INTRODUCTION
According to GLOBOCAN, an international cancer research organization, there were 19.695 million new cases of malignant tumors and 9.737 million cancer-related deaths worldwide in 2022[1]. Cancer cells have extremely unique characteristics of continuous division and proliferation. Under normal circumstances, cell division and proliferation are strictly regulated, but cancer cells divide and proliferate uncontrollably. Moreover, cancer cells infiltrate and metastasize, and they can spread to other parts of the body through the bloodstream or lymphatic system[2]. These characteristics threaten the health and lives of patients[3]. Furthermore, cancer causes a heavy economic burden[4]. The characteristics of cancer include six biological capabilities that are acquired during the multistep developmental process of human tumors, namely maintaining proliferative signals, circumventing growth inhibition, resisting cell death, achieving replicative immortality, inducing angiogenesis, and initiating invasion and metastasis, which play crucial roles in tumorigenesis and cancer progression, and collectively constitute an important organizing principle for complexity of tumor diseases. Genomic instability is at the root of these features. While genomes of normal cells are relatively stable, those of cancer cells contain various mutations and abnormalities. This genomic instability allows cancer cells to adapt constantly to changes in their environment and develop more complex and pernicious biological behaviors. In addition, inflammation promotes the development of many characteristic functions of cancer cells[5]. Although many cancer treatments are currently available, there are still many limitations. For example, surgical treatment can treat only a limited range of cancers, whereas surgery to remove tumors that are located deep within the body or are large is difficult and may cause infections and complications. Some tumors cannot be completely removed, and there is a risk of recurrence of the lesion. In addition, chemotherapy resistance is an important issue. With the extensive use of chemotherapeutic drugs, cancer cells gradually develop resistance to these drugs, resulting in weakened or even ineffective chemotherapy. This resistance limits the use of chemotherapy drugs and increase the difficulty of cancer treatment. Although chemotherapeutic drugs kill cancer cells, they can also cause damage to normal cells, triggering a series of adverse reactions that seriously affect the quality of life of patients and may even lead to treatment interruption[6,7]. These limitations highlight the urgent need to develop novel anticancer drugs.
The search for chemotherapeutic agents with low toxicity and high efficacy, as well as new drug targets, has always been the focus of cancer therapy research. Natural products, many of which are highly efficient, have low toxicity and can work against specific disease targets, have great potential as sources of novel drug candidates. Identifying small-molecule natural product compounds that can be used to treat diseases is one of the key methods for developing antitumor drugs. In the field of traditional Chinese medicine (TCM), the discovery and development of novel natural compounds, active ingredients, single herbs, herb combinations or formulas with therapeutic selectivity have become important areas of cancer treatment research[8]. Approximately 80% of clinically used anticancer drugs are natural products or their derivatives[9].
Cortex Periplocae is the dried root bark of Periploca sepium, a plant of the family Lauraceae, which is an important TCM herb and is widely distributed in North Africa, tropical Africa and East Asia[10]. According to the Chinese Pharmacopoeia, Cortex Periplocae is pungent, warm, bitter and toxic, and it belongs to the liver, kidney and heart meridians; Cortex Periplocae is effective at inducing diuresis, eliminating wind dampness, and strengthening tendons and bones, and it is mainly used to treat lower limb swelling, palpitations, shortness of breath, wind dampness, cold paralysis, and lumbar and knee soreness and weakness, etc[11]. In recent years, many scholars have studied the chemical composition of Cortex Periplocae and reported that it contains various compounds, such as C21 steroids, cardiac glycosides, triterpenoids, aldehydes, oligosaccharides, small-molecular fatty acids, and flavonoids[12,13]. These studies have demonstrated the potential of Cortex Periplocae for use in medicine and have provided research directions for future drug development. In the field of pharmacology, studies on Cortex Periplocae have focused on cardiovascular potentiation, anti-inflammatory effects, antitumor activities, and immune effects[14].
PHARMACOLOGICAL PROPERTIES OF PERIPLOCIN, PERIPLOCYMARIN AND PERIPLOGENIN
Compound overview
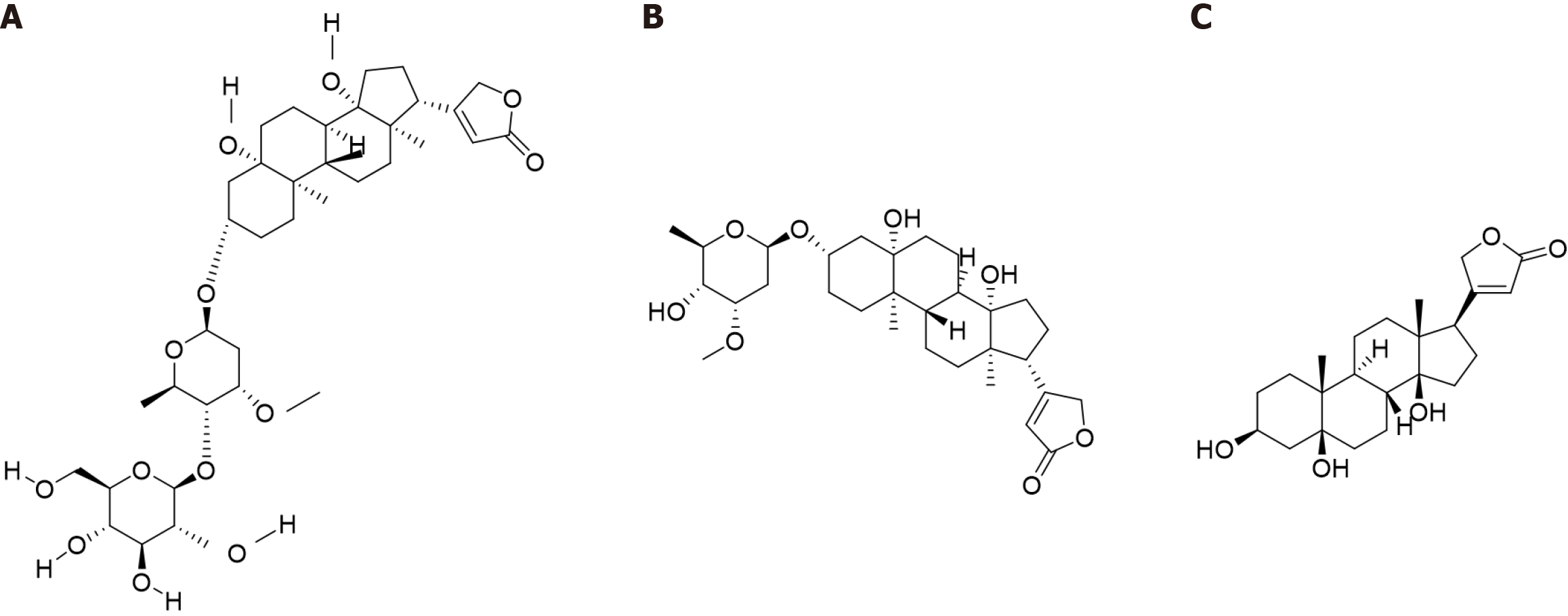
Cortex Periplocae is rich in periplocin, periplocymarin and periplogenin, which are cardiac glycosides and are widely recognized as the main active compounds in Cortex Periplocae[15]. The most cytotoxic constituent of Cortex Periplocae, periplocin is an alpha cardiac glycoside with a steroid core and an unsaturated five-membered lactone ring structure. The structure of periplocin contains periplogenin and a linked glycosyl portion, specifically periplogenin-D-cannabinose-D-glucose. Periplocin has various biological functions, including cardiotonic, diuretic, antitumor, anti-inflammatory, and immunomodulatory effects[16,17]. Owing to a larger number of sugar groups in the molecule, which results in increased water solubility, decreased lipophilicity, and weakened affinity for myocardial cell membrane lipids, the cardiotonic effect of periplocin is less than that of periplocymarin (Figure 1A).
Figure 1 Compound structure.
A: Structural formula of periplocin; B: Structural formula of periplocymarin; C: Structural formula of periplogenin.
Periplocymarin is composed of periplogenin-D-canadine and is a secondary alpha cardiac glycoside, which is produced by the removal of one glucose molecule from periplocin. The activity and toxicity of periplocymarin are greater than those of periplogenin but less than those of other alpha cardiac glycosides because of its greater affinity toward cardiac cell membrane lipids than that of sapogenins. Periplocymarin is generally used as a diuretic[18]. Periplocymarin has a rapid onset and a short duration of action, which facilitates its clinical administration. Moreover, periplocymarin does not accumulate, which reduces the toxic side effects of its long-term use. In addition, periplocymarin has high permeability and does not undergo p-glycoprotein efflux or cytochrome P450 metabolism, which suggests that periplocymarin is a natural drug with great potential (Figure 1B)[19].
Periplogenin is the glycoside portion, i.e., the core structure with the sugar group removed, and the basic unit of alpha cardiac glycosides, including periplocin and periplocymarin. Periplogenin has some biological activities, but they are usually weaker than those of its corresponding glycosides. Periplogenin is often used as an antitumor agent and a diuretic[20]. Several pharmacological studies have shown that periplogenin can be used to treat hyperthyroidism and cardiovascular diseases (Figure 1C)[21].
Extraction and separation methods
Solvent extraction: According to the solubility of components, such as periplocin, in specific solvents, a suitable solvent can be selected for extracting Cortex Periplocae to obtain target compound extracts. The process includes selecting solvents (such as methanol or ethanol), drying and crushing plant material, mixing, extracting, concentrating and washing. Although this method is simple and inexpensive, the purity of the extract is low, and the selectivity is poor. In addition, the yield is strongly affected by the raw material used[22].
High-performance liquid chromatography: According to differences in solubility and adsorption of periplocin, periplocymarin and periplogenin in specific mobile phases[23], separation of Cortex Periplocae components can be achieved via a chromatographic column after cleaning, drying, crushing, and reflux extraction or ultrasonic extraction with ethanol and other solvents to optimize the extraction time and temperature. The extract can be concentrated and purified to remove impurities, and the appropriate pore size, column (Agilent ZORBAX SB-C18 or Venusil PLOW-C18 column) and mobile phase are utilized. The flow rate and detection wavelength are adjusted to ensure the separation and detection of the target compounds. High-performance liquid chromatography has high separation efficiency and a high degree of automation, is easy to operate, and is suitable for high-throughput sample analysis, which requires pretreatment to prevent clogging of the column and ensure sample purity and solubility[24,25].
External and internal metabolic processes
The study of the pharmacokinetics of the active ingredients of Chinese medicines is necessary for their rational use and further development. (1) Zhou et al[26] administered three doses (0.37 mg/kg, 0.74 mg/kg, and 1.48 mg/kg) of a test drug to rats, after the result were fitted to the atrial compartment model, the in vivo process of periplocin conformed to the two-atrial model (weight 1/c), with distribution phase half-lives t1/2α of 1.49 minutes, 2.32 minutes, and 3.48 minutes and elimination phase half-lives t1/2β of 14.00 minutes, 12.37 minutes, and 15.44 minutes, respectively. These results suggest that the distribution and elimination of periplocin in vivo are dose dependent and that the elimination phase half-life is relatively long, which indicates that there may be a certain accumulation effect of periplocin in vivo; and (2) Gao et al[27] reported that after intravenous administration of periplocin to rats, its mass concentration in all tissues decreased in the following order: Liver > blood > kidney > heart > lung > spleen > brain. At 2 minutes after administration, periplocin was rapidly distributed to all tissues, except the brain, and the mass concentration of periplocin was greater in the liver, kidneys, and heart, which are adequately perfused with blood. The fact that periplocin was not detected in the brain at all time points may be related to the strong polarity of periplocin and its inability to easily cross the blood-brain barrier. As a selective permeability barrier, the blood-brain barrier usually restricts the passage of polar molecules and macromolecules. The polar character of periplocin may be the main reason for its inability to enter the central nervous system. These studies suggest that periplocin is rapidly distributed and eliminated in the body.
ANTICANCER ACTIVITY AND MECHANISM OF ACTION
Cell proliferation inhibition
The cell cycle is the entire process from the completion of one cell division to the end of the next, and it is regulated mainly by the cyclin- cyclin-dependent kinase (CDK)-CDK inhibitor (CDKI) signaling regulatory network. This complex regulatory mechanism ensures the orderly progression of the cell cycle, which is crucial for the normal growth and division of cells. However, in studies of tumor pathogenesis, abnormal cell cycle activity and uncontrolled tumor cell proliferation, which are due to aberrant expression of cell cycle proteins, have been reported. Periplocin can affect cell cycle proteins and block cancer cell proliferation by modulating retinoblastoma (Rb)-based signaling pathways. (1) Periplocin downregulates the expression of cyclin E1, cyclin D, and CDK2/4/6, in pancreatic cancer cells, arresting them in the G0/G1 phase[28]; and (2) Periplocin inhibits the proliferation of lymphoma cells and downregulates the expression of the CDK1 and cyclinB1 proteins, leading to cell cycle arrest in the G2/M phase[29]. Other studies have shown that cell proliferation is also regulated by p53, which induces the expression of p21 to regulate cell cycle division. The p21 protein is a CDKI that degrades cyclins, inhibits CDK activity, binds to the cyclin/CDK complex or proliferating cell nuclear antigen, and prevents cells from entering the S phase, thus causing G1 phase arrest[30]. In colorectal cancer cells, periplocymarin significantly increases the proportion of cells in the G0/G1 phase and significantly decreases the proportion of cells in the S phase by increasing the expression of p21[31]. Therefore, blocking cell proliferation is an important tool in the treatment of cancer.
Induction of apoptosis
Apoptosis is an autonomous extinction process that is strictly regulated by cell signaling and can be divided into a cell death phase and a cell clearance phase. Apoptosis involves both endogenous and exogenous pathways. The endogenous pathway is activated by the release of cytochrome c (cyt c) from mitochondria, and the exogenous pathway is triggered by tumour necrosis factor alpha-related apoptosis-inducing ligand receptor (TRAIL) and Fas death receptors, which together activate a series of proteases, including caspase-3, caspase-6, caspase-7, caspase-8, caspase-9, and caspase-10, ultimately leading to cell death[32-34]. Periplocin induces tumor cell death through the endogenous apoptotic pathway. (1) In breast cancer cells, periplocin increases the accumulation of endogenous reactive oxygen species (ROS), which in turn leads to the release of large amounts of cyt c from mitochondria and significantly elevates the expression levels of the apoptosis-associated proteins caspase-3, caspase-8, and caspase-9[35]. Initiator caspases are activated upon acquisition of apoptotic signals by cells, thus triggering the apoptosis cascade response, whereas executioner caspases are activated in response to initiator caspases to execute the final apoptotic program[36]; and (2) Similarly, periplocin induces apoptosis in pancreatic cancer cells by increasing the expression levels of apoptosis-related proteins [Bcl-2-associated X protein (BAX), cleaved caspase-8, and cleaved caspase-3] and decreasing the expression level of B-cell chronic lymphocytic leukemia/lymphoma-2 (BCL-2)[37]. BAX promotes apoptosis and initiates the apoptotic program by increasing mitochondrial membrane permeability and releasing factors, such as cyt c. BCL-2 is an antiapoptotic protein, which is embedded in the mitochondrial membrane, and it captures BAX to prevent the cell from entering the apoptotic program. The main function of BCL-2 is to inhibit proapoptotic factors, such as BAX, and protect cells. BCL-2 and BAX form a heterodimer and together regulate apoptosis. When the expression of BCL-2 is high, the heterodimer formed with BAX is predominant and inhibits apoptosis; when the expression of BAX is high, a homodimer is formed, and apoptosis is induced. The BAX/BCL-2 ratio determines the degree of apoptosis inhibition[38].
Periplocin also induces apoptosis through the exogenous apoptotic pathway. (1) In human mucinous fibrosarcoma cells, periplocin increases the gene expression levels of some death receptors, including BCL-2, TRAIL-R1, and TRAIL-2, which significantly increases the proportion of late-stage apoptotic cancer cells[39]. The TRAIL death receptor, a member of the tumor necrosis factor (TNF) receptor superfamily, specifically binds to TRAIL and transmits apoptotic signals, selectively inducing apoptosis in tumor cells but having no toxic effects on the vast majority of normal cells[40]; and (2) In addition, periplocin promotes liposarcoma cell apoptosis by binding to the Fas receptor via Fas ligand (FasL) and triggering the apoptotic mechanism via Fas-associated death domain (FADD) and caspase-8[41]. Both FasL and Fas are members of the TNF superfamily, and once they are bound, they trigger the trimerization of the Fas receptor and the recruitment of the FADD protein. FADD then binds to the caspase-8 precursor through its death effector structural domain to form a death-inducing signaling complex (DISC). The caspase-8 precursor self-cleaves and is activated by the DISC, which initiates a cascade of caspase reactions and ultimately activates executioner caspases, leading to cellular apoptosis.
Periplogenin and periplocymarin also induce tumor cell death through the endogenous apoptotic pathway. (1) In colorectal cancer cells, periplogenin increases the accumulation of endogenous ROS, promotes the expression of the proapoptotic factor BAX, and decreases the expression of the antiapoptotic factor BCL-2[42]. ROS are unstable and reactive molecules that are produced by oxygen during cellular metabolism. ROS induce DNA damage and genomic instability, are involved in the metabolic reprogramming of tumor cells and promote tumor cell migration and invasion[43]; and (2) In colorectal cancer cells, periplocymarin increases the protein levels of BAX, cleaved caspase-3, caspase-9, and caspase-7, and significantly decreases the protein expression level of BCL-2, thereby effectively promoting apoptosis[44]. Therefore, inducing tumor cells to undergo apoptosis is an important means of cancer treatment.
Relationship between cell cycle and apoptosis
Cell cycle proteins can regulate apoptosis. Cyclin D1 is the first cyclin synthesized by cells that enter the proliferative cycle, and it phosphorylates the G1-phase repressor protein (Rb) by binding to and activating the CDK4 or CDK6, which are specific to the G1 phase. The phosphorylated Rb protein dissociates from its bound E2F transcription factor, and the released E2F transcription factor initiates the transcription of genes involved in the cell cycle, thereby driving the cell cycle from the G1 to the S phase, which in turn promotes cell proliferation[45]. In serum-starved rat fibroblasts, the expression of cyclin D1 through an inducible promoter leads to apoptosis[46]. Similarly, increased expression of cyclin D1 in mammary epithelial cells enhances apoptosis when the cells are subjected to serum starvation[47]. Similarly, cyclin D3 sensitizes tumor cells to TNF-induced apoptosis, further supporting the notion that other cell cycle protein complexes may be involved in mediating the apoptotic response[48]. The p53 is a cell cycle-associated oncogene that is activated when cells are stressed and undergo uncontrolled division and proliferation. When cellular damage is irreparable, p53 upregulates the proapoptotic protein BAX and inhibits the antiapoptotic protein BCL-2, thereby triggering programmed cell death (i.e., apoptosis) as a means of controlling the cell cycle, DNA replication, and uncontrolled cell division during tumor growth[49]. In liver cancer cells, the p53 gene is activated in response to cellular stress and uncontrolled division and proliferation. In hepatocellular carcinoma cells, periplocin treatment significantly increased the level of cyclin B1, which induced cell cycle arrest and activated the mitochondrial apoptotic pathway, resulting in increased levels of cleaved caspase-3, cleaved caspase-9, and cleaved polyadenosine-diphosphate-ribose polymerase, an increase in the BAX/BCL-2 ratio, and induction of apoptosis[50]. In colorectal cancer cells, periplocin treatment downregulated the levels of cyclin B1 and CDK1, leading to cell cycle arrest in the G2/M phase, increased the level of cleaved caspase-3 and decreased the level of BCL-2, inducing apoptosis[51]. Thus, there is a close and complex relationship between the cell cycle and apoptosis in cancer cells.
COMBINATION STUDIES
Combinations with conventional chemotherapeutic agents
Oxaliplatin (OXA) is a third-generation platinum-based anticancer drug, which acts in a manner similar to that of other platinum-based drugs, with the platinum atoms interacting with DNA to block DNA replication and transcription[52,53]. The combination with periplocin increases the sensitivity of cancer cells to OXA. In hepatocellular carcinoma, the combination treatment with periplocin decreases the the half-maximal drug inhibitory concentration value of OXA against an OXA-resistant hepatocellular carcinoma cell line (HepG2/OXA) and increases the protein expression levels of BAX and caspase-3, thus blocking cell proliferation and inducing apoptosis. Moreover, periplocin inhibits M2 macrophage polarization, which increases the resistance of HepG2/OXA cells to OXA, thereby enhancing the sensitivity of HepG2/OXA cells to OXA in a synergistic manner[54].
Combination with targeted anticancer drugs
Tumor drug resistance refers to the phenomenon of increased tumor cell tolerance to chemotherapeutic drugs, targeted drugs and other drugs, which leads to a decrease in their efficacies. Tumor drug resistance is common in the treatment of cancer and has become a major factor in the failure to cure cancer patients. Early chemotherapeutic drugs were initially used with apparent success, but subsequent studies have revealed that despite the ability of chemotherapeutic drugs to shrink tumors and even eliminate them, tumor cells become resistant to the treatment. The emergence of this problem has led researchers to search for new solutions[55]. The solution to the problem of resistance to single-agent chemotherapy is the combination of drugs with nonoverlapping mechanisms of action or multiagent chemotherapy[56].
The combination of periplocin and TRAIL increases the sensitivity of cancer cells to TRAIL. (1) Zhao et al[57] evaluated the inhibitory effect of the combination of periplocin and TRAIL on gastric cancer formation by establishing a gastric cancer model in BALB/c nude mice. Periplocin enhanced the sensitivity of gastric cancer cells to TRAIL by increasing the expression of death receptors Apo Trail R1 (DR4) and death receptors Apo Trail R2 (DR5) at the transcriptional and protein levels, which indicated that periplocin acted as a sensitizer of TRAIL. Similar results have been reported in esophageal squamous cell carcinoma; (2) Han et al[58] evaluated the efficacy of a combination regimen of periplocin with AAV-TRAIL in an esophageal squamous cell carcinoma model in BALB/c nude mice. They reported that periplocin treatment increased the expression levels of DR4 and DR5 but significantly decreased those of FoxP3, and survivin by affecting the nuclear localization of β-catenin and inhibiting the activity of the Wnt/β-catenin pathway in cancer cells; and (3) In addition, Cheng et al[59] reported that the combination of periplocin and TRAIL significantly inhibited the growth of hepatocellular carcinoma in a tumor xenograft model in SCID mice. They reported that the expression levels of DR4 and FADD increased after periplocin treatment and that caspase-3, caspase-8, and caspase-9 were activated, leading to the apoptosis of hepatocellular carcinoma cells. These findings indicate that the combination of periplocin and TRAIL inhibits cancer cell proliferation and induces apoptosis both in vitro and in vivo. This combination provides new ideas for the treatment of other cancers and overcoming the resistance of cancer cells to TRAIL.
SAFETY AND TOXICOLOGICAL EVALUATION
Acute toxicity
Acute toxicity is the deleterious effect on an organism exposed to a high single dose ora repeated dose of a compound within a short period of time, and acute toxicity is important to consider when assessing the safety of compounds. Wang et al[51] reported that mice treated with a high dose of periplocin (15 mg/kg/day) for a short period of time presented no significant toxicity, as indicated by the lack of significant body weight fluctuations during the experimental period. These researchers also reported no significant pathological changes in major organs (heart, liver, spleen, lungs and kidneys) of the mice and no significant pathological organ damage as observed by hematoxylin and eosin staining. Although high doses of periplocin are relatively safe in the short term, potential chronic effects need to be evaluated by long-term exposure at low doses.
Chronic toxicity
Chronic toxicity refers to the harmful effects of repeated exposure of an organism to a low dose of a compound over a long period of time. Chronic toxicity is important when assessing the long-term safety of compounds, and the mechanism of chronic toxicity should be elucidated to provide a basis for subsequent drug optimization. Wang et al[60] reported that compared with those in the normal control group, the serum levels of creatine kinase (CK), lactate dehydrogenase, hydroxybutyrate dehydrogenase, and CK isoenzyme were significantly increased in the periplocin group. In addition, these researchers reported that the levels of cardiac enzymes in rats were normalized after administration of periplocin and Panax notoginseng saponin (PNSs) by gavage. Myocardial fiber degeneration and necrosis (5%-10%), as well as intermyocardial lymphocyte infiltration (5%-10%), were observed in the cardiac tissues of the rats in the periplocin group, and intermyocardial fibers were focally infiltrated by lymphocytes in some cardiac tissues of the rats in the high-dose group (1%-5%). Myocardial fibers were closely arranged in an orderly manner in the normal control, PNS, and low-dose groups, and no degeneration, necrosis or inflammatory cell infiltration was observed. These findings suggest that periplocin gavage for 14 days induces cardiac tissue damage in rats, but myocardial damage is attenuated when periplocin is administered with PNSs. According to the experimental results, periplocin significantly increased the serum levels of cardiac enzymes, suggesting that periplocin can disrupt the integrity of myocardial cell membranes, by generating ROS, which can trigger lipid peroxidation and damage the structure of cell membranes. Histopathological observations of myocardial fiber degeneration and necrosis and lymphocyte infiltration (5%-10%) suggest that periplocin may activate the immune system, leading to inflammatory cell infiltration and further exacerbating myocardial injury. Therefore, periplocin may promote the secretion of inflammatory factors such as TNF-α and interleukin-6, by activating inflammatory pathways such as the nuclear factor-kappa B (NF-κB) pathway. The combination with PNSs significantly attenuated myocardial injury caused by periplocin, and their protective effects may involve the following antioxidant effects: (1) Scavenging free radicals or inhibiting ROS generation to attenuate oxidative stress damage to cardiomyocytes; and (2) Exerting anti-inflammatory effects: By inhibiting the release of inflammatory factors (e.g., downregulating the NF-κB signaling pathway) and reducing lymphocyte infiltration. On the basis of the above mechanisms, the toxicity of periplocin can be reduced via the following strategies: (1) Combination optimization: Combining periplocin with PNSs at specific ratios (e.g., low-dose group) to neutralize toxicity by taking advantage of the antioxidant and anti-inflammatory properties of PNSs; and (2) Drug development: Inhibition of key toxicity pathways: Via development of small-molecule inhibitors to block periplocin -induced ROS generation or inflammatory signaling.
RESEARCH AND PROSPECT
Multiomics techniques have become important tools for revealing the mechanisms of action of anticancer compounds. By integrating these techniques, a comprehensive understanding of drug action at the cellular and molecular levels is possible. Metabolomics can detect changes in cellular metabolites after drug treatment, thus revealing the effects of drugs on cellular metabolism. For example, periplocymarin exerts dual inhibitory effects on glycolysis and mitochondrial oxidative phosphorylation in esophageal squamous cell carcinoma cells through the phosphoinosytol-3 phosphate/protein kinase B and mitogen-activated protein kinase/extracellular signal-regulated kinases signaling pathways, both of which are critical for increasing the efficacy of targeted metabolic drugs[61]. More effective drug dosage forms can be developed through interdisciplinary collaboration. Multiomics technologies can reveal the multitarget mechanisms of action of drugs and provide a basis for developing combination therapeutic regimens. For example, periplogenin inhibits the proliferation of esophageal squamous carcinoma cells by targeting signal transducer and activator of transcription 3 (STAT3) and is more effective than other STAT3-targeting agents are[51]. By optimizing the drug dosage form and dosing regimen, the toxic side effects of the drug can be reduced and patient tolerability can be improved. Genomics and proteomics can be used to screen for drug-sensitive patient groups and achieve personalized treatment. In conclusion, it is highly important to explore the anticancer mechanisms of periplocin, periplocymarin and periplogenin with the help of multiomics technology, optimize the dosages of these drugs through interdisciplinary cooperation, and carry out a larger-scale clinical study to improve the prospects of their anticancer applications.
CONCLUSION
Cancer, also known as a malignant tumor, is characterized by the uncontrolled growth, division and proliferation of cells in the body. Currently, the incidence of and mortality rates from cancer remain high, placing a heavy burden on countless families. Given the current situation with cancer, there is an urgent need to explore effective drugs with low toxicity and few side effects for the treatment or adjuvant therapy of cancer. Tumor development involves multiple associations, pathways, and targets, and the complexity of the interactions of each association is likely to trigger clinical responses, such as limited therapeutic effects and significant side effects. Periplocin, periplocymarin and periplogenin are strong natural product cardiac glycoside analogs from Cortex Periplocae, and they have significant inhibitory effects on many types of tumors. Numerous experimental studies have shown that these Periploca sepium compounds can block malignant cell proliferation by regulating cell cycle proteins, activating caspase proteases and inducing cancer cell apoptosis. With the development of multiomics technology, we can systematically analyze the cardiotoxicity mechanisms (e.g., mitochondrial damage, oxidative stress, and activation of inflammatory pathways) of the periplocin compounds and validate the synergistic toxicity-reducing mechanisms (e.g., antioxidant, anti-inflammatory, and membrane-stabilizing effects) of PNSs and other paired drugs. The difference in the toxicity of periplocymarin and periplogenin compared with that of periplocin were explored, and candidate molecules with lower toxicity and better anticancer activity were identified. The design of prodrugs or sustained-release dosage forms should be optimized through metabolomics analysis to extend the half lives of the drugs and reduce the accumulation of toxic metabolites. In the future, we need to systematically optimize the pharmacokinetic properties and reduce the toxicity of the Periploca sepium compounds by using multiomics technology as the core and combining it with drug delivery strategies. In summary, the Periploca sepium compounds have great potential for application in the field of tumor therapy. By overcoming existing challenges and elucidating the mechanism of action, the barberry compounds are expected to become key drugs in the future of tumor therapy.