Published online Aug 24, 2025. doi: 10.5306/wjco.v16.i8.107306
Revised: May 11, 2025
Accepted: July 3, 2025
Published online: August 24, 2025
Processing time: 153 Days and 3.2 Hours
Ki-67 is a routine test item in clinical pathology departments. However, its prognostic value requires further investigation, especially in the context of research using machine learning (ML), which remains relatively underdeveloped.
To investigate the prognostic value of Ki-67 in cases of colorectal carcinoma (CRC) and explore the potential application of ML algorithms to predict the Ki-67 index.
Case data and pathological sections from two centers were systematically collected. To analyze the prognostic value of the Ki-67 index in CRC, multiple cutoff values were established. Meanwhile, by virtue of the histological features presented in the hematoxylin and eosin-stained CRC images, three mainstream ML algorithms, support vector machine (SVM), random forest (RF), and eXtreme gradient boosting (XGBoost) were employed to construct prediction models. Subsequently, the potential of these algorithms to classify and predict the Ki-67 index was explored.
Non-parametric tests revealed that Ki-67 ≥ 40% correlated with a high histological grade (P = 0.017), deficient mismatch repair protein status associated with ≥ 50%-90% cutoffs (all P ≤ 0.028), and ≥ 80% linked to lymph node metastasis (P = 0.006). Kaplan-Meier analysis showed that Ki-67 ≥ 50% predicted higher survival (log-rank P = 0.0299, hazard ratio = 2.142), with no differences for other cutoffs. COX regression identified the Ki-67 positive rate as a significant predictor (P = 0.027, hazard ratio = 2.583), while other variables had no association. In algorithmic model predictions, the SVM, RF, and XGBoost models achieved training area under the curve (AUC) values of 0.851, 0.948, and 0.872, respectively, with corresponding test set AUC values of 0.795, 0.755, and 0.750, respectively. During external validation, their AUC values for predicting Ki-67 status reached 0.757, 0.749, and 0.783, respectively.
In algorithmic model predictions, the SVM, RF, and XGBoost models achieved training AUC values of 0.851, 0.948, and 0.872, respectively, with corresponding test set AUC values of 0.795, 0.755, and 0.750, respectively. During external validation, their AUC values for predicting Ki-67 status reached 0.757, 0.749, and 0.783, respectively.
Core Tip: This study pioneers the application of machine learning to predict Ki-67 status in colorectal carcinoma directly from hematoxylin and eosin-stained images. By analyzing data, 50% was identified as the optimal Ki-67 cutoff, with high-expression being linked to improved survival rates and low-expression being associated with advanced tumor stage and lymph node metastasis. Predictive models were developed using the support vector machine, random forest, and eXtreme gradient boosting algorithms, achieving area under the curve values (0.851-0.948 in training and 0.750-0.795 in the external validation group). This innovative approach highlights the potential of machine learning to enhance prognostic accuracy.
- Citation: Zeng DT, Li MJ, Lin R, Huang WJ, Li SD, Huang WY, Li B, Li Q, Chen G, Jiang JS. Prognostic role of Ki-67 in colorectal carcinoma: Development and evaluation of machine learning prediction models. World J Clin Oncol 2025; 16(8): 107306
- URL: https://www.wjgnet.com/2218-4333/full/v16/i8/107306.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i8.107306
Colorectal carcinoma (CRC) is one of the most prevalent and lethal cancers globally[1,2]. In the past three decades, a contrasting trend has been observed in the incidence and mortality rates of CRC between the United States and China. While the United States has experienced a decline in these rates, China has seen a consistent increase[3]. Additionally, the proportion of young individuals (< 50 years old) diagnosed with CRC is increasing annually, the disease is more prevalent in the distal colorectum, such as rectal and left-sided colon cancers[4]. Given these circumstances, the exploration of early diagnostic methods, accurate prognostic assessment, and personalized treatment strategies for CRC is of utmost clinical significance.
Numerous studies have identified Ki-67 as a key biomarker in the prognosis of various tumors[5]. As early as 1999, Palmqvist et al[6] discovered that in Duke’s B stage CRC, a low Ki-67 index at the invasive tumor margin was associated with a poor prognosis[6]. On the one hand, some studies have proposed that high Ki-67 expression in CRC is linked to favorable clinical outcomes[7,8]. On the other hand, additional investigations have revealed that high-expression levels of Ki-67 are negatively correlated with the maturation of tertiary lymphoid structures. Intriguingly, the degree of tertiary lymphoid structure maturation is positively associated with patient survival rates, indicating that the role of Ki-67 in the CRC tumor microenvironment might be intricately intertwined with immune responses[9].
Furthermore, high Ki-67 expression has been identified as an adverse indicator of invasiveness in colorectal adenomas and as a risk factor for the development of CRC[10]. Combining the expression levels of Ki-67 with other biomarkers, such as p53 and RNF135, has shown the potential to provide a more precise prediction of patient survival rates and disease progression[11,12]. These collective findings strongly underscore the clinical utility of Ki-67 as an independent prognostic biomarker for CRC. Nevertheless, due to the inconsistent cutoff values of Ki-67 applied across different studies, substantial variations in patient prognoses have been observed. Consequently, future research should focus on determining the optimal prognostic-related cutoff value of Ki-67, which is essential for enhancing the accuracy of clinical interpretation and patient management.
In the realm of detecting Ki-67 expression in tumor tissues to determine post-treatment tumor cell proliferation activity, immunohistochemistry (IHC) is a conventional and reliable method[13]. However, the inherent heterogeneity of tumor cells and the subjectivity of observers invariably give rise to discrepancies during the interpretation of Ki-67 expression rates. The hematoxylin and eosin (HE) stain has stood the test of time as the standard stain for histologic examination of human tissues. This simple dye combination can highlight the fine structures of cells and tissues[14].
In the analysis of HE-stained images using machine learning (ML), domain-agnostic features enable a more effective characterization of image features across diverse diseases and tissue types[15]. Given the assumption that the differences between Ki-67-positive and -negative cells in IHC-stained sections correlate with the histological features of the corresponding region in HE-stained images, there is a need to establish a connection between the cellular feature information in HE images and the IHC staining status. Identifying and inferring such information from HE-stained sections via ML techniques would substantially boost interpretation efficiency and alleviate subjective bias. Moreover, this approach could potentially eliminate the need for IHC staining, thus conserving both economic and human resources.
ML, a pivotal branch of artificial intelligence (AI) technology, extracts features by harnessing domain knowledge, enabling computers to learn and make decisions and predictions regarding real-world events[16]. This process unearths latent information within the experimental data. With the widespread application of computer-aided diagnostic technologies in medical image processing, the practice of pathological diagnosis has undergone a remarkable transformation. Novel tools, such as digital imaging, advanced AI algorithms, and computer-aided diagnostic techniques, increasingly facilitate and augment computational pathology and AI-based diagnostics[17].
The digitization of pathological images not only enables seamless transmission of rich pathological data across remote locations, but can be widely adopted in digital diagnostics, teleconsultations, education, and research[18,19]. The efficacy of computer-assisted digital whole-slide imaging (WSI) algorithms for cancer diagnosis has approached the level of experienced pathologists[20,21]. Additionally, certain algorithms can predict molecular marker statuses[22,23], detect cancer-associated gene mutations[24,25], assess treatment efficacy[26,27], and estimate patient survival rates[28,29]. These investigations reveal the promising potential of AI in extracting comprehensive and detailed information from standard pathological images. Satisfactory outcomes have occurred in numerous cancers, including CRC. However, to date, no applications of AI technology for predicting Ki-67 status from HE images of CRC have emerged.
This study aims to explore the optimal cutoff value of the Ki-67 index in CRC regarding its prognostic value and to construct algorithmic models for Ki-67 classification prediction by leveraging HE image features and ML. The overarching goal is to reduce or even eliminate subjective bias among pathologists through AI-assisted means, enhance work efficiency, reduce medical costs, and achieve a more accurate and standardized prediction of CRC patient prognosis.
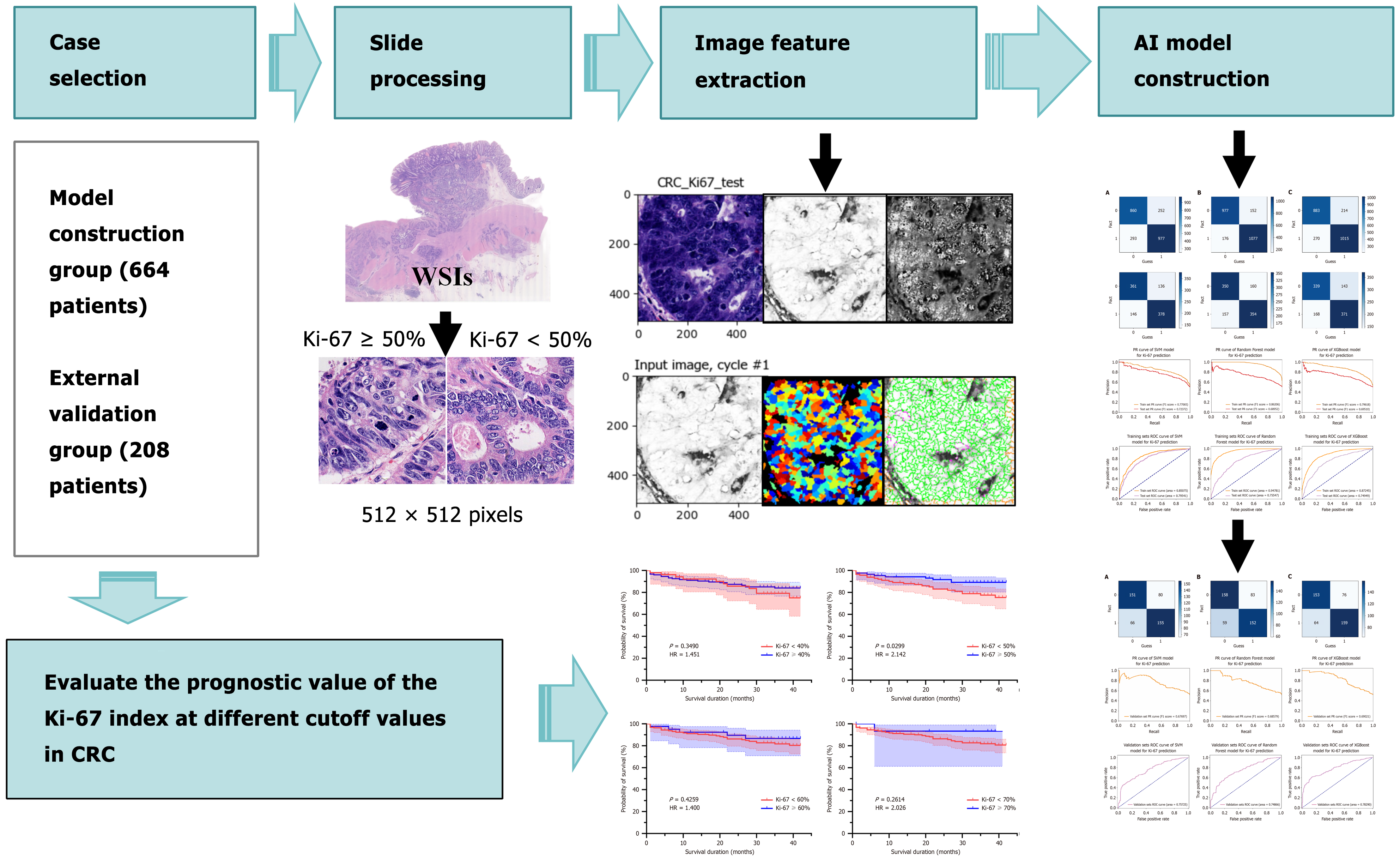
The technical workflow of this study is illustrated in Figure 1.
This retrospective study gathered data from 872 patients diagnosed with primary CRC. Among them, 664 patients had undergone surgical resection at the First Affiliated Hospital of Guangxi Medical University between April 2019 and April 2021, and 208 patients at the Yulin Red Cross Hospital from April 2020 to March 2021. The inclusion criteria consisted of the following: (1) Pathological confirmation of CRC; (2) Radical surgical specimens; (3) Complete clinical and pathological data; and (4) Availability of IHC results for Ki-67 protein. The exclusion criteria encompassed: (1) CRC other than carcinoma or metastatic cancer; (2) Treatment limited to biopsy, submucosal dissection, or simple tumor resection; (3) Incomplete clinical and pathological data; and (4) Absence of IHC results for Ki-67 protein.
Ki-67 expression was evaluated using nine pre-specified cutoff values (10%, 20%, 30%, 40%, 50%, 60%, 70%, 80% and 90%). However, cutoffs of 10%, 20%, and 30% were excluded due to excessively small sample sizes in the lower-expression groups (Table 1), which posed risks of bias and insufficient statistical power. The final analysis included six cutoff values: 40%, 50%, 60%, 70%, 80%, and 90%, with patients stratified into two groups for each cutoff: < cutoff value and ≥ cutoff value.
| Cutoff | < cutoff (n) | ≥ cutoff (n) | Total (n) | Exclusion reason |
| 10% | 3 | 869 | 872 | < 1% in < cutoff group |
| 20% | 9 | 863 | 872 | 1% in < cutoff group |
| 30% | 15 | 857 | 872 | < 2% in ≥ cutoff group |
| 40% | 82 | 790 | 872 | Retained |
| 50% | 200 | 672 | 872 | Retained |
| 60% | 301 | 571 | 872 | Retained |
| 70% | 437 | 435 | 872 | Retained |
| 80% | 578 | 294 | 872 | Retained |
| 90% | 728 | 144 | 872 | Retained |
Normality of clinical pathological parameters - gender, age, tumor stage, tumor location, number of lesions, histological type, histological grade, vascular invasion, nerve invasion, lymph node metastasis, and mismatch repair (MMR) protein status - was assessed using the Shapiro-Wilk test. Since all parameters failed to meet the normal distribution assumption, non-parametric tests were employed. The Mann-Whitney U test (equivalent to the Wilcoxon rank-sum test) was used to compare differences between the two groups (< cutoff vs ≥ cutoff) for each continuous or ordinal variable. The Wilcoxon W statistic, a value derived from the Mann-Whitney U test, and the standardized Z score were reported. Statistical significance was defined as a two-tailed P value < 0.05. All analyses were performed using the SPSS software (Version 26.0, IBM).
A total of 208 CRC patients from the Yulin Red Cross Hospital were included in this survival analysis. The patients were initially stratified into seven Ki-67 expression cutoff groups: 20%, 30%, 40%, 50%, 60%, 70%, and 80%. Groups with < 3% of cases in the lower cutoff category (i.e., < 20%, < 30%, and ≥ 80%) were excluded due to increased risk of bias and insufficient statistical power (Table 2). The remaining four cutoffs (40%, 50%, 60%, and 70%) were used for Kaplan-Meier survival analysis. Survival curves were compared using the log-rank test hazard ratios (HRs) with 95% confidence intervals were calculated to assess the associations between Ki-67 expression and patient survival. Statistical significance was defined as P < 0.05. All the analyses mentioned above were conducted using GraphPad Prism (Version 8.0). To comprehensively assess the influence of Ki-67 expression and other potential confounding factors - gender, age, tumor budding, pathological grade, clinical stage, mismatch repair function, and lymph node invasion - on patient survival, a COX proportional-hazards regression analysis was conducted using SPSS software (Version 26.0, IBM).
| Cutoff | < cutoff (n) | ≥ cutoff (n) | Total (n) | Exclusion reason |
| 20% | 2 | 206 | 208 | < 1% in < cutoff group |
| 30% | 6 | 202 | 208 | < 3% in < cutoff group |
| 40% | 54 | 154 | 208 | Retained |
| 50% | 117 | 91 | 208 | Retained |
| 60% | 165 | 43 | 208 | Retained |
| 70% | 190 | 18 | 208 | Retained |
| 80% | 204 | 4 | 208 | < 2% in ≥ cutoff group |
| 20% | 2 | 206 | 208 | < 1% in < cutoff group |
| 30% | 6 | 202 | 208 | < 3% in < cutoff group |
Pathologists with over five years of experience screened all HE-stained sections from the included cases based on pathological results and reviewed them under a microscope to ensure accuracy. The selection criteria demanded complete tissue within the HE-stained section, clear and uniform staining, good contrast, and absence of wrinkles and impurities. A digital pathology slide scanner (model: KF-PRO-020) scanned the HE-stained sections. Operators correctly set all scanner parameters, and precisely scanned the images at a magnification of 400 ×, then saved them as high-resolution WSIs in tagged image file format.
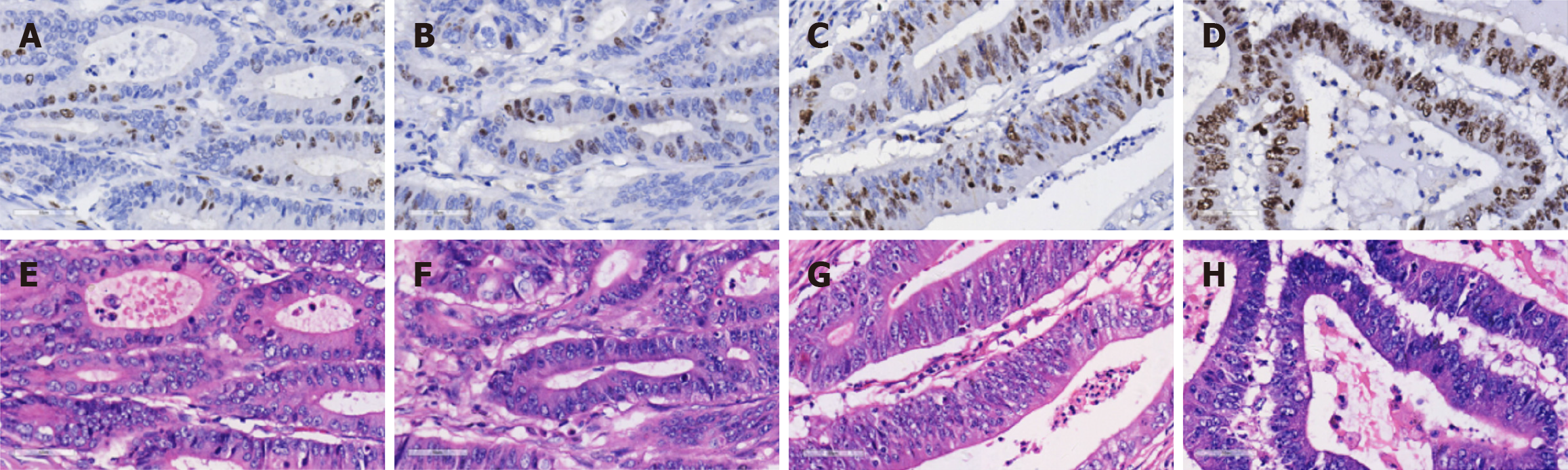
Based on previous studies on Ki-67 cutoff values[30], we selected the most frequently reported 50% as the threshold for the ML-based predictive grouping of Ki-67 expression. When the proportion of positively stained CRC cell nuclei reaches or exceeds 50%, it indicates high expression; otherwise, it indicates low expression. To mitigate the impact of the non-uniform distribution of Ki-67 hotspots caused by tumor heterogeneity, two senior pathologists first identified regions with Ki-67 positivity rates closest to those reported in pathological diagnoses on IHC sections of CRC, with consensus reached through joint evaluation. Subsequently, corresponding tumor regions were localized in the WSI of HE-stained sections from the same tissue samples to facilitate targeted image acquisition. Figure 2 displays representative cases across the low, moderate, and high Ki-67 expression ranges.
Leveraging image characteristics, we employed the open-source software Cellprofiler 4.2.5 to extract 350 quantitative image features, such as “area shape”, “intensity”, “location”, “radial distribution”, and “texture”. After eliminating non-valid image features, we selected 225 quantitative image features that demonstrated statistically significant differences between the high and low Ki-67 expression groups for subsequent analysis. We trained a random forest (RF) model using these feature datasets and computed feature importance to identify the 31 most crucial image features in the dataset (Supplementary Table 1). We averaged the target image features and utilized SPSS 26.0 for data analysis, organizing the data in Microsoft Excel (combining data from both groups and excluding features without significant differences). To achieve a higher level of statistical significance, we consistently chose feature values with P < 0.01. The correlation between the selected 31 features (such as “Texture_SumVariance-Hematoylin”) and Ki-67 expression was tested using the Spearman rank test (P < 0.01), combined with RF feature importance analysis, and biological significance features related to nuclear morphology and staining intensity were prioritized for preservation.
During the training phase of the cases, we predefined the computational parameters of the support vector machine (SVM), RF, and eXtreme gradient boosting (XGBoost) algorithms and furnished the computer with image feature data for recognition. Based on these recognition results, we classified the images into either high or low Ki-67 expression groups, thus constructing a preliminary AI prediction model for CRC Ki-67 classification. We evaluated the performance of the model by generating receiver operating characteristic (ROC) curves and calculating the area under the curve (AUC). We partitioned the samples from the model development cohort into training and testing datasets in a 7:3 ratio with a random seed of 6588. We then optimized the initial parameters of the model using the training dataset and then evaluated the model’s generalization ability on the testing dataset.
Additionally, we selected quantitative image features from the external validation group, the same as those from the model construction group, for external validation of the SVM, RF, and XGBoost algorithms. The goal was to prove the universal applicability of the three models in predicting Ki-67 expression classification in colorectal cancer under different conditions. We appraised the diagnostic performance of the models through ROC curves and AUC, further analyzed algorithm parameters, and ultimately constructed an AI prediction model for CRC Ki-67 classification. The SVM model used the radial basis function as the kernel function, which has good classification performance in nonlinear feature space and is suitable for processing high-dimensional image feature data. GridSearchCV was used to optimize SVM and RF model hyperparameters, and the Python package “optuna” was used to optimize XGBoost model hyperparameters to avoid model overfitting. We implemented the algorithms SVM, RF, and XGBoost algorithms using Python (version 3.11).
Table 3 summarizes the results of the non-parametric tests for six Ki-67 cutoffs (40%-90%). Most parameters showed no significant association with Ki-67 expression at the P < 0.05 threshold. Notable findings included: (1) Histological grade (40% cutoff): Patients with Ki-67 ≥ 40% had a significant association with a high histological grade (Mann-Whitney U = 28922.000, Z = -2.383, P = 0.017), indicating that higher Ki-67 expression was linked to more aggressive tumor differentiation; (2) MMR status (50%, 60%, 70%, 80%, 90% cutoffs): Deficient MMR (dMMR) was significantly associated with Ki-67 ≥ 50% (P = 0.028), ≥ 60% (P = 0.005), ≥ 70% (P < 0.001), ≥ 80% (P < 0.001), and ≥ 90% (P < 0.001). This suggests that dMMR tumors are more likely to exhibit higher Ki-67 expression across these cutoffs; and (3) Lymph node metastasis (80% cutoff): A significant association was observed between Ki-67 ≥ 80% and lymph node metastasis (Mann-Whitney U = 76857.000, Z = -2.744, P = 0.006), indicating that patients with higher Ki-67 expression (≥ 80%) had a greater likelihood of lymphatic spread. No significant associations were found for gender, age, tumor stage, location, lesion number, vascular invasion, or nerve invasion across any of the analyzed cutoffs (all P > 0.05).
| Ki-67 expression groups | Clinicopathological indicators | Mann-Whitney U value | Wilcoxon W value | Z value | Asymptotic significance (two-tailed) P value |
| < 40 vs ≥ 40 | Gender (male, female) | 31612.000 | 344057.000 | -0.425 | 0.671 |
| Age (> 50, ≤ 50) | 28845.000 | 341290.000 | -2.655 | 0.008 | |
| Stage (I and II, III and IV) | 29815.000 | 33218.000 | -1.370 | 0.171 | |
| Tumor location (right colon, left colon, rectum) | 30872.500 | 34275.500 | -0.746 | 0.455 | |
| Number of lesions (1, > 1) | 31917.000 | 344362.000 | -0.679 | 0.497 | |
| Histological type (non-specific type, specific type) | 32110.000 | 35513.000 | -0.294 | 0.769 | |
| Histological grade (low-grade, high-grade) | 28922.000 | 341367.000 | -2.383 | 0.017 | |
| Vascular invasion (no, yes) | 30354.000 | 33757.000 | -1.413 | 0.158 | |
| Nerve invasion (no, yes) | 30897.000 | 343342.000 | -1.039 | 0.299 | |
| Lymph node metastasis (no, yes) | 31463.000 | 34866.000 | -0.508 | 0.611 | |
| MMR (pMMR, dMMR) | 27290.000 | 30140.000 | -1.563 | 0.118 | |
| < 50 vs ≥ 50 | Gender (male, female) | 66972.000 | 293100.000 | -0.086 | 0.931 |
| Age (> 50, ≤ 50) | 63892.000 | 290020.000 | -1.720 | 0.085 | |
| Stage (I and II, III and IV) | 65120.000 | 85220.000 | -0.768 | 0.442 | |
| Tumor location (right colon, left colon, rectum) | 64225.000 | 84325.000 | -1.016 | 0.310 | |
| Number of lesions (1, > 1) | 66812.000 | 292940.000 | -0.387 | 0.699 | |
| Histological type (non-specific type, specific type) | 65788.000 | 291916.000 | -1.030 | 0.303 | |
| Histological grade (low-grade, high-grade) | 64452.000 | 290580.000 | -1.311 | 0.190 | |
| Vascular invasion (no, yes) | 66860.000 | 86960.000 | -0.164 | 0.870 | |
| Nerve invasion (no, yes) | 64780.000 | 84880.000 | -1.169 | 0.242 | |
| Lymph node metastasis (no, yes) | 66728.000 | 292856.000 | -0.180 | 0.857 | |
| MMR (pMMR, dMMR) | 63276.000 | 83376.000 | -2.191 | 0.028 | |
| < 60 vs ≥ 60 | Gender (male, female) | 85356.000 | 130807.500 | -0.194 | 0.846 |
| Age (> 50, ≤ 50) | 83525.000 | 246831.000 | -1.109 | 0.268 | |
| Stage (I and II, III and IV) | 84560.000 | 130011.000 | -0.449 | 0.653 | |
| Tumor location (right colon, left colon, rectum) | 84505.500 | 247811.500 | -0.432 | 0.666 | |
| Number of lesions (1, > 1) | 85805.000 | 249111.000 | -0.115 | 0.908 | |
| Histological type (non-specific type, specific type) | 85373.500 | 248679.500 | -0.363 | 0.717 | |
| Histological grade (low-grade, high-grade) | 84727.500 | 248033.500 | -0.510 | 0.610 | |
| Vascular invasion (no, yes) | 85433.500 | 248739.500 | -0.214 | 0.831 | |
| Nerve invasion (no, yes) | 82664.000 | 128115.000 | -1.397 | 0.162 | |
| Lymph node metastasis (no, yes) | 82995.000 | 246301.000 | -0.989 | 0.322 | |
| MMR (pMMR, dMMR) | 80296.000 | 125747.000 | -2.806 | 0.005 | |
| < 70 vs ≥ 70 | Gender (male, female) | 94780.000 | 190483.500 | -0.085 | 0.932 |
| Age (> 50, ≤ 50) | 92855.000 | 187415.000 | -1.077 | 0.282 | |
| Stage (I and II, III and IV) | 92867.000 | 187697.000 | -0.677 | 0.498 | |
| Tumor location (right colon, left colon, rectum) | 94707.500 | 189537.500 | -0.098 | 0.922 | |
| Number of lesions (1, > 1) | 93942.000 | 189645.000 | -0.927 | 0.354 | |
| Histological type (non-specific type, specific type) | 93333.500 | 188163.500 | -1.051 | 0.293 | |
| Histological grade (low-grade, high-grade) | 94255.500 | 189085.500 | -0.318 | 0.751 | |
| Vascular invasion (no, yes) | 94253.500 | 189083.500 | -0.322 | 0.748 | |
| Nerve invasion (no, yes) | 94907.000 | 189737.000 | -0.057 | 0.955 | |
| Lymph node metastasis (no, yes) | 91071.000 | 185901.000 | -1.272 | 0.203 | |
| MMR (pMMR, dMMR) | 87145.000 | 182848.500 | -3.724 | 0.000 | |
| < 80 vs ≥ 80 | Gender (male, female) | 84512.000 | 127877.000 | -0.153 | 0.878 |
| Age (> 50, ≤ 50) | 82565.000 | 125930.000 | -1.110 | 0.267 | |
| Stage (I and II, III and IV) | 81625.000 | 124990.000 | -1.097 | 0.273 | |
| Tumor location (right colon, left colon, rectum) | 84876.000 | 128241.000 | -0.027 | 0.978 | |
| Number of lesions (1, > 1) | 84769.000 | 128134.000 | -0.175 | 0.861 | |
| Histological type (non-specific type, specific type) | 83122.000 | 126487.000 | -1.196 | 0.232 | |
| Histological grade (low-grade, high-grade) | 84070.000 | 251401.000 | -0.380 | 0.704 | |
| Vascular invasion (no, yes) | 84270.000 | 127635.000 | -0.298 | 0.766 | |
| Nerve invasion (no, yes) | 84853.000 | 128218.000 | -0.049 | 0.961 | |
| Lymph node metastasis (no, yes) | 76857.000 | 120222.000 | -2.744 | 0.006 | |
| MMR (pMMR, dMMR) | 76568.000 | 243899.000 | -4.220 | 0.000 | |
| < 90 vs ≥ 90 | Gender (male, female) | 52336.000 | 62776.000 | -0.034 | 0973 |
| Age (> 50, ≤ 50) | 50368.000 | 60808.000 | -1.206 | 0.228 | |
| Stage (I and II, III and IV) | 51616.000 | 62056.000 | -0.335 | 0.738 | |
| Tumor location (right colon, left colon, rectum) | 50731.000 | 61171.000 | -0.652 | 0.515 | |
| Number of lesions (1, > 1) | 51928.000 | 62368.000 | -0.551 | 0.582 | |
| Histological type (non-specific type, specific type) | 51584.000 | 62024.000 | -0.687 | 0.492 | |
| Histological grade (low-grade, high-grade) | 49548.000 | 314904.000 | -1.549 | 0.121 | |
| Vascular invasion (no, yes) | 50776.000 | 61216.000 | -0.895 | 0.371 | |
| Nerve invasion (no, yes) | 49540.000 | 59980.000 | -1.573 | 0.116 | |
| Lymph node metastasis (no, yes) | 49948.000 | 60388.000 | -1.063 | 0.288 | |
| MMR (pMMR, dMMR) | 45368.000 | 310724.000 | -4.473 | 0.000 |
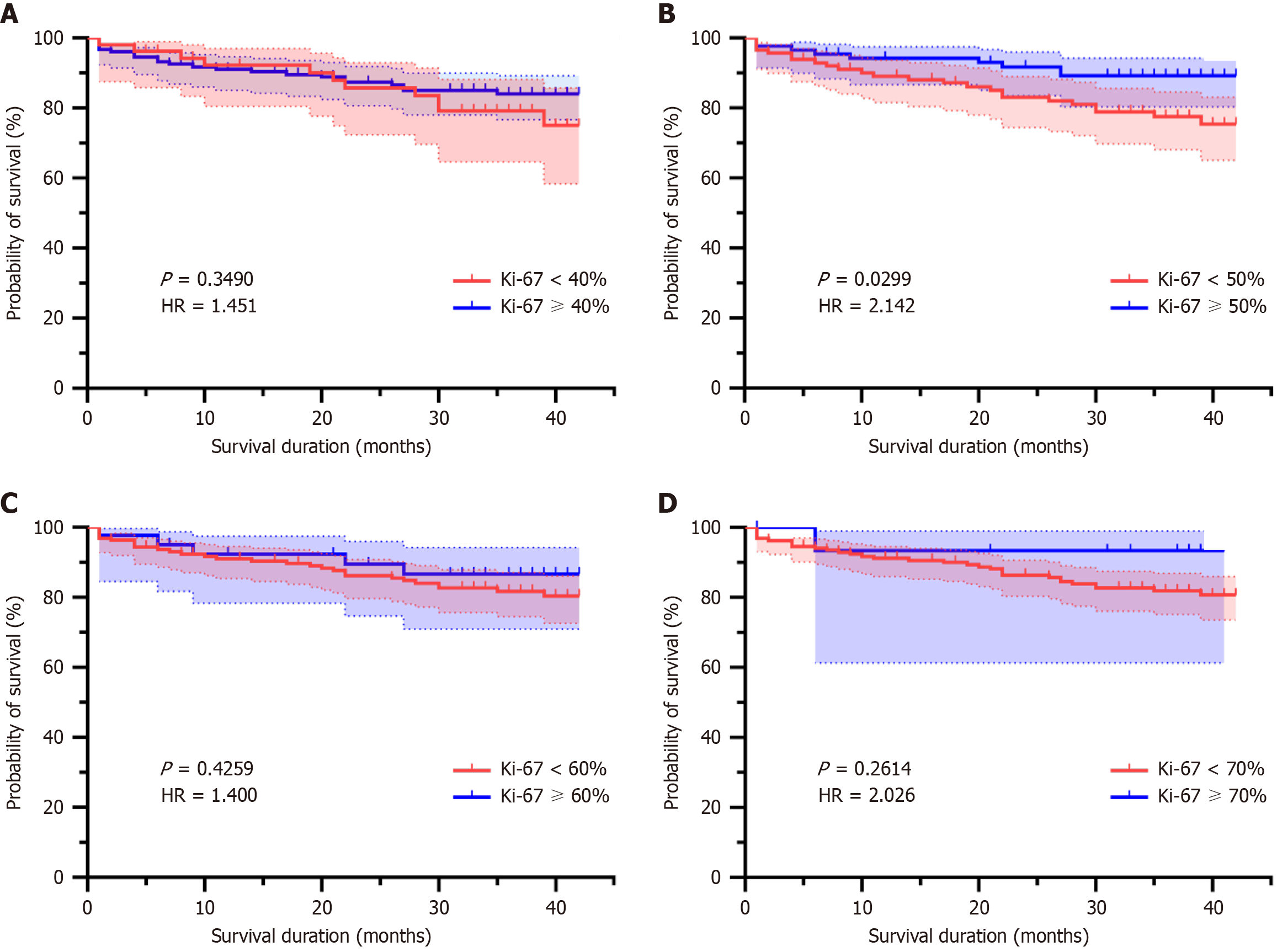
After excluding groups with inadequate sample sizes (20%, 30%, 80% cutoffs, where the lower-expression groups comprised < 3% of cases), four valid cutoff groups were retained: 40%, 50%, 60%, and 70%. Patients with Ki-67 ≥ 50% exhibited a higher survival rate compared to those with Ki-67 < 50% (log-rank P = 0.0299, HR = 2.142). No significant differences was observed for the 40% (P = 0.3490; Figure 3A). Kaplan-Meier analysis revealed that only the 50% cutoff demonstrated statistical significance (Figure 3B). There were also no significant differences observed for the 60% (P = 0.4259; Figure 3C), or 70% (P = 0.2614; Figure 3D) cutoffs, where survival curves overlapped substantially and HRs lacked statistical significance.
Overall model test: The Omnibus test of the model coefficients showed that the significance values of the overall model and each change part (0.144 and 0.139, respectively) were both greater than 0.05. This indicates that when combined the independent variables included in the model, when combined, had no statistically significant impact on the dependent variable, suggesting limited explanatory power of the overall model.
Variable analysis: Ki67 positive rate: The significance level of 0.027 (< 0.05) was statistically significant. The regression coefficient (B) was 0.949, and the HR Exp(B) was 2.583. That is, for every one-unit increase in the Ki67 positive rate, the risk was approximately 2.583 times the original. The 95% confidence interval (1.114-5.990) did not include 1. Other variables: Variables such as gender, age, tumor budding, clinical stage, mismatch repair function, pathological grade, and lymph node invasion all had significance values greater than 0.05, showing no statistical significance and having no clear association with risk.
Based on IHC test results, we divided patients in the model construction group into high-expression (581 cases) and low-expression (83 cases) groups. Given the significant difference in the number of cases between the two groups, to prevent overfitting, we cut an average of three tiles per sample in the high-expression group, amounting to a total of 1743 tiles, and an average of 20 tiles per sample in the low-expression group, resulting in a total of 1660 tiles. We divided the external validation group into high-expression (91 cases, with an average of three tiles per sample, amounting to 273 tiles) and low-expression (117 cases, with an average of three tiles per sample, resulting in 351 tiles) groups according to the same criteria.
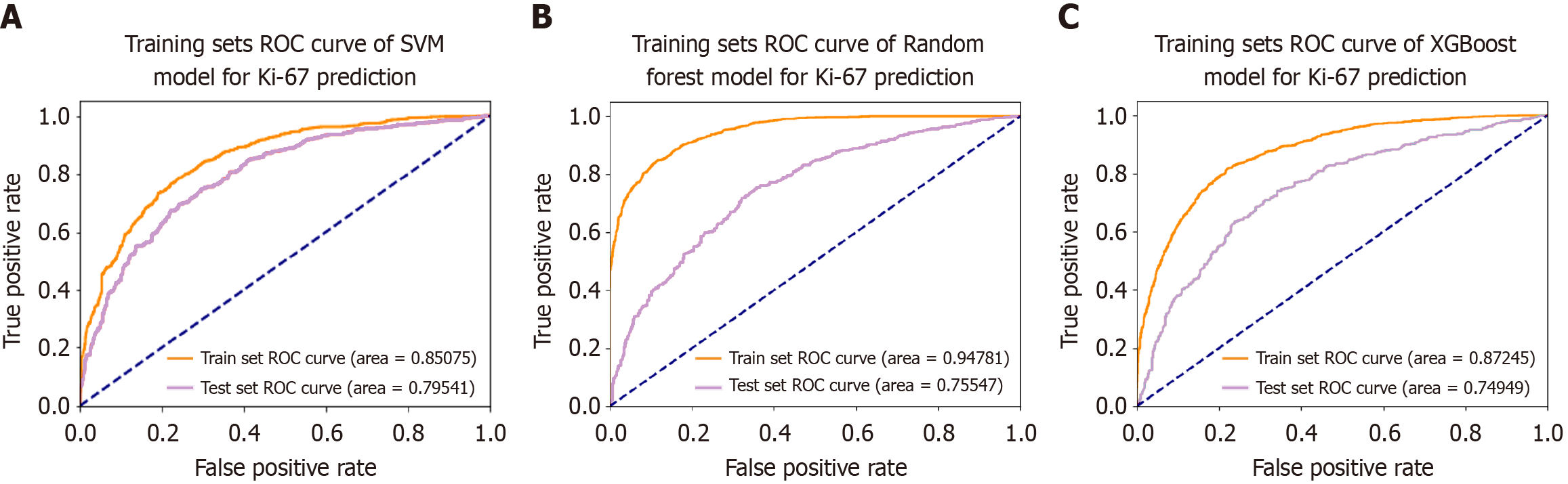
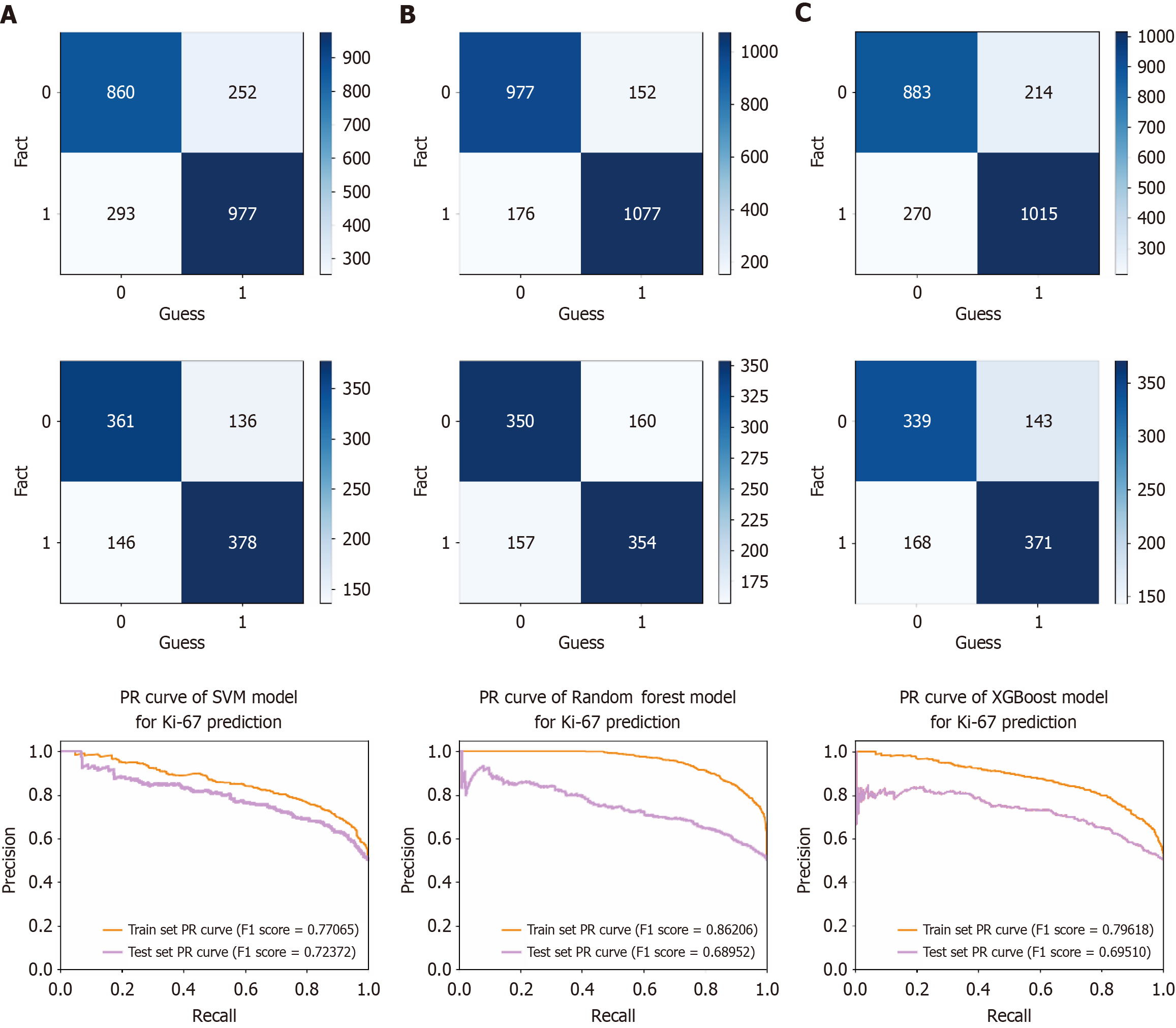
Based on the cellular characteristics in tumor images, a Ki-67 classification prediction model for CRC was constructed using 664 CRC samples from the First Affiliated Hospital of Guangxi Medical University. The findings revealed that the AUC values for the SVM, RF, and XGBoost models in the training dataset reached 0.851 (accuracy = 0.771, sensitivity = 0.795 and specificity = 0.746), 0.948 (accuracy = 0.862, sensitivity = 0.847 and specificity = 0.876), and 0.872 (accuracy = 0.797, sensitivity = 0.826 and specificity = 0.766), respectively. In contrast, the AUC values of the SVM, RF, and XGBoost models in the test dataset attained 0.795 (accuracy = 0.724, sensitivity = 0.735 and specificity = 0.712), 0.755 (accuracy = 0.690, sensitivity = 0.689 and specificity = 0.690), and 0.750 (accuracy = 0.750, sensitivity = 0.722 and specificity = 0.669), respectively (Figure 4). The performance comparison of each model is shown in the confusion matrix and precision recall curve in Figure 5. The accuracy of the 5-fold cross-validation of the SVM, RF, and XGBoost models is 0.51, 0.65, and 0.66, respectively. GridSearchCV and Python package “optuna” were used to avoid model overfitting. Exceptional proficiency in classification prediction was demonstrated by each of the three models.
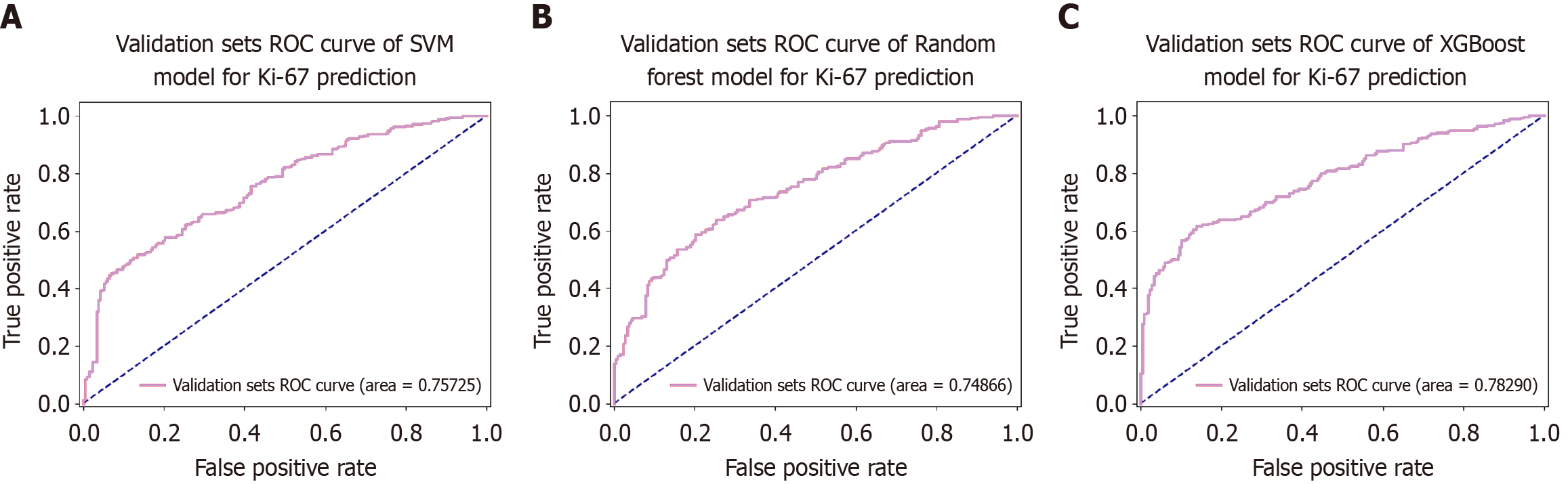
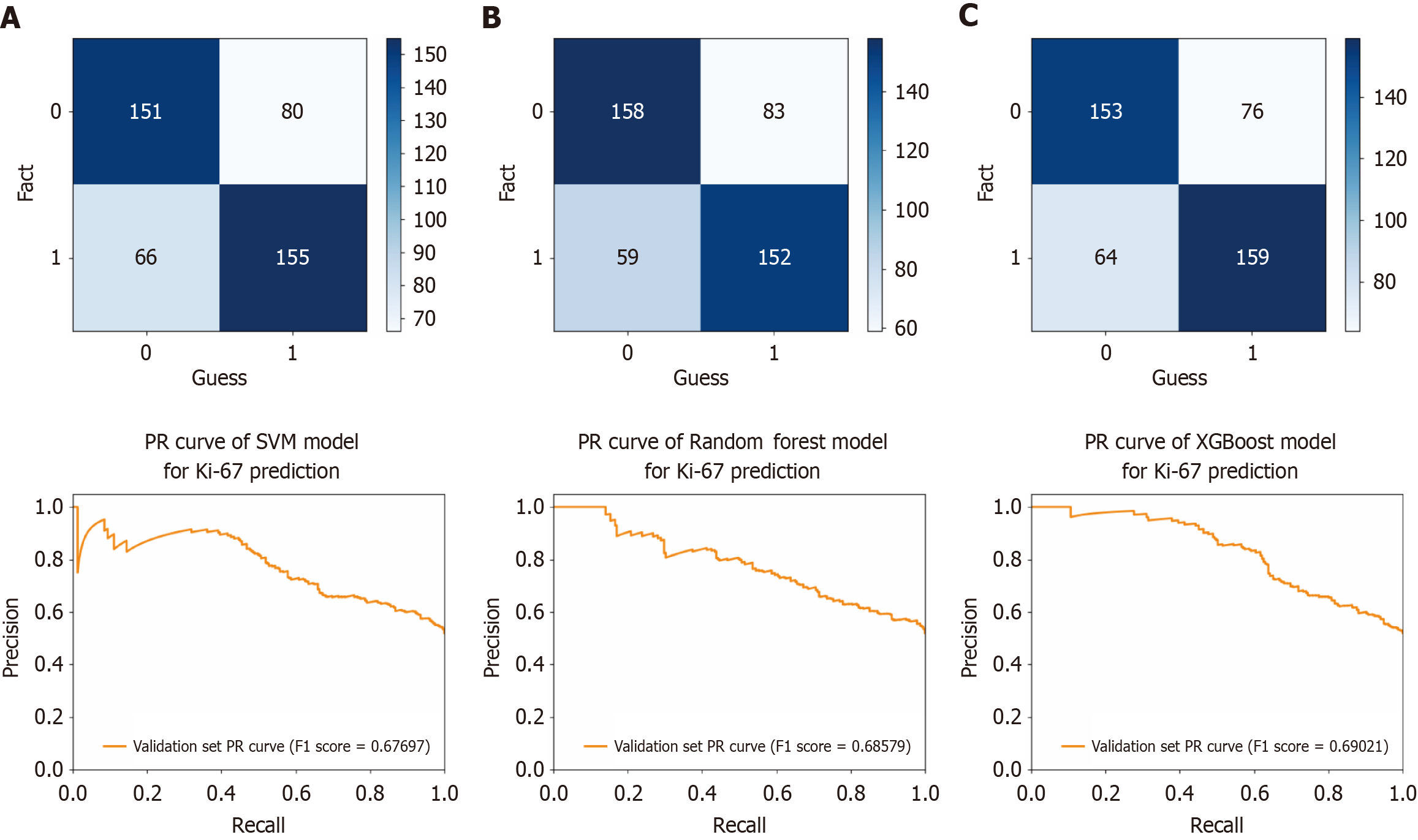
An external validation model for predicting Ki-67 classification in CRC was developed using the SVM, RF, and XGBoost algorithms. A dataset consisting of 208 CRC samples from Yulin Red Cross Hospital was utilized. ROC curves were generated from the testing results to evaluate the efficacy of the models (Figure 6). The AUC values of the respective models were 0.757 (accuracy = 0.677, sensitivity = 0.660, specificity = 0.696), 0.749 (accuracy = 0.686, sensitivity = 0.647, specificity = 0.728), and 0.783 (accuracy = 0.686, sensitivity = 0.677, specificity = 0.705), respectively. The performance comparison of these models on the validation set is shown in the confusion matrix and precision recall curve in Figure 7. These results indicated that the models possessed excellent classification capabilities and demonstrated good generalizability and stability during the external validation process.
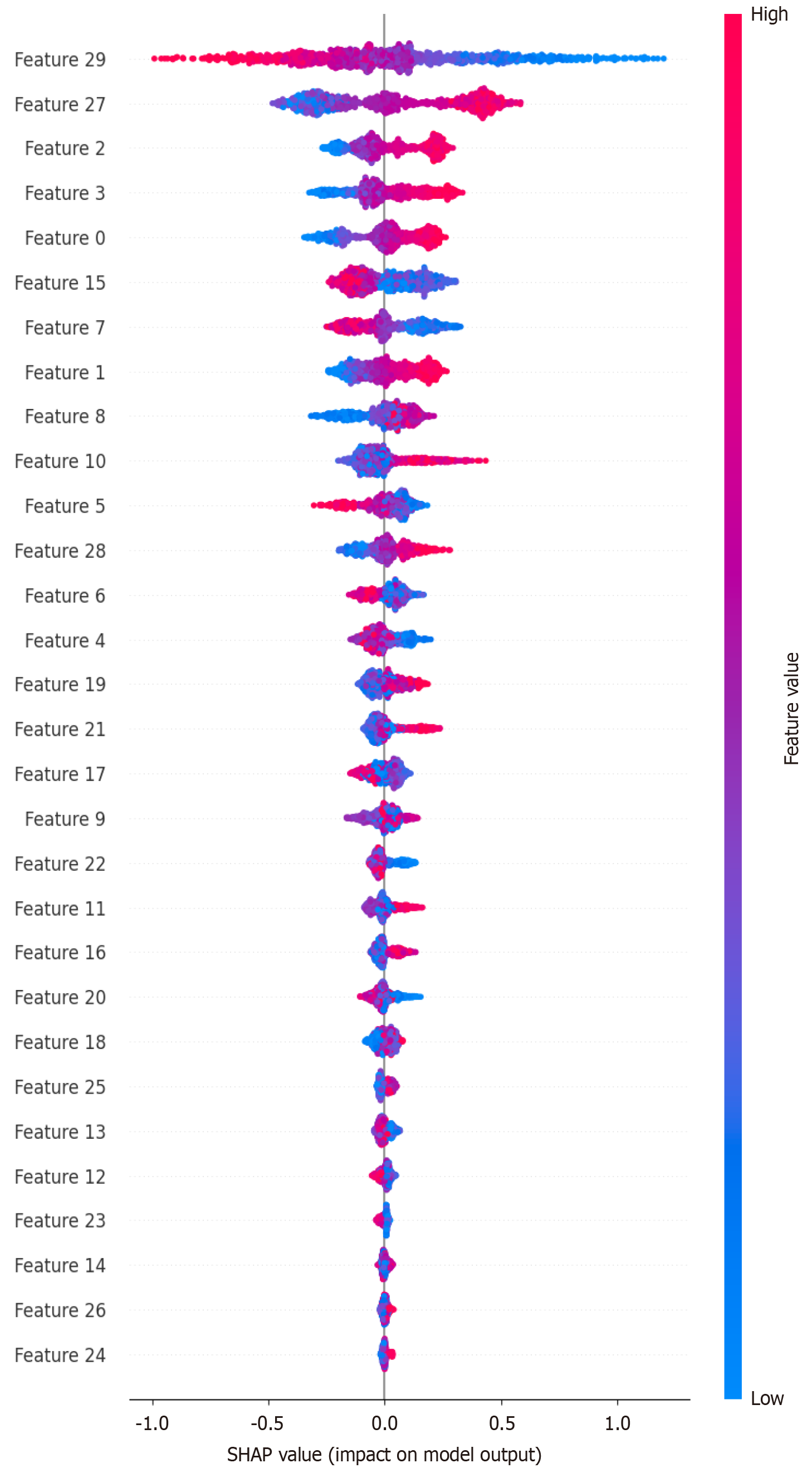
Based on the XGBoost model, we achieved the highest external validation AUC. We used the “SHAP” package to rank the features of the model and analyze its interpretability. After comparing the data, the top five features included the following: “AreaShape_MaxFeretDiameter”; “Intensity_IntegratedIntensityEdge_Hematoxylin”
This study explored the association between Ki-67 expression and clinicopathological features across multiple cutoffs (40%-90%), revealing distinct patterns. A key finding was the significant link between high Ki-67 expression (≥ 40%) and high histological grade, consistent with Ki-67’s role as a marker of cellular proliferation and tumor aggressiveness. This is consistent with prior research indicating that increased proliferative activity, as measured by Ki-67, correlates with poorer differentiation and more aggressive tumor behavior[9,31,32].
Notably, dMMR status showed a strong association with higher Ki-67 cutoffs (50%-90%), suggesting that dMMR tumors, characterized by high tumor mutational burden and microsatellite instability, exhibit heightened proliferative activity. This may reflect oncogenic stress and replicative demand in hypermutated tumors, driving elevated cell cycle progression. Conversely, lymph node metastasis was only significantly associated with the highest cutoff (≥ 80%), implying that extremely high proliferative activity may be a critical threshold for lymphatic invasion. This finding could inform risk stratification for patients with aggressive diseases.
Survival analysis highlighted the 50% cutoff as the only significant threshold, where patients with Ki-67 ≥ 50% exhibited a higher survival rate (HR = 2.142, P = 0.0299). This finding appears to align with the results from some previous studies. For instance, Li et al[33] established a cutoff value of 50% for Ki-67 and discovered that patients with stage III CRC exhibiting high Ki-67 expression had superior three-year disease-free survival and overall survival rates. Furthermore, among stage IV CRC patients, a significant enhancement in three-year progression-free survival was observed.
Similarly, Fodor et al[34] adopted the median value as the cutoff for Ki-67 and reported an improved five-year overall survival rate in the high Ki-67 subgroup within Dukes C stage patients, who also appeared to derive greater benefits from chemotherapy[34]. However, we believe this finding warrants cautious interpretation. This may reflect selection bias due to excluded small sample groups (e.g., 80% cutoff) or interactions with other biological pathways, such as immune cell infiltration in high-proliferation tumors, which is known to correlate with improved outcomes in CRC[35]. The lack of significance in other cutoffs underscores the importance of validating optimal Ki-67 thresholds in larger heterogeneous cohorts.
With the aid of deep learning technology, Liu et al[36] managed to estimate the number of Ki-67-positive cells in HE-stained images[36]. Their study no longer relied on IHC-stained images. There have been studies using AI technology to identify and predict the Ki-67 index in pathology images of breast cancer[37], brain tumors[38], and gastrointestinal neuroendocrine tumors[39]. This study aimed to develop a Ki-67 classification prediction model for CRC by leveraging three algorithms: SVM, RF, and XGBoost. To the best of our current knowledge, this might be among the early attempts to construct such a model using these specific algorithms for CRC globally. The analysis of the training dataset de
During external validation, the SVM, RF, and XGBoost models yielded AUC values of 0.757, 0.749, and 0.783, respectively - performance metrics that approached the reported inter pathologist agreement levels for Ki-67 interpretation in the literature[40]. These findings suggest that the models demonstrate reasonable generalizability across external datasets. Nevertheless, the generalizability and reliability of the models cannot be firmly established at this stage, as the external validation results could be influenced by various factors such as differences in patient characteristics, data collection protocols, and histological assessment criteria between the external dataset and the training and test datasets.
Based on the SHAP score, we sorted the data to identify the top five features that contribute the most to the model. One “AreaShape_MaxFeretDiameter”, one “Intensity_IntegratedIntensityEdge”, and two “Texture_SumAverage”. Based on these features, to some extent, they reflect the size, staining depth, and texture of the cell nucleus. The importance of the “AreaShape_MaxFeretDiameter” indicated that the expression of Ki-67 was positively correlated with the shape change of the nucleus. This is consistent with the known function of Ki-67, which is closely related to mitosis[41]. “Intensity_IntegratedIntensityEdge” shows that the expression levels of Ki-67 may have had a correlation with the intensity. The intensity of the nucleus is related to the degree of nuclear staining. The deep staining of the nucleus also reflects the malignant degree of the tumor, which is consistent with the way the high expression of Ki-67 can reflect the malignant degree of CRC. Although the importance of the feature “Texture” indicates that the Ki-67 Level may be related to the nuclear texture, to our knowledge, there is no relevant study. We hope to validate this result in future research. The reliability of our ML model was explained to some degree by the confirmed correlations between Ki-67 and pathomics features. The unproven correlation would be the goal of our next step. Tumor nuclei from high-risk tiles tended to exhibit increased size, more distorted shapes, and anomalous textures.
Several limitations merit consideration. First, the survival analysis excluded cutoffs with small sample sizes, potentially limiting the generalizability of threshold-specific outcomes. Second, traditional IHC may have differences in readings due to differences between slide producers and readers. Therefore, Ki-67 cannot become an important biomarker for CRC prognosis in traditional IHC and can be applied in clinical practice. Our ML models may be able to avoid these errors caused by humans, making Ki-67 a better indicator for CRC prognosis. Third, while the ML models demonstrated promise, their reliance on manually curated tiles and single-institution training data may affect scalability. Prospective studies with multi-center datasets and the integration of genomic/transcriptomic data could further refine these models. Additionally, exploring the mechanistic link between dMMR status and Ki-67 expression - e.g., via pathways like mismatch repair deficiency–induced replicative stress - would deepen our understanding of tumor biology.
This study identified clinically relevant associations between Ki-67 expression and histopathological features, particularly highlighting dMMR status as a key correlate of high proliferative activity. The development of robust ML models for Ki-67 prediction represents a step toward automated, precision pathology, with the potential to enhance diagnostic consistency and guide personalized treatment strategies in CRC.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64589] [Article Influence: 16147.3] [Reference Citation Analysis (176)] |
| 2. | Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75:10-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 444] [Article Influence: 444.0] [Reference Citation Analysis (0)] |
| 3. | Li Q, Wu H, Cao M, Li H, He S, Yang F, Yan X, Zhang S, Teng Y, Xia C, Peng J, Chen W. Colorectal cancer burden, trends and risk factors in China: A review and comparison with the United States. Chin J Cancer Res. 2022;34:483-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Kasi PM, Shahjehan F, Cochuyt JJ, Li Z, Colibaseanu DT, Merchea A. Rising Proportion of Young Individuals With Rectal and Colon Cancer. Clin Colorectal Cancer. 2019;18:e87-e95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 5. | Li LT, Jiang G, Chen Q, Zheng JN. Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol Med Rep. 2015;11:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 550] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 6. | Palmqvist R, Sellberg P, Oberg A, Tavelin B, Rutegård JN, Stenling R. Low tumour cell proliferation at the invasive margin is associated with a poor prognosis in Dukes' stage B colorectal cancers. Br J Cancer. 1999;79:577-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Melling N, Kowitz CM, Simon R, Bokemeyer C, Terracciano L, Sauter G, Izbicki JR, Marx AH. High Ki67 expression is an independent good prognostic marker in colorectal cancer. J Clin Pathol. 2016;69:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Salminen E, Palmu S, Vahlberg T, Roberts PJ, Söderström KO. Increased proliferation activity measured by immunoreactive Ki67 is associated with survival improvement in rectal/recto sigmoid cancer. World J Gastroenterol. 2005;11:3245-3249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Mori N, Dorjkhorloo G, Shiraishi T, Erkhem-Ochir B, Okami H, Yamaguchi A, Shioi I, Komine C, Endo M, Seki T, Hosoi N, Nakazawa N, Shibasaki Y, Okada T, Osone K, Sano A, Sakai M, Sohda M, Yokobori T, Shirabe K, Saeki H. A Mature Tertiary Lymphoid Structure with a Ki-67-Positive Proliferating Germinal Center Is Associated with a Good Prognosis and High Intratumoral Immune Cell Infiltration in Advanced Colorectal Cancer. Cancers (Basel). 2024;16:2684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Ahmed Nor EM, Saied EM, Mina SN, Shareef MM, Abdelaziz DM. Clinicopathological Value Of Epidermal Growth Factor Receptor (EGFR) And Ki-67 Expression In Colorectal Adenoma And Adenocarcinoma. J Pak Med Assoc. 2023;73 (Suppl 4):S124-S130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Sui X, Guo Y, Ni W, Jin H, Lin H, Xie T. Molecular profiling analysis for colorectal cancer patients with Pi-Xu or Shi-Re syndrome. Integr Med Res. 2019;8:21-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Qiu Z, He S, Lu B, Sun Y, Zhang T, Lv W, Shen D. The E3 ubiquitin ligase RNF135 modulates chemotherapy resistance to oxaliplatin for colorectal cancer by modulating autophagy. Tissue Cell. 2024;86:102282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 13. | Ma YL, Peng JY, Zhang P, Liu WJ, Huang L, Qin HL. Immunohistochemical analysis revealed CD34 and Ki67 protein expression as significant prognostic factors in colorectal cancer. Med Oncol. 2010;27:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Chan JK. The wonderful colors of the hematoxylin-eosin stain in diagnostic surgical pathology. Int J Surg Pathol. 2014;22:12-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 15. | Shafi S, Parwani AV. Artificial intelligence in diagnostic pathology. Diagn Pathol. 2023;18:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 80] [Reference Citation Analysis (0)] |
| 16. | Badillo S, Banfai B, Birzele F, Davydov II, Hutchinson L, Kam-Thong T, Siebourg-Polster J, Steiert B, Zhang JD. An Introduction to Machine Learning. Clin Pharmacol Ther. 2020;107:871-885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 164] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 17. | Rakha EA, Toss M, Shiino S, Gamble P, Jaroensri R, Mermel CH, Chen PC. Current and future applications of artificial intelligence in pathology: a clinical perspective. J Clin Pathol. 2021;74:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 18. | Madabhushi A, Lee G. Image analysis and machine learning in digital pathology: Challenges and opportunities. Med Image Anal. 2016;33:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 537] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 19. | Kumar N, Gupta R, Gupta S. Whole Slide Imaging (WSI) in Pathology: Current Perspectives and Future Directions. J Digit Imaging. 2020;33:1034-1040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 121] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 20. | Ehteshami Bejnordi B, Veta M, Johannes van Diest P, van Ginneken B, Karssemeijer N, Litjens G, van der Laak JAWM; the CAMELYON16 Consortium, Hermsen M, Manson QF, Balkenhol M, Geessink O, Stathonikos N, van Dijk MC, Bult P, Beca F, Beck AH, Wang D, Khosla A, Gargeya R, Irshad H, Zhong A, Dou Q, Li Q, Chen H, Lin HJ, Heng PA, Haß C, Bruni E, Wong Q, Halici U, Öner MÜ, Cetin-Atalay R, Berseth M, Khvatkov V, Vylegzhanin A, Kraus O, Shaban M, Rajpoot N, Awan R, Sirinukunwattana K, Qaiser T, Tsang YW, Tellez D, Annuscheit J, Hufnagl P, Valkonen M, Kartasalo K, Latonen L, Ruusuvuori P, Liimatainen K, Albarqouni S, Mungal B, George A, Demirci S, Navab N, Watanabe S, Seno S, Takenaka Y, Matsuda H, Ahmady Phoulady H, Kovalev V, Kalinovsky A, Liauchuk V, Bueno G, Fernandez-Carrobles MM, Serrano I, Deniz O, Racoceanu D, Venâncio R. Diagnostic Assessment of Deep Learning Algorithms for Detection of Lymph Node Metastases in Women With Breast Cancer. JAMA. 2017;318:2199-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1871] [Cited by in RCA: 1557] [Article Influence: 194.6] [Reference Citation Analysis (0)] |
| 21. | Coudray N, Ocampo PS, Sakellaropoulos T, Narula N, Snuderl M, Fenyö D, Moreira AL, Razavian N, Tsirigos A. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24:1559-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1542] [Article Influence: 220.3] [Reference Citation Analysis (0)] |
| 22. | Jin L, Shi F, Chun Q, Chen H, Ma Y, Wu S, Hameed NUF, Mei C, Lu J, Zhang J, Aibaidula A, Shen D, Wu J. Artificial intelligence neuropathologist for glioma classification using deep learning on hematoxylin and eosin stained slide images and molecular markers. Neuro Oncol. 2021;23:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 23. | Naik N, Madani A, Esteva A, Keskar NS, Press MF, Ruderman D, Agus DB, Socher R. Deep learning-enabled breast cancer hormonal receptor status determination from base-level H&E stains. Nat Commun. 2020;11:5727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 24. | Chen M, Zhang B, Topatana W, Cao J, Zhu H, Juengpanich S, Mao Q, Yu H, Cai X. Classification and mutation prediction based on histopathology H&E images in liver cancer using deep learning. NPJ Precis Oncol. 2020;4:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 25. | Kather JN, Heij LR, Grabsch HI, Loeffler C, Echle A, Muti HS, Krause J, Niehues JM, Sommer KAJ, Bankhead P, Kooreman LFS, Schulte JJ, Cipriani NA, Buelow RD, Boor P, Ortiz-Brüchle NN, Hanby AM, Speirs V, Kochanny S, Patnaik A, Srisuwananukorn A, Brenner H, Hoffmeister M, van den Brandt PA, Jäger D, Trautwein C, Pearson AT, Luedde T. Pan-cancer image-based detection of clinically actionable genetic alterations. Nat Cancer. 2020;1:789-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 343] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 26. | Farahmand S, Fernandez AI, Ahmed FS, Rimm DL, Chuang JH, Reisenbichler E, Zarringhalam K. Deep learning trained on hematoxylin and eosin tumor region of Interest predicts HER2 status and trastuzumab treatment response in HER2+ breast cancer. Mod Pathol. 2022;35:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 27. | Li F, Yang Y, Wei Y, He P, Chen J, Zheng Z, Bu H. Deep learning-based predictive biomarker of pathological complete response to neoadjuvant chemotherapy from histological images in breast cancer. J Transl Med. 2021;19:348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 28. | Shi JY, Wang X, Ding GY, Dong Z, Han J, Guan Z, Ma LJ, Zheng Y, Zhang L, Yu GZ, Wang XY, Ding ZB, Ke AW, Yang H, Wang L, Ai L, Cao Y, Zhou J, Fan J, Liu X, Gao Q. Exploring prognostic indicators in the pathological images of hepatocellular carcinoma based on deep learning. Gut. 2021;70:951-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 29. | Wang X, Chen Y, Gao Y, Zhang H, Guan Z, Dong Z, Zheng Y, Jiang J, Yang H, Wang L, Huang X, Ai L, Yu W, Li H, Dong C, Zhou Z, Liu X, Yu G. Predicting gastric cancer outcome from resected lymph node histopathology images using deep learning. Nat Commun. 2021;12:1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 30. | Luo ZW, Zhu MG, Zhang ZQ, Ye FJ, Huang WH, Luo XZ. Increased expression of Ki-67 is a poor prognostic marker for colorectal cancer patients: a meta analysis. BMC Cancer. 2019;19:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 31. | Zhang M, Meng L, Zhang Z, Wu J, Chen X, Wang Y, He J. The relationships of OSBPL3 expression with KI-67 expression and KRAS mutations in CRC: implications for diagnosis and prognosis. BMC Med Genomics. 2022;15:259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 32. | Li W, Zhang G, Wang HL, Wang L. Analysis of expression of cyclin E, p27kip1 and Ki67 protein in colorectal cancer tissues and its value for diagnosis, treatment and prognosis of disease. Eur Rev Med Pharmacol Sci. 2016;20:4874-4879. [PubMed] |
| 33. | Li P, Xiao ZT, Braciak TA, Ou QJ, Chen G, Oduncu FS. Association between Ki67 Index and Clinicopathological Features in Colorectal Cancer. Oncol Res Treat. 2016;39:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Fodor IK, Hutchins GG, Espiritu C, Quirke P, Jubb AM. Prognostic and predictive significance of proliferation in 867 colorectal cancers. J Clin Pathol. 2012;65:989-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Boutin M, Gill S. Controversies and management of deficient mismatch repair gastrointestinal cancers in the neoadjuvant setting. Ther Adv Med Oncol. 2023;15:17588359231162577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 36. | Liu Y, Li X, Zheng A, Zhu X, Liu S, Hu M, Luo Q, Liao H, Liu M, He Y, Chen Y. Predict Ki-67 Positive Cells in H&E-Stained Images Using Deep Learning Independently From IHC-Stained Images. Front Mol Biosci. 2020;7:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Swiderska-chadaj Z, Gallego J, Gonzalez-lopez L, Bueno G. Detection of Ki67 Hot-Spots of Invasive Breast Cancer Based on Convolutional Neural Networks Applied to Mutual Information of H&E and Ki67 Whole Slide Images. Appl Sci. 2020;10:7761. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Swiderska-chadaj Z, Markiewicz T, Gallego J, Bueno G, Grala B, Lorent M. Deep learning for damaged tissue detection and segmentation in Ki-67 brain tumor specimens based on the U-net model. Bull Pol Acad Sci Tech. 2018;66:849-856. [DOI] [Full Text] |
| 39. | Govind D, Jen KY, Matsukuma K, Gao G, Olson KA, Gui D, Wilding GE, Border SP, Sarder P. Improving the accuracy of gastrointestinal neuroendocrine tumor grading with deep learning. Sci Rep. 2020;10:11064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 40. | Guo R, Ma L, Bai X, Miao L, Li Z, Yang J. A Scoring Method for Immunohistochemical Staining on Ki67. Appl Immunohistochem Mol Morphol. 2021;29:e20-e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 41. | Andrés-Sánchez N, Fisher D, Krasinska L. Physiological functions and roles in cancer of the proliferation marker Ki-67. J Cell Sci. 2022;135:jcs258932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |