarkers, imaging, neoadjuvant treatment) | MSI-L status, BRAF and KRAS negative, absence of circulating tumour cells, increase in CD8+ T cell cell-free DNA, ct DNA, DRM-1cm, CRM-1 mm in MRI, good response to NACRT |
| Surgeon factors | Surgeon’s training, specialized colorectal surgeon, high volume colorectal cancer center |
This review summarizes the predictive factors determining sphincter preservation surgery for low rectal cancer. Various factors such as BMI, pre-operative sphincter function, distance from the anal verge, CRM, DRM, EAS/LAM involvement, response to NACRT, and various markers like CEA, IL-6, MMR status, KRAS, and BRAF involvement determine the feasibility of SPS. Newer imaging modalities, radiomics, and circulating tumor markers, along with the application of AI, are a few of the newer tools that can be used to accurately predict the outcomes of SPS in low rectal cancer. There are some well-established factors/predictors in the literature, but many more are likely to emerge in the future. Colorectal surgeons need to understand these factors before contemplating sphincter preservation surgery.
| 1. | Ernest Miles W. A method of performing abdominoperineal resection for cancer of the rectum and pelvic colon. Lancet. 1908;172:1812-1813. [RCA] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 462] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 2. | Dixon CF. Anterior Resection for Malignant Lesions of the Upper Part of the Rectum and Lower Part of the Sigmoid. Ann Surg. 1948;128:425-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998;133:894-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1037] [Cited by in RCA: 1056] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 4. | Fain SN, Patin CS, Morgenstern L. Use of a mechanical suturing apparatus in low colorectal anastomosis. Arch Surg. 1975;110:1079-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 99] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Ravitch MM. The use of stapling instruments in surgery of the gastrointestinal tract, with a note on a new instrument for end-to-end low rectal and oesophagojejunal anastomoses. Aust N Z J Surg. 1978;48:444-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | GOLIGHER JC, DUKES CE, BUSSEY HJ. Local recurrences after sphincter saving excisions for carcinoma of the rectum and rectosigmoid. Br J Surg. 1951;39:199-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 278] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 7. | Pollett WG, Nicholls RJ. The relationship between the extent of distal clearance and survival and local recurrence rates after curative anterior resection for carcinoma of the rectum. Ann Surg. 1983;198:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 289] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Kang DW, Kwak HD, Sung NS, Yang IS, Baek SJ, Kwak JM, Kim J, Kim SH. Oncologic outcomes in rectal cancer patients with a ≤1-cm distal resection margin. Int J Colorectal Dis. 2017;32:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Akagi Y, Shirouzu K, Ogata Y, Kinugasa T. Oncologic outcomes of intersphincteric resection without preoperative chemoradiotherapy for very low rectal cancer. Surg Oncol. 2013;22:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Peeters KC, Tollenaar RA, Marijnen CA, Klein Kranenbarg E, Steup WH, Wiggers T, Rutten HJ, van de Velde CJ; Dutch Colorectal Cancer Group. Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. Br J Surg. 2005;92:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 508] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 11. | You YN, Baxter NN, Stewart A, Nelson H. Is the increasing rate of local excision for stage I rectal cancer in the United States justified?: a nationwide cohort study from the National Cancer Database. Ann Surg. 2007;245:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 243] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | Nash GM, Weiss A, Dasgupta R, Gonen M, Guillem JG, Wong WD. Close distal margin and rectal cancer recurrence after sphincter-preserving rectal resection. Dis Colon Rectum. 2010;53:1365-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Kim JC, Yu CS, Lim SB, Kim CW, Kim JH, Kim TW. Abdominoperineal resection and low anterior resection: comparison of long-term oncologic outcome in matched patients with lower rectal cancer. Int J Colorectal Dis. 2013;28:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Partl R, Magyar M, Hassler E, Langsenlehner T, Kapp KS. Clinical parameters predictive for sphincter-preserving surgery and prognostic outcome in patients with locally advanced low rectal cancer. Radiat Oncol. 2020;15:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Temple LK, Romanus D, Niland J, Veer AT, Weiser MR, Skibber J, Wilson J, Rajput A, Benson A, Wong YN, Schrag D. Factors associated with sphincter-preserving surgery for rectal cancer at national comprehensive cancer network centers. Ann Surg. 2009;250:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Sun Z, Yu X, Wang H, Ma M, Zhao Z, Wang Q. Factors affecting sphincter-preserving resection treatment for patients with low rectal cancer. Exp Ther Med. 2015;10:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Akiyoshi T, Kuroyanagi H, Oya M, Konishi T, Fukuda M, Fujimoto Y, Ueno M, Miyata S, Yamaguchi T. Factors affecting the difficulty of laparoscopic total mesorectal excision with double stapling technique anastomosis for low rectal cancer. Surgery. 2009;146:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 18. | Park IJ, You YN, Skibber JM, Rodriguez-Bigas MA, Das P, Eng C, Kopetz S, Wolff RA, Crane CH, Krishnan S, Minsky B, Hu CY, Nguyen S, Chang GJ. Oncologic and Functional Hazards of Obesity Among Patients With Locally Advanced Rectal Cancer Following Neoadjuvant Chemoradiation Therapy. Am J Clin Oncol. 2017;40:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Li Z, Wang Q, Feng Q, Wang X, Xu F, Xie M. Laparoscopic intersphincteric resection vs. transanal total mesorectal excision in overweight patients with low rectal cancer. Front Surg. 2022;9:984680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Diefenhardt M, Ludmir EB, Hofheinz RD, Ghadimi M, Minsky BD, Fleischmann M, Fokas E, Rödel C. Impact of body-mass index on treatment and outcome in locally advanced rectal cancer: A secondary, post-hoc analysis of the CAO/ARO/AIO-04 randomized phase III trial. Radiother Oncol. 2021;164:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 21. | Meyerhardt JA, Tepper JE, Niedzwiecki D, Hollis DR, McCollum AD, Brady D, O'Connell MJ, Mayer RJ, Cummings B, Willett C, Macdonald JS, Benson AB 3rd, Fuchs CS. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. J Clin Oncol. 2004;22:648-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 210] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Weiser MR, Quah HM, Shia J, Guillem JG, Paty PB, Temple LK, Goodman KA, Minsky BD, Wong WD. Sphincter preservation in low rectal cancer is facilitated by preoperative chemoradiation and intersphincteric dissection. Ann Surg. 2009;249:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 23. | Rullier E, Zerbib F, Laurent C, Bonnel C, Caudry M, Saric J, Parneix M. Intersphincteric resection with excision of internal anal sphincter for conservative treatment of very low rectal cancer. Dis Colon Rectum. 1999;42:1168-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 118] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Akagi Y, Kinugasa T, Shirouzu K. Intersphincteric resection for very low rectal cancer: a systematic review. Surg Today. 2013;43:838-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 25. | Vorobiev GI, Odaryuk TS, Tsarkov PV, Talalakin AI, Rybakov EG. Resection of the rectum and total excision of the internal anal sphincter with smooth muscle plasty and colonic pouch for treatment of ultralow rectal carcinoma. Br J Surg. 2004;91:1506-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Schiessel R, Novi G, Holzer B, Rosen HR, Renner K, Hölbling N, Feil W, Urban M. Technique and long-term results of intersphincteric resection for low rectal cancer. Dis Colon Rectum. 2005;48:1858-65; discussion 1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Rullier E, Laurent C, Bretagnol F, Rullier A, Vendrely V, Zerbib F. Sphincter-saving resection for all rectal carcinomas: the end of the 2-cm distal rule. Ann Surg. 2005;241:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 258] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 28. | Hohenberger W, Merkel S, Matzel K, Bittorf B, Papadopoulos T, Göhl J. The influence of abdomino-peranal (intersphincteric) resection of lower third rectal carcinoma on the rates of sphincter preservation and locoregional recurrence. Colorectal Dis. 2006;8:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Chamlou R, Parc Y, Simon T, Bennis M, Dehni N, Parc R, Tiret E. Long-term results of intersphincteric resection for low rectal cancer. Ann Surg. 2007;246:916-21; discussion 921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 30. | Portier G, Ghouti L, Kirzin S, Guimbaud R, Rives M, Lazorthes F. Oncological outcome of ultra-low coloanal anastomosis with and without intersphincteric resection for low rectal adenocarcinoma. Br J Surg. 2007;94:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Akasu T, Takawa M, Yamamoto S, Ishiguro S, Yamaguchi T, Fujita S, Moriya Y, Nakanishi Y. Intersphincteric resection for very low rectal adenocarcinoma: univariate and multivariate analyses of risk factors for recurrence. Ann Surg Oncol. 2008;15:2668-2676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Saito N, Sugito M, Ito M, Kobayashi A, Nishizawa Y, Yoneyama Y, Nishizawa Y, Minagawa N. Oncologic outcome of intersphincteric resection for very low rectal cancer. World J Surg. 2009;33:1750-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Han JG, Wei GH, Gao ZG, Zheng Y, Wang ZJ. Intersphincteric resection with direct coloanal anastomosis for ultralow rectal cancer: the experience of People's Republic of China. Dis Colon Rectum. 2009;52:950-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Yamada K, Ogata S, Saiki Y, Fukunaga M, Tsuji Y, Takano M. Long-term results of intersphincteric resection for low rectal cancer. Dis Colon Rectum. 2009;52:1065-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Krand O, Yalti T, Tellioglu G, Kara M, Berber I, Titiz MI. Use of smooth muscle plasty after intersphincteric rectal resection to replace a partially resected internal anal sphincter: long-term follow-up. Dis Colon Rectum. 2009;52:1895-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Park JS, Choi GS, Jun SH, Hasegawa S, Sakai Y. Laparoscopic versus open intersphincteric resection and coloanal anastomosis for low rectal cancer: intermediate-term oncologic outcomes. Ann Surg. 2011;254:941-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Kuo LJ, Hung CS, Wu CH, Wang W, Tam KW, Liang HH, Chang YJ, Wei PL. Oncological and functional outcomes of intersphincteric resection for low rectal cancer. J Surg Res. 2011;170:e93-e98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Gong X, Jin Z, Zheng Q. Anorectal function after partial intersphincteric resection in ultra-low rectal cancer. Colorectal Dis. 2012;14:e802-e806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Martin ST, Heneghan HM, Winter DC. Systematic review of outcomes after intersphincteric resection for low rectal cancer. Br J Surg. 2012;99:603-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 40. | Laurent C, Paumet T, Leblanc F, Denost Q, Rullier E. Intersphincteric resection for low rectal cancer: laparoscopic vs open surgery approach. Colorectal Dis. 2012;14:35-41; discussion 42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Zhang YJ, Yin L, Huang L, Zhang HB, Han Y, Lin MB. Long-term results of intersphincteric resection for low rectal cancer. J Invest Surg. 2013;26:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Saito N, Ito M, Kobayashi A, Nishizawa Y, Kojima M, Nishizawa Y, Sugito M. Long-term outcomes after intersphincteric resection for low-lying rectal cancer. Ann Surg Oncol. 2014;21:3608-3615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 43. | Abdel-Gawad W, Zaghloul A, Fakhr I, Sakr M, Shabana A, Lotayef M, Mansour O. Evaluation of the frequency and pattern of local recurrence following intersphincteric resection for ultra-low rectal cancer. J Egypt Natl Canc Inst. 2014;26:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Koyama M, Murata A, Sakamoto Y, Morohashi H, Takahashi S, Yoshida E, Hakamada K. Long-term clinical and functional results of intersphincteric resection for lower rectal cancer. Ann Surg Oncol. 2014;21 Suppl 3:S422-S428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Kanso F, Maggiori L, Debove C, Chau A, Ferron M, Panis Y. Perineal or Abdominal Approach First During Intersphincteric Resection for Low Rectal Cancer: Which Is the Best Strategy? Dis Colon Rectum. 2015;58:637-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Lee SY, Jo JS, Kim HJ, Kim CH, Kim YJ, Kim HR. Prognostic factors for low rectal cancer patients undergoing intersphincteric resection after neoadjuvant chemoradiation. J Surg Oncol. 2015;111:1054-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Mahalingam S, Seshadri RA, Veeraiah S. Long-Term Functional and Oncological Outcomes Following Intersphincteric Resection for Low Rectal Cancers. Indian J Surg Oncol. 2017;8:457-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Klose J, Tarantino I, Kulu Y, Bruckner T, Trefz S, Schmidt T, Schneider M, Hackert T, Büchler MW, Ulrich A. Sphincter-Preserving Surgery for Low Rectal Cancer: Do We Overshoot the Mark? J Gastrointest Surg. 2017;21:885-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Yamada K, Saiki Y, Takano S, Iwamoto K, Tanaka M, Fukunaga M, Noguchi T, Nakamura Y, Hisano S, Fukami K, Kuwahara D, Tsuji Y, Takano M, Usuku K, Ikeda T, Sugihara K. Long-term results of intersphincteric resection for low rectal cancer in Japan. Surg Today. 2019;49:275-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 50. | Shahjehan F, Kasi PM, Habermann E, Day CN, Colibaseanu DT, Mathis KL, Larson DW, Merchea A. Trends and outcomes of sphincter-preserving surgery for rectal cancer: a national cancer database study. Int J Colorectal Dis. 2019;34:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Molnar C, Nicolescu C, Grigorescu BL, Botoncea M, Butiurca VO, Petrişor MD, Gurzu S. Comparative oncological outcomes and survival following surgery for low rectal cancer - a single center experience. Rom J Morphol Embryol. 2019;60:847-852. [PubMed] |
| 52. | Park JS, Park SY, Kim HJ, Cho SH, Kwak SG, Choi GS. Long-term Oncologic Outcomes After Neoadjuvant Chemoradiation Followed by Intersphincteric Resection With Coloanal Anastomosis for Locally Advanced Low Rectal Cancer. Dis Colon Rectum. 2019;62:408-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Piozzi GN, Park H, Lee TH, Kim JS, Choi HB, Baek SJ, Kwak JM, Kim J, Kim SH. Risk factors for local recurrence and long term survival after minimally invasive intersphincteric resection for very low rectal cancer: Multivariate analysis in 161 patients. Eur J Surg Oncol. 2021;47:2069-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Kim JC, Kim CW, Lee JL, Yoon YS, Park IJ, Kim JR, Kim J, Park SH. Complete intersphincteric longitudinal muscle excision May Be key to reducing local recurrence during intersphincteric resection. Eur J Surg Oncol. 2021;47:1629-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Jiang WZ, Xu JM, Xing JD, Qiu HZ, Wang ZQ, Kang L, Deng HJ, Chen WP, Zhang QT, Du XH, Yang CK, Guo YC, Zhong M, Ye K, You J, Xu DB, Li XX, Xiong ZG, Tao KX, Ding KF, Zang WD, Feng Y, Pan ZZ, Wu AW, Huang F, Huang Y, Wei Y, Su XQ, Chi P; LASRE trial investigators. Short-term Outcomes of Laparoscopy-Assisted vs Open Surgery for Patients With Low Rectal Cancer: The LASRE Randomized Clinical Trial. JAMA Oncol. 2022;8:1607-1615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 56. | Taylor FG, Quirke P, Heald RJ, Moran BJ, Blomqvist L, Swift IR, Sebag-Montefiore D, Tekkis P, Brown G; Magnetic Resonance Imaging in Rectal Cancer European Equivalence Study Study Group. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014;32:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 443] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 57. | Battersby NJ, How P, Moran B, Stelzner S, West NP, Branagan G, Strassburg J, Quirke P, Tekkis P, Pedersen BG, Gudgeon M, Heald B, Brown G; MERCURY II Study Group. Prospective Validation of a Low Rectal Cancer Magnetic Resonance Imaging Staging System and Development of a Local Recurrence Risk Stratification Model: The MERCURY II Study. Ann Surg. 2016;263:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 233] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 58. | Krdzalic J, Beets-Tan RGH, Engelen SME, van Griethuysen J, Lahaye MJ, Lambregts DMJ, Bakers FCH, Vliegen RFA, Beets GL, Maas M. MRI predicts increased eligibility for sphincter preservation after CRT in low rectal cancer. Radiother Oncol. 2020;145:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Kim SJ, Lee YJ, Park MY, Yang SY, Han YD, Cho MS, Hur H, Lee KY, Lim JS, Min BS. Postchemoradiation magnetic resonance imaging circumferential resection margin predicts treatment failure after multidisciplinary directed sphincter preservation in low rectal cancer. J Surg Oncol. 2023;128:1365-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 60. | Popiţa AR, Rusu A, Muntean V, Cadariu PA, Irimie A, Lisencu C, Pop B, Resiga L, Fekete Z, Badea R. Preoperative MRI accuracy after neoadjuvant chemoradiation for locally advanced rectal cancer. Med Pharm Rep. 2023;96:258-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 61. | Gu J, Bo XF, Xiong CY, Wu AW, Zhang XP, Li M, An Q, Fang J, Li J, Zhang X, Wang HY, Gao F, You WC. Defining pelvic factors in sphincter-preservation of low rectal cancer with a three-dimensional digital model of pelvis. Dis Colon Rectum. 2006;49:1517-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Zhou X, Su M, Hu K, Su Y, Ye Y, Huang C, Yu Z, Li X, Zhou H, Ni Y, Jiang Y. Applications of computed tomography pelvimetry and clinical-pathological parameters in sphincter preservation of mid-low rectal cancer. Int J Clin Exp Med. 2015;8:2174-2181. [PubMed] |
| 63. | Hamabe A, Takemasa I, Ishii M, Okuya K, Hida K, Nishizaki D, Sumii A, Arizono S, Kohno S, Tokunaga K, Nakai H, Sakai Y, Watanabe M. The potential of an artificial intelligence for diagnosing MRI images in rectal cancer: multicenter collaborative trial. J Gastroenterol. 2024;59:896-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 64. | García-Figueiras R, Baleato-González S, Padhani AR, Luna-Alcalá A, Marhuenda A, Vilanova JC, Osorio-Vázquez I, Martínez-de-Alegría A, Gómez-Caamaño A. Advanced Imaging Techniques in Evaluation of Colorectal Cancer. Radiographics. 2018;38:740-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 65. | Martens MH, Lambregts DM, Papanikolaou N, Alefantinou S, Maas M, Manikis GC, Marias K, Riedl RG, Beets GL, Beets-Tan RG. Magnetization transfer imaging to assess tumour response after chemoradiotherapy in rectal cancer. Eur Radiol. 2016;26:390-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Alberda WJ, Dassen HP, Dwarkasing RS, Willemssen FE, van der Pool AE, de Wilt JH, Burger JW, Verhoef C. Prediction of tumor stage and lymph node involvement with dynamic contrast-enhanced MRI after chemoradiotherapy for locally advanced rectal cancer. Int J Colorectal Dis. 2013;28:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Gu J, Khong PL, Wang S, Chan Q, Wu EX, Law W, Liu RK, Zhang J. Dynamic contrast-enhanced MRI of primary rectal cancer: quantitative correlation with positron emission tomography/computed tomography. J Magn Reson Imaging. 2011;33:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 68. | Fischer MA, Vrugt B, Alkadhi H, Hahnloser D, Hany TF, Veit-Haibach P. Integrated ¹⁸F-FDG PET/perfusion CT for the monitoring of neoadjuvant chemoradiotherapy in rectal carcinoma: correlation with histopathology. Eur J Nucl Med Mol Imaging. 2014;41:1563-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Partl R, Lukasiak K, Stranz B, Hassler E, Magyar M, Stranzl-Lawatsch H, Langsenlehner T. Can Pre-Treatment Inflammatory Parameters Predict the Probability of Sphincter-Preserving Surgery in Patients with Locally Advanced Low-Lying Rectal Cancer? Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 70. | Teng F, Meng X, Kong L, Mu D, Zhu H, Liu S, Zhang J, Yu J. Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl Res. 2015;166:721-732.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 71. | Jin C, Yu H, Ke J, Ding P, Yi Y, Jiang X, Duan X, Tang J, Chang DT, Wu X, Gao F, Li R. Predicting treatment response from longitudinal images using multi-task deep learning. Nat Commun. 2021;12:1851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 72. | Tada N, Tsuno NH, Kawai K, Murono K, Nirei T, Ishihara S, Sunami E, Kitayama J, Watanabe T. Changes in the plasma levels of cytokines/chemokines for predicting the response to chemoradiation therapy in rectal cancer patients. Oncol Rep. 2014;31:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Ryan JE, Warrier SK, Lynch AC, Ramsay RG, Phillips WA, Heriot AG. Predicting pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a systematic review. Colorectal Dis. 2016;18:234-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 74. | Thierry AR, Mouliere F, El Messaoudi S, Mollevi C, Lopez-Crapez E, Rolet F, Gillet B, Gongora C, Dechelotte P, Robert B, Del Rio M, Lamy PJ, Bibeau F, Nouaille M, Loriot V, Jarrousse AS, Molina F, Mathonnet M, Pezet D, Ychou M. Clinical validation of the detection of KRAS and BRAF mutations from circulating tumor DNA. Nat Med. 2014;20:430-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 523] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 75. | Ji D, Yi H, Zhang D, Zhan T, Li Z, Li M, Jia J, Qiao M, Xia J, Zhai Z, Song C, Gu J. Somatic Mutations and Immune Alternation in Rectal Cancer Following Neoadjuvant Chemoradiotherapy. Cancer Immunol Res. 2018;6:1401-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 76. | Hasan S, Renz P, Wegner RE, Finley G, Raj M, Monga D, McCormick J, Kirichenko A. Microsatellite Instability (MSI) as an Independent Predictor of Pathologic Complete Response (PCR) in Locally Advanced Rectal Cancer: A National Cancer Database (NCDB) Analysis. Ann Surg. 2020;271:716-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 77. | Alkan A, Hofving T, Angenete E, Yrlid U. Biomarkers and cell-based models to predict the outcome of neoadjuvant therapy for rectal cancer patients. Biomark Res. 2021;9:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 78. | Ocanto A, Teja M, Amorelli F, Couñago F, Gomez Palacios A, Alcaraz D, Cantero R. Landscape of Biomarkers and Pathologic Response in Rectal Cancer: Where We Stand? Cancers (Basel). 2024;16:4047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 79. | Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, de Chaisemartin C, Meunier B, Mehrdad J, Cotte E, Desrame J, Karoui M, Benoist S, Kirzin S, Berger A, Panis Y, Piessen G, Saudemont A, Prudhomme M, Peschaud F, Dubois A, Loriau J, Tuech JJ, Meurette G, Lupinacci R, Goasgen N, Parc Y, Simon T, Tiret E. Effect of Interval (7 or 11 weeks) Between Neoadjuvant Radiochemotherapy and Surgery on Complete Pathologic Response in Rectal Cancer: A Multicenter, Randomized, Controlled Trial (GRECCAR-6). J Clin Oncol. 2016;34:3773-3780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 327] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 80. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R; German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4463] [Article Influence: 212.5] [Reference Citation Analysis (1)] |
| 81. | Rullier A, Gourgou-Bourgade S, Jarlier M, Bibeau F, Chassagne-Clément C, Hennequin C, Tisseau L, Leroux A, Ettore F, Peoc'h M, Diebold MA, Robin YM, Kleinclaus I, Mineur L, Petitjean C, Mosnier JF, Soubeyran I, Padilla N, Lemaistre AI, Bérille J, Denis B, Conroy T, Gérard JP. Predictive factors of positive circumferential resection margin after radiochemotherapy for rectal cancer: the French randomised trial ACCORD12/0405 PRODIGE 2. Eur J Cancer. 2013;49:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 82. | Wibe A, Rendedal PR, Svensson E, Norstein J, Eide TJ, Myrvold HE, Søreide O. Prognostic significance of the circumferential resection margin following total mesorectal excision for rectal cancer. Br J Surg. 2002;89:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 523] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 83. | Crane CH, Skibber JM, Feig BW, Vauthey JN, Thames HD, Curley SA, Rodriguez-Bigas MA, Wolff RA, Ellis LM, Delclos ME, Lin EH, Janjan NA. Response to preoperative chemoradiation increases the use of sphincter-preserving surgery in patients with locally advanced low rectal carcinoma. Cancer. 2003;97:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 145] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 84. | Ito M, Saito N, Sugito M, Kobayashi A, Nishizawa Y, Tsunoda Y. Analysis of clinical factors associated with anal function after intersphincteric resection for very low rectal cancer. Dis Colon Rectum. 2009;52:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 85. | Kang H, Bahk HJ, Shim J, Kim NK. Management of long-term colorectal cancer survivors in Korea. J Korean Med Assoc. 2016;59:276. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 86. | Gervaz P, Rotholtz N, Wexner SD, You SY, Saigusa N, Kaplan E, Secic M, Weiss EG, Nogueras JJ, Belin B. Colonic J-pouch function in rectal cancer patients: impact of adjuvant chemoradiotherapy. Dis Colon Rectum. 2001;44:1667-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 87. | Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3104] [Cited by in RCA: 3119] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 88. | Gerard JP, Chapet O, Nemoz C, Hartweig J, Romestaing P, Coquard R, Barbet N, Maingon P, Mahe M, Baulieux J, Partensky C, Papillon M, Glehen O, Crozet B, Grandjean JP, Adeleine P. Improved sphincter preservation in low rectal cancer with high-dose preoperative radiotherapy: the lyon R96-02 randomized trial. J Clin Oncol. 2004;22:2404-2409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 257] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 89. | Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, Rutten H, Pahlman L, Glimelius B, Leer JW, van de Velde CJ; Dutch Colorectal Cancer Group. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 875] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 90. | van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1346] [Article Influence: 96.1] [Reference Citation Analysis (0)] |
| 91. | Ortholan C, Romestaing P, Chapet O, Gerard JP. Correlation in rectal cancer between clinical tumor response after neoadjuvant radiotherapy and sphincter or organ preservation: 10-year results of the Lyon R 96-02 randomized trial. Int J Radiat Oncol Biol Phys. 2012;83:e165-e171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 92. | Wang T, Wang J, Deng Y, Wu X, Wang L. Neoadjuvant therapy followed by local excision and two-stage total mesorectal excision: a new strategy for sphincter preservation in locally advanced ultra-low rectal cancer. Gastroenterol Rep (Oxf). 2014;2:37-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 93. | Lin JZ, Peng JH, Qdaisat A, Lu ZH, Wu XJ, Chen G, Ding PR, Li LR, Gao YH, Zeng ZF, Wan DS, Pan ZZ. Preoperative chemoradiotherapy creates an opportunity to perform sphincter preserving resection for low-lying locally advanced rectal cancer based on an oncologic outcome study. Oncotarget. 2016;7:57317-57326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 94. | Huang Y, Huang SH, Chi P, Wang XJ, Lin HM, Lu XR, Ye DX, Lin Y, Deng Y. [Rectum-preserving surgery after consolidation neoadjuvant therapy or totally neoadjuvant therapy for low rectal cancer: a preliminary report]. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 95. | Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, Roodvoets AGH, Nagtegaal ID, Beets-Tan RGH, Blomqvist LK, Fokstuen T, Ten Tije AJ, Capdevila J, Hendriks MP, Edhemovic I, Cervantes A, Nilsson PJ, Glimelius B, van de Velde CJH, Hospers GAP; RAPIDO collaborative investigators. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 959] [Article Influence: 239.8] [Reference Citation Analysis (0)] |
| 96. | Williams NS, Dixon MF, Johnston D. Reappraisal of the 5 centimetre rule of distal excision for carcinoma of the rectum: a study of distal intramural spread and of patients' survival. Br J Surg. 1983;70:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 371] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 97. | Andreola S, Leo E, Belli F, Lavarino C, Bufalino R, Tomasic G, Baldini MT, Valvo F, Navarria P, Lombardi F. Distal intramural spread in adenocarcinoma of the lower third of the rectum treated with total rectal resection and coloanal anastomosis. Dis Colon Rectum. 1997;40:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 98. | Shirouzu K, Isomoto H, Kakegawa T. Distal spread of rectal cancer and optimal distal margin of resection for sphincter-preserving surgery. Cancer. 1995;76:388-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 99. | Yan H, Wang PY, Wu YC, Liu YC. Is a Distal Resection Margin of ≤ 1 cm Safe in Patients with Intermediate- to Low-Lying Rectal Cancer? A Systematic Review and Meta-Analysis. J Gastrointest Surg. 2022;26:1791-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 100. | Moore HG, Riedel E, Minsky BD, Saltz L, Paty P, Wong D, Cohen AM, Guillem JG. Adequacy of 1-cm distal margin after restorative rectal cancer resection with sharp mesorectal excision and preoperative combined-modality therapy. Ann Surg Oncol. 2003;10:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 159] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 101. | Mezhir JJ, Shia J, Riedel E, Temple LK, Nash GM, Weiser MR, Paty PB, Wong WD, Guillem JG. Whole-mount pathologic analysis of rectal cancer following neoadjuvant therapy: implications of margin status on long-term oncologic outcome. Ann Surg. 2012;256:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 102. | Kuvshinoff B, Maghfoor I, Miedema B, Bryer M, Westgate S, Wilkes J, Ota D. Distal margin requirements after preoperative chemoradiotherapy for distal rectal carcinomas: are < or = 1 cm distal margins sufficient? Ann Surg Oncol. 2001;8:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 103. | Guillem JG, Chessin DB, Shia J, Suriawinata A, Riedel E, Moore HG, Minsky BD, Wong WD. A prospective pathologic analysis using whole-mount sections of rectal cancer following preoperative combined modality therapy: implications for sphincter preservation. Ann Surg. 2007;245:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 104. | Pahlman L, Bujko K, Rutkowski A, Michalski W. Altering the therapeutic paradigm towards a distal bowel margin of < 1 cm in patients with low-lying rectal cancer: a systematic review and commentary. Colorectal Dis. 2013;15:e166-e174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 105. | Manegold P, Taukert J, Neeff H, Fichtner-Feigl S, Thomusch O. The minimum distal resection margin in rectal cancer surgery and its impact on local recurrence - A retrospective cohort analysis. Int J Surg. 2019;69:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 106. | Kwak JY, Kim CW, Lim SB, Yu CS, Kim TW, Kim JH, Jang SJ, Kim JC. Oncologically safe distal resection margins in rectal cancer patients treated with chemoradiotherapy. J Gastrointest Surg. 2012;16:1947-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 107. | Yellinek S, Krizzuk D, Gilshtein H, Freund MR, Wexner SD, Berho M. Distal Tumor Spread in Rectal Cancer-How Low Should We Go? Am Surg. 2023;89:5553-5558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 108. | Goldstein NS, Soman A, Sacksner J. Disparate surgical margin lengths of colorectal resection specimens between in vivo and in vitro measurements. The effects of surgical resection and formalin fixation on organ shrinkage. Am J Clin Pathol. 1999;111:349-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 107] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 109. | Simon HL, de Paula TR, Profeta da Luz MM, Kiran RP, Keller DS. Predictors of Positive Circumferential Resection Margin in Rectal Cancer: A Current Audit of the National Cancer Database. Dis Colon Rectum. 2021;64:1096-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 110. | Mazaki J, Tsukamoto S, Miyake M, Moritani K, Sakamoto R, Shida D, Kanemitsu Y. Circumferential Resection Margin Status as a Predictive Factor for Recurrence in Preoperative MRI for Advanced Lower Rectal Cancer Without Preoperative Therapy. Dis Colon Rectum. 2021;64:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 111. | Rengan R, Paty PB, Wong WD, Guillem JG, Weiser M, Temple L, Saltz L, Minsky BD. Ten-year results of preoperative radiation followed by sphincter preservation for rectal cancer: increased local failure rate in nonresponders. Clin Colorectal Cancer. 2006;5:413-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 112. | Lee YC, Hsieh CC, Chuang JP. Prognostic significance of partial tumor regression after preoperative chemoradiotherapy for rectal cancer: a meta-analysis. Dis Colon Rectum. 2013;56:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 113. | Agger EA, Jörgren FH, Lydrup MA, Buchwald PL. Risk of local recurrence of rectal cancer and circumferential resection margin: population-based cohort study. Br J Surg. 2020;107:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 114. | Patel S, Kazi M, Desouza AL, Sukumar V, Gori J, Bal M, Saklani A. Outcomes of rectal cancer patients with a positive pathological circumferential resection margin. Langenbecks Arch Surg. 2022;407:1151-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 115. | Chin RI, Schiff JP, Shetty AS, Pedersen KS, Aranha O, Huang Y, Hunt SR, Glasgow SC, Tan BR, Wise PE, Silviera ML, Smith RK, Suresh R, Byrnes K, Samson PP, Badiyan SN, Henke LE, Mutch MG, Kim H. Circumferential Resection Margin as Predictor of Nonclinical Complete Response in Nonoperative Management of Rectal Cancer. Dis Colon Rectum. 2023;66:973-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 116. | Nissan A, Guillem JG, Paty PB, Douglas Wong W, Minsky B, Saltz L, Cohen AM. Abdominoperineal resection for rectal cancer at a specialty center. Dis Colon Rectum. 2001;44:27-35; discussion 35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 117. | Lim SB, Heo SC, Lee MR, Kang SB, Park YJ, Park KJ, Choi HS, Jeong SY, Park JG. Changes in outcome with sphincter preserving surgery for rectal cancer in Korea, 1991-2000. Eur J Surg Oncol. 2005;31:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 118. | Hodgson DC, Zhang W, Zaslavsky AM, Fuchs CS, Wright WE, Ayanian JZ. Relation of hospital volume to colostomy rates and survival for patients with rectal cancer. J Natl Cancer Inst. 2003;95:708-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 119. | Schrag D, Panageas KS, Riedel E, Cramer LD, Guillem JG, Bach PB, Begg CB. Hospital and surgeon procedure volume as predictors of outcome following rectal cancer resection. Ann Surg. 2002;236:583-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 270] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 120. | Martling A, Holm T, Rutqvist LE, Johansson H, Moran BJ, Heald RJ, Cedermark B. Impact of a surgical training programme on rectal cancer outcomes in Stockholm. Br J Surg. 2005;92:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 121. | Kapiteijn E, Putter H, van de Velde CJ; Cooperative investigators of the Dutch ColoRectal Cancer Group. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg. 2002;89:1142-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 377] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 122. | Meyerhardt JA, Tepper JE, Niedzwiecki D, Hollis DR, Schrag D, Ayanian JZ, O'Connell MJ, Weeks JC, Mayer RJ, Willett CG, MacDonald JS, Benson AB 3rd, Fuchs CS. Impact of hospital procedure volume on surgical operation and long-term outcomes in high-risk curatively resected rectal cancer: findings from the Intergroup 0114 Study. J Clin Oncol. 2004;22:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 123. | Purves H, Pietrobon R, Hervey S, Guller U, Miller W, Ludwig K. Relationship between surgeon caseload and sphincter preservation in patients with rectal cancer. Dis Colon Rectum. 2005;48:195-202; discussion 202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 124. | Harling H, Bülow S, Møller LN, Jørgensen T; Danish Colorectal Cancer Group. Hospital volume and outcome of rectal cancer surgery in Denmark 1994-99. Colorectal Dis. 2005;7:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 125. | Heldenberg E, Vishne TH, Onaka N, Dreznik Z. Rectal cancer: the impact of a colorectal unit on the preservation of the anal sphincter. Isr Med Assoc J. 2004;6:471-473. [PubMed] |
| 126. | Kuo LJ, Lin YK, Chang CC, Tai CJ, Chiou JF, Chang YJ. Clinical outcomes of robot-assisted intersphincteric resection for low rectal cancer: comparison with conventional laparoscopy and multifactorial analysis of the learning curve for robotic surgery. Int J Colorectal Dis. 2014;29:555-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 127. | Kuo LJ, Hung CS, Wang W, Tam KW, Lee HC, Liang HH, Chang YJ, Huang MT, Wei PL. Intersphincteric resection for very low rectal cancer: clinical outcomes of open versus laparoscopic approach and multidimensional analysis of the learning curve for laparoscopic surgery. J Surg Res. 2013;183:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 128. | Zheng K, Hu Q, Yu G, Zhou L, Yao Y, Zhou Y, Wang H, Hao L, Yu E, Lou Z, Zhang Y, Qiu H, Meng R, Zhang W. Trends of sphincter-preserving surgeries for low lying rectal cancer: A 20-year experience in China. Front Oncol. 2022;12:996866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 129. | Tilney HS, Heriot AG, Purkayastha S, Antoniou A, Aylin P, Darzi AW, Tekkis PP. A national perspective on the decline of abdominoperineal resection for rectal cancer. Ann Surg. 2008;247:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 130. | Warschkow R, Ebinger SM, Brunner W, Schmied BM, Marti L. Survival after Abdominoperineal and Sphincter-Preserving Resection in Nonmetastatic Rectal Cancer: A Population-Based Time-Trend and Propensity Score-Matched SEER Analysis. Gastroenterol Res Pract. 2017;2017:6058907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 131. | Shibutani M, Fukuoka T, Iseki Y, Kasashima H, Kitayama K, Maeda K. Impact of a circular powered stapler on preventing anastomotic leakage in patients with left-sided colorectal cancer: a retrospective study. BMC Surg. 2023;23:205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 132. | Pla-Martí V, Martín-Arévalo J, Moro-Valdezate D, García-Botello S, Pérez-Santiago L, Barrachina-Martinez I, González-de-Julián S, Vivas-Consuelo D, Espí-Macías A. Incidence of anastomotic leakage using powered circular staplers versus manual circular staplers for left colorectal anastomosis: a cost-effectiveness analysis. Tech Coloproctol. 2024;28:76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 133. | Lee SH, Kim DH, Lim SW. Robotic versus laparoscopic intersphincteric resection for low rectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2018;33:1741-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 134. | Chen H, Ma B, Gao P, Wang H, Song Y, Tong L, Li P, Wang Z. Laparoscopic intersphincteric resection versus an open approach for low rectal cancer: a meta-analysis. World J Surg Oncol. 2017;15:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 135. | Kim JC, Lee JL, Bong JW, Seo JH, Kim CW, Park SH, Kim J. Oncological and anorectal functional outcomes of robot-assisted intersphincteric resection in lower rectal cancer, particularly the extent of sphincter resection and sphincter saving. Surg Endosc. 2020;34:2082-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 136. | Zhu H, Zou J, Pan H, Huang Y, Chi P. Comparison of laparoscopic versus robot-assisted sugery for rectal cancer after neo-adjuvant therapy: a large volume single center experience. BMC Surg. 2025;25:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 137. | Petropoulou T, Theodoraki K, Kitsanta P, Amin S. Efficiency of the Robotic Platform in Improving the Rate of Sphincter Preservation in Patients With Mid and Low Rectal Cancer. World J Oncol. 2023;14:499-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 138. | Feng Q, Yuan W, Li T, Tang B, Jia B, Zhou Y, Zhang W, Zhao R, Zhang C, Cheng L, Zhang X, Liang F, He G, Wei Y, Xu J; REAL Study Group. Robotic versus laparoscopic surgery for middle and low rectal cancer (REAL): short-term outcomes of a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol. 2022;7:991-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 232] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 139. | Ansari SA, Javed MA, Hedayat F, Harris C, Gill M, Sheikh A. Real-world comparison of curative open, laparoscopic and robotic resections for sigmoid and rectal cancer-single center experience. J Robot Surg. 2022;16:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 140. | Rouanet P, Bertrand MM, Jarlier M, Mourregot A, Traore D, Taoum C, de Forges H, Colombo PE. Robotic Versus Laparoscopic Total Mesorectal Excision for Sphincter-Saving Surgery: Results of a Single-Center Series of 400 Consecutive Patients and Perspectives. Ann Surg Oncol. 2018;25:3572-3579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 141. | Gebhardt JM, Werner N, Stroux A, Förster F, Pozios I, Seifarth C, Schineis C, Weixler B, Beyer K, Lauscher JC. Robotic-Assisted versus Laparoscopic Surgery for Rectal Cancer: An Analysis of Clinical and Financial Outcomes from a Tertiary Referral Center. J Clin Med. 2024;13:1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 142. | Tuech JJ, Karoui M, Lelong B, De Chaisemartin C, Bridoux V, Manceau G, Delpero JR, Hanoun L, Michot F. A step toward NOTES total mesorectal excision for rectal cancer: endoscopic transanal proctectomy. Ann Surg. 2015;261:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 143. | Fernández-Hevia M, Delgado S, Castells A, Tasende M, Momblan D, Díaz del Gobbo G, DeLacy B, Balust J, Lacy AM. Transanal total mesorectal excision in rectal cancer: short-term outcomes in comparison with laparoscopic surgery. Ann Surg. 2015;261:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 144. | de Lacy AM, Rattner DW, Adelsdorfer C, Tasende MM, Fernández M, Delgado S, Sylla P, Martínez-Palli G. Transanal natural orifice transluminal endoscopic surgery (NOTES) rectal resection: "down-to-up" total mesorectal excision (TME)--short-term outcomes in the first 20 cases. Surg Endosc. 2013;27:3165-3172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 195] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 145. | Atallah S. Transanal total mesorectal excision: full steam ahead. Tech Coloproctol. 2015;19:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 146. | Hompes R, Guy R, Jones O, Lindsey I, Mortensen N, Cunningham C. Transanal total mesorectal excision with a side-to-end stapled anastomosis - a video vignette. Colorectal Dis. 2014;16:567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 147. | Atallah S, Martin-Perez B, Pinan J, Quinteros F, Schoonyoung H, Albert M, Larach S. Robotic transanal total mesorectal excision: a pilot study. Tech Coloproctol. 2014;18:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 148. | Atallah S, Nassif G, Polavarapu H, deBeche-Adams T, Ouyang J, Albert M, Larach S. Robotic-assisted transanal surgery for total mesorectal excision (RATS-TME): a description of a novel surgical approach with video demonstration. Tech Coloproctol. 2013;17:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 149. | Gómez Ruiz M, Parra IM, Palazuelos CM, Martín JA, Fernández CC, Diego JC, Fleitas MG. Robotic-assisted laparoscopic transanal total mesorectal excision for rectal cancer: a prospective pilot study. Dis Colon Rectum. 2015;58:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 150. | Buchs NC, Nicholson GA, Ris F, Mortensen NJ, Hompes R. Transanal total mesorectal excision: A valid option for rectal cancer? World J Gastroenterol. 2015;21:11700-11708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 151. | Jia LL, Zheng QY, Tian JH, He DL, Zhao JX, Zhao LP, Huang G. Artificial intelligence with magnetic resonance imaging for prediction of pathological complete response to neoadjuvant chemoradiotherapy in rectal cancer: A systematic review and meta-analysis. Front Oncol. 2022;12:1026216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 152. | Kim J, Oh JE, Lee J, Kim MJ, Hur BY, Sohn DK, Lee B. Rectal cancer: Toward fully automatic discrimination of T2 and T3 rectal cancers using deep convolutional neural network. Int J Imag Syst Tech. 2019;29:247-259. [DOI] [Full Text] |
| 153. | Wu QY, Liu SL, Sun P, Li Y, Liu GW, Liu SS, Hu JL, Niu TY, Lu Y. Establishment and clinical application value of an automatic diagnosis platform for rectal cancer T-staging based on a deep neural network. Chin Med J (Engl). 2021;134:821-828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 154. | Ding L, Liu GW, Zhao BC, Zhou YP, Li S, Zhang ZD, Guo YT, Li AQ, Lu Y, Yao HW, Yuan WT, Wang GY, Zhang DL, Wang L. Artificial intelligence system of faster region-based convolutional neural network surpassing senior radiologists in evaluation of metastatic lymph nodes of rectal cancer. Chin Med J (Engl). 2019;132:379-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 155. | Kim HJ, Choi GS, Park JS, Park SY, Cho SH, Seo AN, Yoon GS. S122: impact of fluorescence and 3D images to completeness of lateral pelvic node dissection. Surg Endosc. 2020;34:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 156. | Schwyzer M, Martini K, Benz DC, Burger IA, Ferraro DA, Kudura K, Treyer V, von Schulthess GK, Kaufmann PA, Huellner MW, Messerli M. Artificial intelligence for detecting small FDG-positive lung nodules in digital PET/CT: impact of image reconstructions on diagnostic performance. Eur Radiol. 2020;30:2031-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 157. | Kudo SE, Ichimasa K, Villard B, Mori Y, Misawa M, Saito S, Hotta K, Saito Y, Matsuda T, Yamada K, Mitani T, Ohtsuka K, Chino A, Ide D, Imai K, Kishida Y, Nakamura K, Saiki Y, Tanaka M, Hoteya S, Yamashita S, Kinugasa Y, Fukuda M, Kudo T, Miyachi H, Ishida F, Itoh H, Oda M, Mori K. Artificial Intelligence System to Determine Risk of T1 Colorectal Cancer Metastasis to Lymph Node. Gastroenterology. 2021;160:1075-1084.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 158. | Su J, Liu Z, Li H, Kang L, Huang K, Wu J, Huang H, Ling F, Yao X, Huang C. Artificial intelligence-based model to predict recurrence after local excision in T1 rectal cancer. Eur J Surg Oncol. 2025;51:109717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 159. | Kuo CY, Kuo LJ, Lin YK. Artificial intelligence based system for predicting permanent stoma after sphincter saving operations. Sci Rep. 2023;13:16039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 160. | Wang X, Jiang W, Deng Y, Chen Z, Zheng Z, Sun Y, Xie Z, Lu X, Huang S, Lin Y, Huang Y, Chi P. Unraveling variations and enhancing prediction of successful sphincter-preserving resection for low rectal cancer: a post hoc analysis of the multicentre LASRE randomized clinical trial. Int J Surg. 2024;110:4031-4042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 161. | Baltussen EJM, Kok END, Brouwer de Koning SG, Sanders J, Aalbers AGJ, Kok NFM, Beets GL, Flohil CC, Bruin SC, Kuhlmann KFD, Sterenborg HJCM, Ruers TJM. Hyperspectral imaging for tissue classification, a way toward smart laparoscopic colorectal surgery. J Biomed Opt. 2019;24:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 162. | Echle A, Ghaffari Laleh N, Quirke P, Grabsch HI, Muti HS, Saldanha OL, Brockmoeller SF, van den Brandt PA, Hutchins GGA, Richman SD, Horisberger K, Galata C, Ebert MP, Eckardt M, Boutros M, Horst D, Reissfelder C, Alwers E, Brinker TJ, Langer R, Jenniskens JCA, Offermans K, Mueller W, Gray R, Gruber SB, Greenson JK, Rennert G, Bonner JD, Schmolze D, Chang-Claude J, Brenner H, Trautwein C, Boor P, Jaeger D, Gaisa NT, Hoffmeister M, West NP, Kather JN. Artificial intelligence for detection of microsatellite instability in colorectal cancer-a multicentric analysis of a pre-screening tool for clinical application. ESMO Open. 2022;7:100400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 163. | Cao R, Yang F, Ma SC, Liu L, Zhao Y, Li Y, Wu DH, Wang T, Lu WJ, Cai WJ, Zhu HB, Guo XJ, Lu YW, Kuang JJ, Huan WJ, Tang WM, Huang K, Huang J, Yao J, Dong ZY. Development and interpretation of a pathomics-based model for the prediction of microsatellite instability in Colorectal Cancer. Theranostics. 2020;10:11080-11091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 164. | González-castro V, Cernadas E, Huelga E, Fernández-delgado M, Porto J, Antunez JR, Souto-bayarri M. CT Radiomics in Colorectal Cancer: Detection of KRAS Mutation Using Texture Analysis and Machine Learning. Appl Sci. 2020;10:6214. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 165. | Xiong X, Wang C, Wang B, Shen Z, Jiang K, Gao Z, Ye Y. Can transanal endoscopic microsurgery effectively treat T1 or T2 rectal cancer?A systematic review and meta-analysis. Surg Oncol. 2021;37:101561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 166. | Garcia-Aguilar J, Mellgren A, Sirivongs P, Buie D, Madoff RD, Rothenberger DA. Local excision of rectal cancer without adjuvant therapy: a word of caution. Ann Surg. 2000;231:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 234] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 167. | Rullier E, Rouanet P, Tuech JJ, Valverde A, Lelong B, Rivoire M, Faucheron JL, Jafari M, Portier G, Meunier B, Sileznieff I, Prudhomme M, Marchal F, Pocard M, Pezet D, Rullier A, Vendrely V, Denost Q, Asselineau J, Doussau A. Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open-label, multicentre, phase 3 trial. Lancet. 2017;390:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 253] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 168. | Garcia-Aguilar J, Renfro LA, Chow OS, Shi Q, Carrero XW, Lynn PB, Thomas CR Jr, Chan E, Cataldo PA, Marcet JE, Medich DS, Johnson CS, Oommen SC, Wolff BG, Pigazzi A, McNevin SM, Pons RK, Bleday R. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol. 2015;16:1537-1546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 297] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 169. | Al-Sukhni E, Milot L, Fruitman M, Beyene J, Victor JC, Schmocker S, Brown G, McLeod R, Kennedy E. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2012;19:2212-2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 396] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 170. | Puli SR, Reddy JB, Bechtold ML, Choudhary A, Antillon MR, Brugge WR. Accuracy of endoscopic ultrasound to diagnose nodal invasion by rectal cancers: a meta-analysis and systematic review. Ann Surg Oncol. 2009;16:1255-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 171. | Nascimbeni R, Burgart LJ, Nivatvongs S, Larson DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002;45:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 467] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 172. | Yamamoto S, Watanabe M, Hasegawa H, Baba H, Yoshinare K, Shiraishi J, Kitajima M. The risk of lymph node metastasis in T1 colorectal carcinoma. Hepatogastroenterology. 2004;51:998-1000. [PubMed] |
| 173. | Halverson AL, Morris AM, Cleary RK, Chang GJ. For Patients with Early Rectal Cancer, Does Local Excision Have an Impact on Recurrence, Survival, and Quality of Life Relative to Radical Resection? Ann Surg Oncol. 2019;26:2497-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 174. | Cong ZJ, Hu LH, Xing JJ, Zhang W, Fu CG, Yu ED, Zhong M. Risk factors associated with sphincter-preserving resection in patients with low rectal cancer. Int Surg. 2014;99:330-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 175. | Yeom SS, Park IJ, Jung SW, Oh SH, Lee JL, Yoon YS, Kim CW, Lim SB, Kim N, Yu CS, Kim JC. Outcomes of patients with abdominoperineal resection (APR) and low anterior resection (LAR) who had very low rectal cancer. Medicine (Baltimore). 2017;96:e8249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 176. | Huang SH, Tsai KY, Tsai TY, You JF, Yeh CY, Hsieh PS, Tang R, Chiang JM, Tsai WS. Preoperative risk stratification of permanent stoma in patients with non-metastatic mid and low rectal cancer undergoing curative resection and a temporary stoma. Langenbecks Arch Surg. 2022;407:1991-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 177. | Paquette IM, Kemp JA, Finlayson SR. Patient and hospital factors associated with use of sphincter-sparing surgery for rectal cancer. Dis Colon Rectum. 2010;53:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 178. | Wang XJ, Chi P, Lin HM, Lu XR, Huang Y, Xu ZB, Huang SH, Sun YW, Ye DX. [Effects of neoadjuvant chemoradiotherapy on the rates of sphincter preserving surgery in lower rectal cancer and analysis of their prognostic factors]. Zhonghua Wai Ke Za Zhi. 2016;54:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 179. | Tang C, He F, Yang F, Chen D, Xiong J, Zou Y, Qian K. Development and validation of a nomogram for preoperatively predicting permanent stoma after rectal cancer surgery with ileostomy: a retrospective cohort study. BMC Cancer. 2024;24:874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 180. | He F, Tang C, Yang F, Chen D, Xiong J, Zou Y, Zhao D, Qian K. Preoperative risk factors and cumulative incidence of temporary ileostomy non-closure after sphincter-preserving surgery for rectal cancer: a meta-analysis. World J Surg Oncol. 2024;22:94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 181. | Hu G, Liu JG, Qiu WL, Mei SW, Wang X, Tang JQ. [Risk factor and nomogram for predicting the probability of a permanent stoma after laparoscopic intersphincteric resection for ultralow rectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2022;25:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 182. | Liu JR, Zhang J, Duan XL. Risk factors influencing sphincter preservation in laparoscopic radical rectal cancer surgery. World J Gastrointest Surg. 2025;17:101061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 183. | Huang CY, Bai MH, Shen JW, Sun QQ, Feng YR, Chen QP, Mao W, Ju HX, Zhu J. Anus preservation in low rectal adenocarcinoma based on MMR/MSI status (APRAM): a study protocol for a randomised, controlled, open-label, multicentre phase III trial. BMC Cancer. 2024;24:57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |