Published online Aug 24, 2025. doi: 10.5306/wjco.v16.i8.106873
Revised: April 23, 2025
Accepted: June 24, 2025
Published online: August 24, 2025
Processing time: 163 Days and 17.4 Hours
In recent years, emerging clinical research has prioritized assessment of combined therapeutic efficacy and safety parameters when programmed death 1 or its ligand (PD-1/L1) inhibitors are incorporated into first-line standard-of-care (SOC) therapy for metastatic colorectal cancer (mCRC). However, data obtained from these trials demonstrated conflicting evidence concerning survival benefits and clinical outcomes.
To evaluate the therapeutic impact and safety parameters of combining PD-1/L1 inhibitors with SOC protocols as first-line treatment for mCRC.
Four biomedical databases (PubMed, Embase, Cochrane Library, Web of Science) were systematically interrogated to identify eligible studies published up to October 12, 2024. The analysis focused on evaluating the primary outcome of overall survival (OS) in the mCRC population with secondary outcomes of progression-free survival (PFS), overall response rate (ORR), and incidence rate of grade ≥ 3 adverse events. Additionally, we performed exploratory analyses in the microsatellite stable/mismatch repair-proficient (MSS/pMMR) subpopulation, based on a subset of the included studies. Subgroup analyses according to PD-1/L1 inhibitor use were conducted in both the overall population and the MSS/pMMR subgroup.
This pooled analysis incorporated six randomized controlled trials involving 675 patients with mCRC receiving first-line therapy. The combination of PD-1/L1 inhibitors with SOC regimens demonstrated a significant PFS advantage over SOC monotherapy in intention-to-treat populations [hazard ratio (HR) = 0.8, 95% confidence interval (CI): 0.65-0.98, P = 0.033]. Nevertheless, the MSS/pMMR subgroup showed no PFS benefit (HR = 0.83, 95%CI: 0.67-1.03, P = 0.091), and no cohort exhibited OS improvement (intention-to-treat: HR = 0.84, 95%CI: 0.66-1.05, P = 0.124; MSS/pMMR: HR = 0.79, 95%CI: 0.60-1.03, P = 0.083). Comparable outcomes were observed for ORR (risk ratio = 1.03, 95%CI: 0.90-1.17, P = 0.711) and incidence rate of grade ≥ 3 adverse events (risk ratio = 1.12, 95%CI: 0.93-1.36, P = 0.245) between treatment arms.
The findings indicated that integrating PD-1/L1 blocking agents with SOC regimens for mCRC as first-line treatment failed to demonstrate significant improvements in ORR. Existing clinical data remain inadequate to establish OS advantages, particularly in patients with MSS/pMMR, despite exhibiting manageable toxicity profiles. Subsequent confirmation through rigorously designed phase III clinical trials remains essential to verify these therapeutic outcomes.
Core Tip: We performed a meta-analysis to evaluate the efficacy and safety of combining inhibitors of programmed death 1 or its ligand with standard-of-care therapy as first-line treatment for metastatic colorectal cancer. Results showed integrating inhibitors of programmed death 1 or its ligand failed to demonstrate significant improvements in the overall response rate. Existing clinical data remain inadequate to establish overall survival advantages, particularly in patients with microsatellite stable/mismatch repair-proficient, despite exhibiting manageable toxicity profiles.
- Citation: Zheng T, Li XX, Zhou L, Jin JJ. Adding programmed death 1/programmed death ligand 1 inhibitors to first-line standard-of-care therapy for metastatic colorectal cancer: A meta-analysis. World J Clin Oncol 2025; 16(8): 106873
- URL: https://www.wjgnet.com/2218-4333/full/v16/i8/106873.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i8.106873
Globally, colorectal cancer (CRC) ranks third in gastrointestinal malignancy incidence and is the second most prevalent cause of cancer mortality[1]. Epidemiological models predict annual CRC diagnoses will escalate to approximately 2.5 million cases worldwide by 2035[2]. The asymptomatic nature of early-stage disease results in metastatic presentation at diagnosis for 20% of patients with CRC[3], while 40% of those with initially localized tumors experience recurrence post-curative intervention[4].
Current therapeutic interventions for metastatic CRC (mCRC) continue to yield suboptimal outcomes, demonstrating median progression-free survival (PFS) approaching 10 months, overall survival (OS) around 30 months[5,6], overall response rate (ORR) of 38%[5], and 5-year survival below 14%[7]. First-line management historically relied on fluo
Immune checkpoint inhibitors (ICIs) have been integrated into therapeutic strategies for a unique mCRC subgroup (approximately 5% incidence) exhibiting DNA mismatch repair (dMMR) and subsequent microsatellite instability-high (MSI-H) characteristics[11]. Therapeutic interventions employing programmed death 1 (PD-1) blockade through nivolumab monotherapy[12] or its synergistic combination with cytotoxic T lymphocyte antigen 4 inhibitor ipilimumab[13] have demonstrated sustained therapeutic responses in patients with MSI-H/dMMR mCRC. These therapeutic approaches have received endorsement from the National Comprehensive Cancer Network clinical guidelines as primary treatment modalities for this molecularly defined patient population[14,15].
Microsatellite stable (MSS)/mismatch repair-proficient (pMMR) mCRC tumors (representing the majority) exhibit intrinsic resistance to ICIs[16], attributable to fundamental immunological disparities. While MSI-H/dMMR malignancies display elevated tumor mutational burden (TMB), abundant immunogenic neoantigens, and robust CD8+ T cell infiltration, MSS/pMMR counterparts typically manifest immunologically inert or hostile microenvironments with scarce activated CD8+ T lymphocytes and reduced checkpoint protein expression on malignant cells[17,18]. Strategic combination approaches integrating ICIs with immunomodulatory cytotoxic agents may potentiate antitumor immunity in MSS/pMMR contexts[19]. Such cytotoxic compounds can trigger immunogenic cell death via tumor-associated antigen release, subsequently activating CD8+ T cells and remodeling the tumor microenvironment toward immune permi
Emerging evidence suggests that incorporating inhibitors of PD-1/programmed death ligand 1 (PD-L1) (PD-1/L1) into first-line standard-of-care (SOC) protocols could enhance therapeutic outcomes for mCRC as demonstrated in multi-cancer clinical investigations[21-23]. Divergent outcomes have been reported across clinical investigations with certain trials indicating no therapeutic advantage from this combination strategy, while others documented enhanced clinical responses. Notably, treatment regimens combining PD-1/L1 inhibitors with conventional SOC demonstrated heightened incidence of treatment-related toxicities relative to SOC monotherapy. To reconcile these conflicting observations, we systematically evaluated aggregated clinical trial data through meta-analysis methodologies to determine the clinical utility and safety profile of PD-1/L1 inhibitor-SOC combination therapy in mCRC frontline management.
This meta-analysis was rigorously conducted using previously published studies that had received institutional ethics approval. As all analyzed data were derived from publicly accessible research outputs with no original clinical data accessed or utilized, formal ethics committee review was deemed unnecessary for this investigation. The methodology strictly adhered to PRISMA reporting standards[24]. The research protocol was prospectively registered with PROSPERO (CRD42024622747), accessible via: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024622747.
Systematic literature retrieval was performed in four principal biomedical repositories (Embase, PubMed, Cochrane Library, Web of Science) and restricted to English language clinical investigations published prior to October 12, 2024. Search strategies were developed utilizing controlled terminologies (“colorectal cancer”, “PD-1/L1 inhibitor”, “first-line”) augmented with free-text keyword searches without imposing temporal or geographic limitations. For duplicated publications identified across repositories, priority was given to studies with the most comprehensive datasets for inclusion in quantitative synthesis. Full search methodologies have been detailed in Supplementary Tables 1-4.
Inclusion criteria: (1) Histologically confirmed diagnosis of unresectable treatment-naïve mCRC in adults (≥ 18 years); (2) Comparative evaluation of PD-1/L1 inhibitor-SOC combination vs SOC monotherapy as initial systemic treatment; (3) Documentation of primary endpoints including OS, PFS, ORR, or severe adverse events (AEs) (grade ≥ 3); and (4) Randomized controlled trials with English language full-text availability.
Exclusion criteria: (1) Non-randomized controlled trial publications including conference abstracts, case series, review articles, preclinical investigations, or single-arm study designs; and (2) Trials with overlapping patient cohorts across multiple publications.
Study characteristics were independently retrieved from eligible publications by two researchers (Zheng T and Li XX). Discrepancies in data interpretation underwent independent adjudication through third-party full-text analysis. Documented parameters encompassed first author (year), study name, country, study design, phase, treatment regimens, case, cases of pMMR/dMMR/unknown, mean age, the proportion of male, and registration ID. Survival metrics [hazard ratios (HRs) with 95% confidence intervals (CIs)] were collected for both intention-to-treat (ITT) populations and MSS/pMMR subgroups regarding PFS and OS endpoints. Additional parameters included ORR values and grade ≥ 3 AEs frequencies, prioritizing the most recent data in cases of multiple reporting instances. Methodological rigor evaluation utilized the Cochrane risk of bias assessment framework[25] with dual independent quality appraisals performed by investigators Zheng T and Li XX.
Statistical computations were executed via STATA 15.1 and R 4.4 platforms employing meta-analysis modules. OS in patients with mCRC constituted the primary outcome with secondary outcomes comprising PFS, ORR, and incidence rate of grade ≥ 3 AEs. Exploratory evaluations focused on MSS/pMMR subcohorts derived from available trial data. PD-1/L1 inhibitor-specific subgroup analyses were implemented across both the ITT and MSS/pMMR subgroups. HRs with 95%CIs served as primary effect measures for PFS/OS, whereas risk ratios (RRs) with 95%CIs quantified ORR and toxicity profiles. Where reported, HRs and CIs were directly obtained from source publications; otherwise, survival curves were digitized using Engauge Digitizer 10.11 with subsequent HR estimation per Tierney’s methodology[26]. Interstudy heterogeneity was quantified through Cochrane’s Q-test and I2 metrics[27]. Random-effects models were applied under significant heterogeneity thresholds (P < 0.1 or I2 ≥ 50%), supplemented by leave-one-out sensitivity analyses to verify result stability. Fixed-effects models governed analyses below these thresholds. Statistical significance was defined as two-tailed P < 0.05. Publication bias was evaluated via Begg’s rank correlation and Egger’s regression tests[28,29].
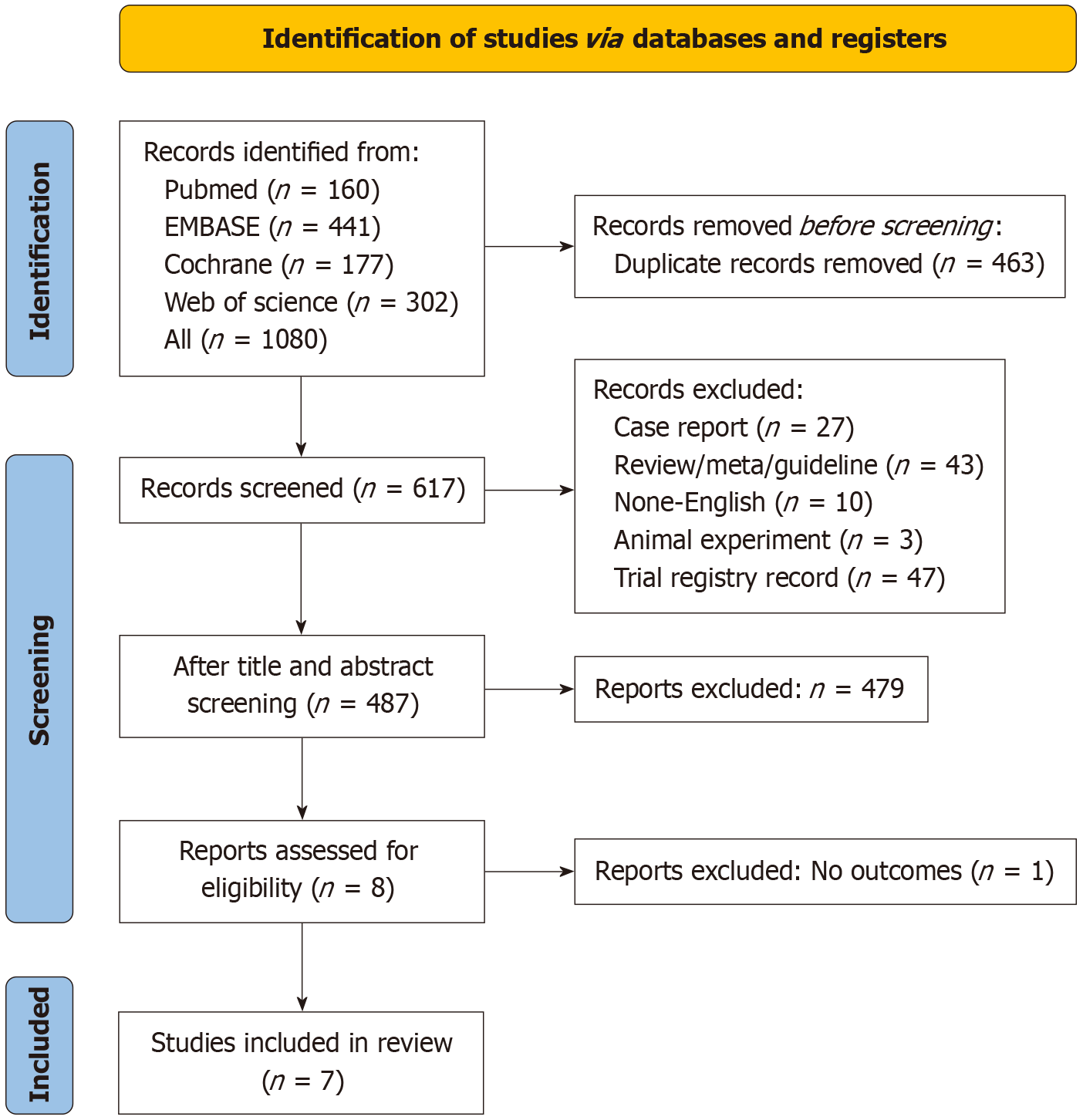
The initial database search yielded 1080 potentially relevant publications. Following deduplication and exclusion of non-conforming publications through title/abstract screening (article type relevance assessment), eight candidate articles progressed to full-text evaluation. One publication was subsequently excluded due to absence of relevant outcome measures. Ultimately, six eligible clinical trials comprising seven publications[30-36] met inclusion criteria (Figure 1).
A systematic compilation of baseline trial characteristics is presented in Table 1. The meta-analysis encompassed seven publications corresponding to six globally conducted randomized controlled trials published between 2022 and 2024. Enrollment comprised 675 patients with mCRC, including a predominant MSS/pMMR cohort (n = 622). Therapeutic allocation comprised 403 participants administered PD-1/L1 inhibitor-SOC combination therapy vs 272 undergoing SOC monotherapy. Investigational agents included two PD-1 inhibitors (nivolumab, serplulimab) and three PD-L1 inhibitors (atezolizumab, avelumab, durvalumab) across the trials. Methodological limitations were noted in one abstract-form publication with incomplete bias risk documentation. Open-label designs introduced elevated performance bias risks across most trials although other bias domains remained low. Comprehensive quality appraisal outcomes are detailed in Table 2.
| Ref. | Study name | Country | Study design | Phase | Regimens | Cases | pMMR/dMMR/unknown | Mean age (range) | Male (%) | Registration ID |
| Lenz et al[30], 2024 | CheckMate 9X8 | Canada, Japan, Spain, United States | RCT | Phase II/III | Nivolumab + mFOLFOX6/BEV | 127 | 121/6/0 | 58.0 (24.0-85.0) | 55.0 | NCT03414983 |
| mFOLFOX6/BEV | 68 | 61/7/0 | 56.0 (24.0-78.0) | 72.0 | ||||||
| Antoniotti et al[31], 2022 | ATEZOTRIBE | Italian | RCT | Phase II | Atezolizumab + FOLFOXIRI/bevacizumab | 145 | 134/8/3 | 60.0 (52.0-67.0) | 57.2 | NCT03721653 |
| Antoniotti et al[32], 2024 | FOLFOXIRI/bevacizumab | 73 | 68/5/0 | 61.0 (54.0-66.0) | 57.5 | - | ||||
| Wang et al[33], 2024 | ASTRUM-015 | China | RCT | Phase II/III | Serplulimab + HLX04 + XELOX | 57 | 40/4/13 | 61.0 (25.0-74.0) | 77.2 | NCT04547166 |
| Placebo + bevacizumab + XELOX | 57 | 50/0/7 | 58.0 (26.0-73.0) | 68.4 | ||||||
| Redman et al[34], 2022 | NA | United States | RCT | Phase II | Avelumab + AdCEA vaccine + FOLFOX6 | 10 | 10/0/0 | NA | 60.0 | NCT03050814 |
| FOLFOX6 | 10 | 10/0/0 | NA | 30.0 | ||||||
| Ree et al[35], 2024 | METIMMOX | Norway | RCT | Phase II | Nivolumab + FLOX | 38 | 38/0/0 | 60.5 (43.0-80.0) | 47.0 | NCT03388190 |
| FLOX | 38 | 38/0/0 | 65.0 (38.0-79.0) | 61.0 | ||||||
| Segal et al[36], 2024 | COLUMBIA-1 | Australia, Canada, France, Spain, United States | RCT | Phase II | Durvalumab + oleclumab + FOLFOX + bevacizumab | 26 | 26/0/0 | 63.5 (41.0-80.0) | 57.7 | NCT04068610 |
| FOLFOX + bevacizumab | 26 | 26/0/0 | 56.0 (22.0-72.0) | 73.1 |
| Ref. | Random sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Selective reporting | Other bias |
| Lenz et al[30], 2024 | L | L | H | L | L | L |
| Antoniotti et al[31], 2022 | L | L | H | L | L | L |
| Antoniotti et al[32], 2024 | ||||||
| Wang et al[33], 2024 | L | U | L | L | L | L |
| Redman et al[34], 2022 | L | L | H | L | L | L |
| Ree et al[35], 2024 | L | L | H | L | L | L |
| Segal et al[36], 2024 | L | L | H | L | L | L |
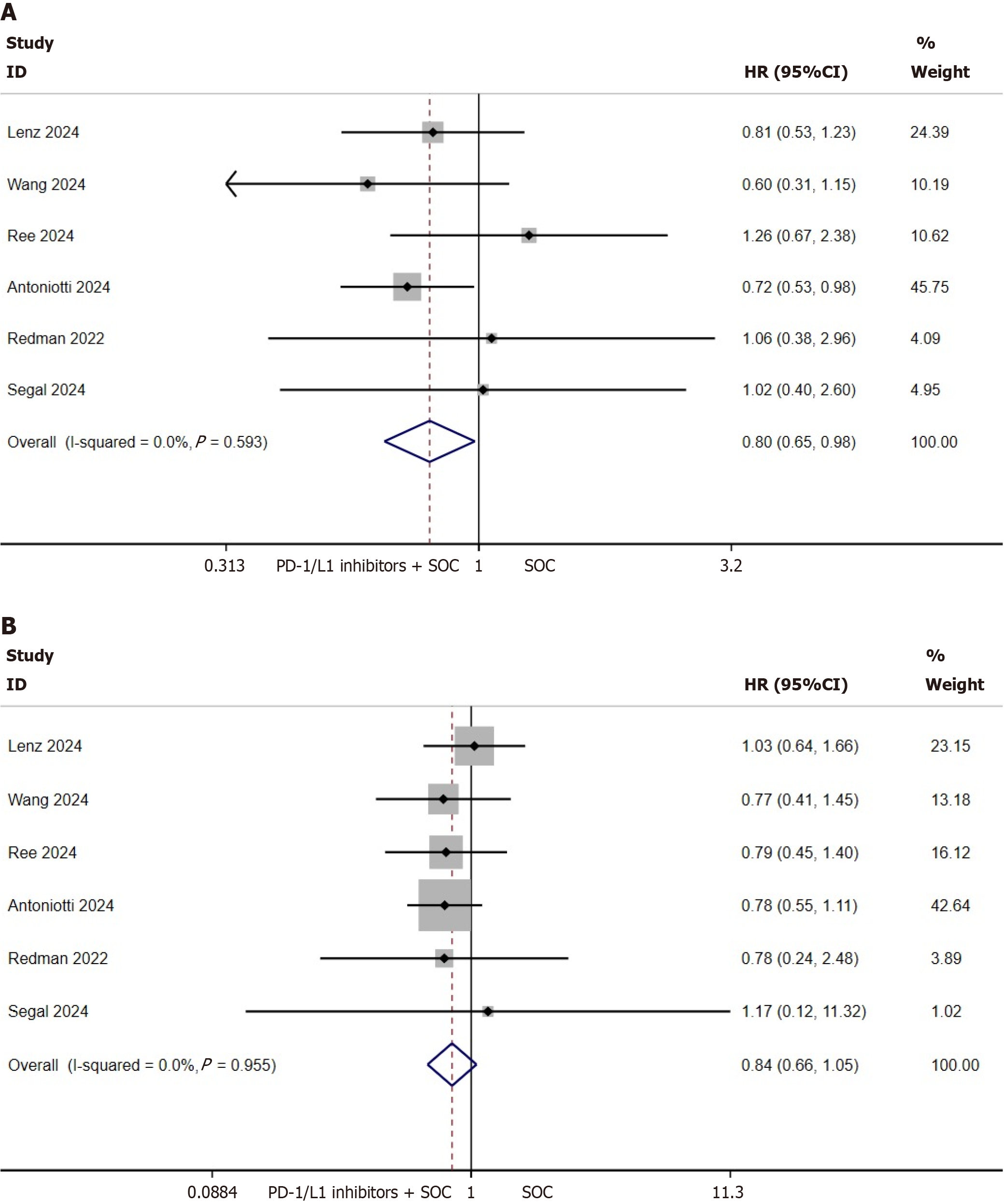
PFS and OS of the ITT population: The present investigation primarily evaluated PFS and OS within the ITT cohort to determine the prognostic implications of integrating PD-1/L1 inhibitors with standard first-line therapy in mCRC. Pooled analysis of six clinical trials involving 675 mCRC cases demonstrated a statistically significant PFS advantage for PD-1/L1 inhibitor-SOC combination therapy over SOC monotherapy in ITT populations (HR = 0.8, 95%CI: 0.65-0.98, P = 0.033; heterogeneity I2 = 0%, P = 0.593; Figure 2A). Absolute median PFS enhancement was calculated as 1.13 months (95%CI: -0.10 to 2.37; Supplementary Figure 1). Conversely, no significant OS benefit was observed with combination therapy (HR = 0.84, 95%CI: 0.66-1.05, P = 0.124; I2 = 0%, P = 0.95; Figure 2B).
Subgroup stratification by PD-1 vs PD-L1 inhibitor types revealed comparable outcomes: Neither PFS (PD-1 subgroup: P = 0.268, Supplementary Figure 2A; PD-L1 subgroup: P = 0.061, Supplementary Figure 2B) nor OS (PD-1: P = 0.439, Supplementary Figure 2C; PD-L1: P = 0.157, Supplementary Figure 2D) demonstrated statistically significant improvements in the ITT population.
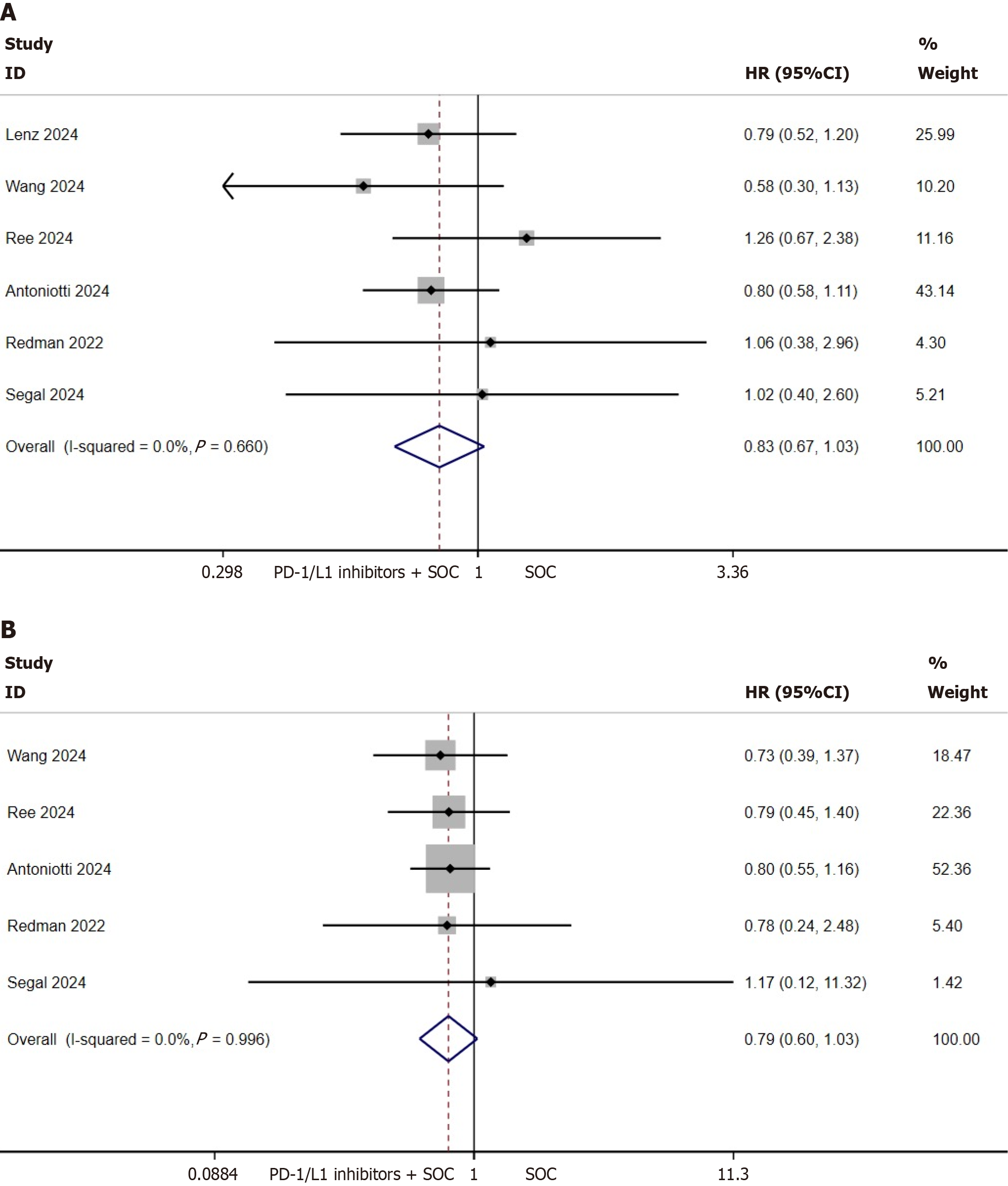
PFS and OS of the MSS/pMMR population: This investigation focused on evaluating the prognostic correlation between MMR status and survival outcomes in mCRC by analyzing PFS/OS data from molecularly defined subgroups. Pooled analysis incorporated six trials (n = 622 MSS/pMMR cases) for PFS quantification and five trials (n = 440 MSS/pMMR cases) for OS evaluation. Results demonstrated comparable outcomes between PD-1/L1 inhibitor-SOC combination therapy and SOC monotherapy in MSS/pMMR cohorts: PFS (HR = 0.83, 95%CI: 0.67-1.03, P = 0.091; I2 = 0%, P = 0.660; Figure 3A) and OS (HR = 0.79, 95%CI: 0.60-1.03, P = 0.083; I2 = 0%, P = 0.996; Figure 3B).
Subanalyses stratified by PD-1 vs PD-L1 inhibitor types revealed similar findings: Neither PFS (PD-1: P = 0.224, Supplementary Figure 3A; PD-L1: P = 0.240, Supplementary Figure 3B) nor OS (PD-1: P = 0.208, Supplementary Figure 3C; PD-L1: P = 0.226, Supplementary Figure 3D) showed statistical significance across subgroups in the MSS/pMMR population.
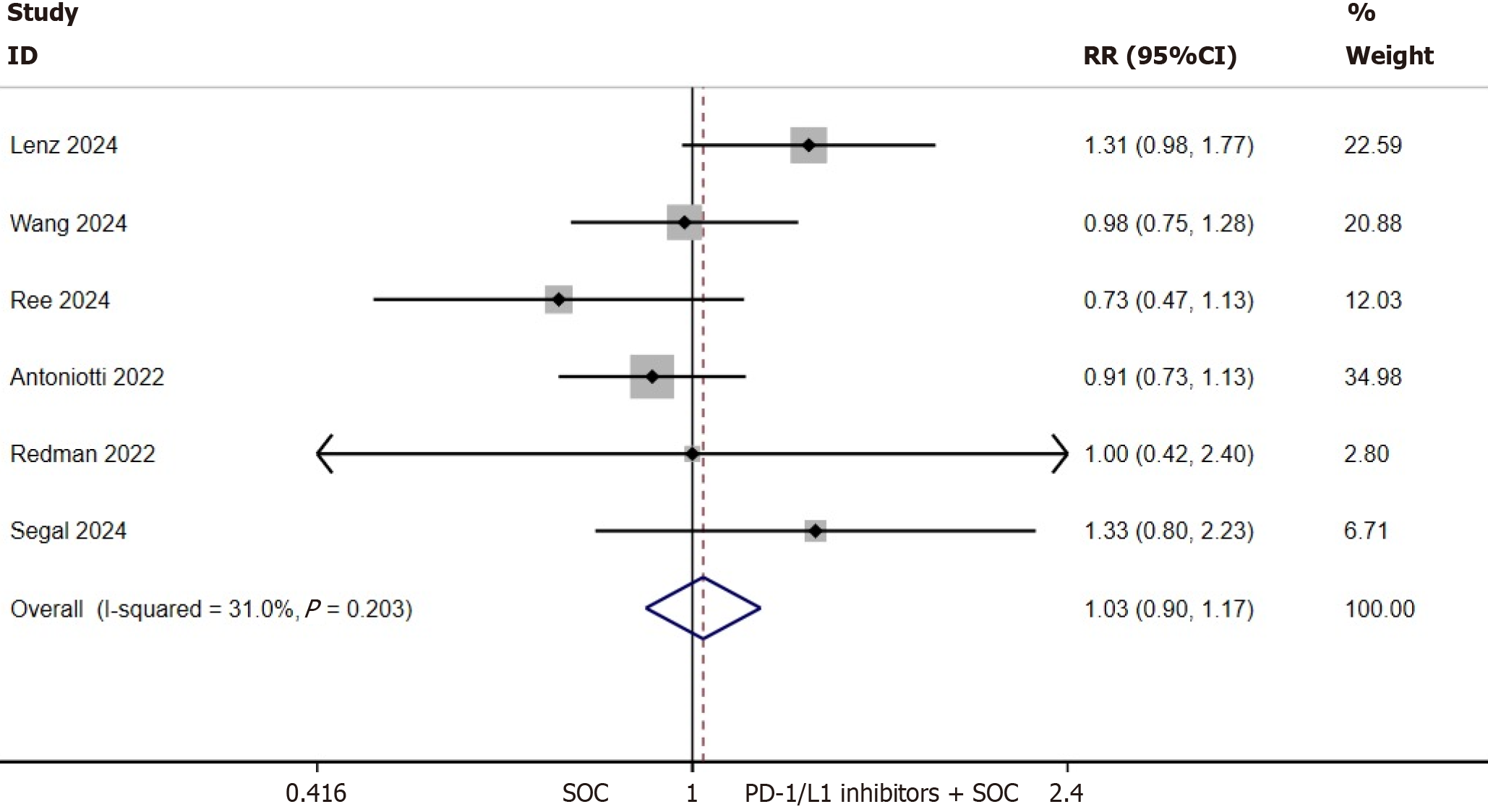
ORR of the ITT population: ORR data were documented across all eligible trials. Quantitative synthesis of ORR outcomes was conducted using a fixed-effects analytical model due to the absence of significant interstudy heterogeneity (I2 = 31%, P = 0.203). The incorporation of PD-1/L1 inhibitors into first-line SOC regimens did not demonstrate statistically significant enhancement in ORR for patients with mCRC (RR = 1.03, 95%CI: 0.90-1.17, P = 0.711; Figure 4).
Treatment-related AEs: Quantitative safety evaluation encompassed 589 patients with mCRC from five clinical trials. Comparative analysis demonstrated comparable safety profiles between PD-1/L1 inhibitor-SOC combination therapy and SOC monotherapy regarding grade ≥ 3 treatment-emergent AEs (RR = 1.12, 95%CI: 0.93-1.36, P = 0.245; Supplementary Figure 4A). A statistical model utilizing random effects was implemented due to moderate between-study heterogeneity (I2 = 60.1%, P = 0.040).
To address observed heterogeneity in AE reporting, sequential exclusion sensitivity analyses were performed. The Lenz 2024 trial emerged as a key heterogeneity source; its exclusion reduced pooled RR from 1.12 (95%CI: 0.93-1.36) to 1.06 (95%CI: 0.92-1.23) with concurrent I² decrease from 60.1% to 4.9% (Supplementary Figure 4B). Immune-related toxicity analysis revealed pooled incidence rates of grade ≥ 3 AEs of 1.1% (95%CI: 0.1%-2.8%) for pneumonitis and 2.8% (95%CI: 0.2%-7.4%) for transaminase elevation.
Publication bias: Publication bias assessment employing Begg’s rank correlation and Egger’s linear regression methodologies revealed no statistically significant bias across analyzed outcomes. In the ITT population this lack of bias was identified for PFS (Begg’s P = 0.452, Egger’s P = 0.274) and OS (Begg’s P = 0.452, Egger’s P = 0.736). Comparable findings were observed in the MSS/pMMR subgroup regarding PFS (Begg’s P = 0.452, Egger’s P = 0.482) and OS (Begg’s P = 1.000, Egger’s P = 0.501). Extended analysis demonstrated absence of significant bias for ORR (Begg’s P = 0.707, Egger’s P = 0.801) and AEs (Begg’s P = 0.806, Egger’s P = 0.801).
CRC constitutes a prominent contributor to the global cancer burden, demonstrating substantial incidence and mortality worldwide[1]. A substantial proportion of CRC cases are diagnosed at advanced metastatic stages, wherein systemic therapy for these patients primarily involves standardized chemotherapeutic protocols[37]. Although demonstrating established clinical utility in mCRC management, current chemotherapeutic approaches fail to translate to substantial improvements in long-term survival outcomes, with 5-year survival rates remaining suboptimal[38]. Immunotherapeutic strategies have emerged as transformative approaches in oncology[39], particularly for mCRC management, generating considerable enthusiasm in clinical research circles. However, recent investigations reveal that immune microenvironment heterogeneity substantially modulates treatment efficacy[40].
To our knowledge this study represents the pioneering systematic evaluation comparing PD-1/L1 inhibitor-SOC combination vs SOC monotherapy as frontline treatment for mCRC, incorporating six clinical trials comprising 675 mCRC cases. Our analysis demonstrated a statistically significant PFS advantage for combination therapy exclusively in the ITT cohort (HR = 0.8, 95%CI: 0.65-0.98), though the marginally extended median PFS of 1.13 months may lack substantial clinical relevance for general populations. Clinicians must rigorously evaluate risk-benefit profiles, considering treatment-related toxicities, financial implications, and patient-specific preferences. While potentially beneficial in immunogenically favorable subgroups, widespread clinical adoption necessitates judicious patient selection. Notably, combination regimens failed to demonstrate significant OS benefits in either ITT or MSS/pMMR populations (HR = 0.84 and 0.79 respectively) nor did they improve PFS in MSS/pMMR subgroups or ORR across cohorts. Safety assessments revealed comparable grade ≥ 3 AE incidence between treatment arms (RR = 1.12, 95%CI: 0.93-1.36), suggesting acceptable toxicity profiles for combination therapy.
mCRC manifests two molecularly distinct subtypes: MSS and MSI variants. These subgroups exhibit differential responsiveness to immunotherapeutic agents due to inherent biological disparities. Approximately 10% of patients with mCRC present with MSI features. CRC exhibiting MSI demonstrates marked tumor neoantigen expression and increased mutational burden, mechanisms that potentiate immune activation and antitumor immunity[41]. Notably, immune checkpoint blockers targeting the PD-1/L1 axis have shown therapeutic benefits in MSI-H or dMMR mCRC populations. Illustrating this, pembrolizumab administration in the KEYNOTE-177 trial demonstrated a two-fold prolongation in median PFS compared with conventional chemotherapy[42].
The CheckMate-142 trial[13] further substantiated these findings, reporting enhanced ORR and complete remission rates with nivolumab-ipilimumab combination therapy. These clinical outcomes correlate with the characteristic T cell-enriched tumor microenvironment observed in patients with MSI-H mCRC[43]. This unique tumor milieu exhibits reduced sensitivity to fluorouracil-based chemotherapy yet displays heightened responsiveness to PD-1-targeted monoclonal antibodies[44], rendering immunotherapies particularly effective in this molecular subset.
In contrast MSS-type mCRC accounts for approximately 90% of metastatic cases. Characterized by low TMB, MSS tumors harbor immunosuppressive cellular infiltrates (regulatory T cells, myeloid-derived suppressor cells) with deficient CD8⁺ T cell activation and diminished checkpoint molecule expression, collectively contributing to suboptimal immunotherapy responses[17,18,45,46]. Cytotoxic chemotherapeutics induce immunogenic tumor cell apoptosis, subsequently activating CD8⁺ T cell populations through tumor-associated neoantigen release, thereby modulating the immune microenvironment[20,47,48]. This immunostimulatory phenomenon reaches maximal potency when employing intensive first-line regimens like triplet chemotherapy. Clinical evidence from resectable liver metastasis cohorts revealed enhanced tumor immunogenicity following FOLFOXIRI-based treatment[49]. Bevacizumab exerts multifaceted immunomodulatory effects: Facilitating dendritic cell maturation to amplify CD8⁺ T-cell priming; and normalizing tumor vasculature to promote lymphocyte infiltration. These synergistic mechanisms establish an immunologically permissive microenvironment favoring antitumor immunity[50].
Over the past 5 years, clinical trial data have predominantly demonstrated limited therapeutic effectiveness in MSS/pMMR mCRC cases[51]. The Keynote-016 trial revealed that none of the 18 enrolled patients achieved ORR following pembrolizumab treatment[52]. Similarly, a separate study involving 73 patients with mCRC reported no ORR after combination therapy with regorafenib and pembrolizumab[53]. These findings collectively suggest that immunotherapy fails to produce satisfactory clinical responses in most patients with MSS/pMMR mCRC[54]. The prevailing theory attributes this phenomenon to a “cold” tumor microenvironment, which is believed to restrict the therapeutic potential of ICIs. The clinical benefits of ICIs for patients with mCRC with varying microsatellite statuses remain controversial, highlighting the need for further investigation in this field.
A prior systematic evaluation conducted by Huang et al[55] investigated frontline PD-1/L1-targeted therapies in metastatic CRC management. Their findings demonstrated superior clinical efficacy with favorable safety profiles when these immunotherapeutics were implemented as initial treatment for dMMR mCRC. Therapeutic regimens combining PD-1/L1 inhibitors, vascular endothelial growth factor-neutralizing antibodies, and cytotoxic agents in MSS/pMMR populations were associated with enhanced objective response rates. Nevertheless, longitudinal survival outcomes remained unimproved, aligning with our current observations. It should be noted that their analysis[55] predominantly incorporated non-comparative single-arm investigations, limiting direct interventional comparisons across therapeutic strategies.
Our meta-analysis exclusively incorporated randomized controlled trials comparing PD-1/L1 inhibitor-SOC combination regimens against SOC monotherapy, providing high-level evidence through analysis of both ITT cohorts and MSS/pMMR mCRC subpopulations. Aligned with the National Comprehensive Cancer Network guideline re
Several limitations should be acknowledged in this investigation. First, this investigation incorporated a constrained number of clinical trials despite comprehensive searches across four major English databases, resulting in limited statistical power for outcome assessment. Reduced sample sizes inherently constrain statistical power for evaluating longitudinal survival metrics like OS, necessitating future large-scale prospective studies with extended follow-up periods. Second, substantial methodological heterogeneity was observed among included trials regarding study designs, participant demographics, and therapeutic protocols. Third, analytical constraints arose from reliance on published aggregate data rather than individual patient-level records, compounded by minimal implementation of blinding procedures. Fourth, critical knowledge gaps persist regarding primary determinants influencing ICI efficacy. Insufficient experimental data precluded exploration of correlations between clinical variables (sex, age, TMB, tumor neoantigen burden, PD-L1 expression levels, metastatic patterns) and treatment outcomes/safety profiles. These limitations warrant cautious interpretation of the synthesized evidence.
This meta-analysis of randomized controlled trials demonstrated that first-line PD-1/L1 blockade combined with SOC regimens significantly enhanced PFS exclusively in the ITT mCRC cohort. Therapeutic augmentation with ICIs failed to demonstrate statistically significant improvements in OS for either ITT or MSS/pMMR subgroups. Furthermore, ORR and PFS in MSS/pMMR patients remained comparable between treatment arms. Safety assessments revealed comparable incidence of grade ≥ 3 AEs between investigational and control groups, indicating acceptable toxicity profiles for combination therapy. These evidence-based insights establish a crucial framework for optimizing therapeutic strategies in mCRC management. Validation through large-scale multicenter randomized trials warrants implementation to refine clinical practice guidelines.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64590] [Article Influence: 16147.5] [Reference Citation Analysis (176)] |
| 2. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 3304] [Article Influence: 413.0] [Reference Citation Analysis (3)] |
| 3. | Cronin KA, Scott S, Firth AU, Sung H, Henley SJ, Sherman RL, Siegel RL, Anderson RN, Kohler BA, Benard VB, Negoita S, Wiggins C, Cance WG, Jemal A. Annual report to the nation on the status of cancer, part 1: National cancer statistics. Cancer. 2022;128:4251-4284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 316] [Article Influence: 105.3] [Reference Citation Analysis (0)] |
| 4. | Kahi CJ, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Lieberman D, Levin TR, Robertson DJ, Rex DK; United States Multi-Society Task Force on Colorectal Cancer. Colonoscopy Surveillance After Colorectal Cancer Resection: Recommendations of the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2016;150:758-768.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 5. | Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 2270] [Article Influence: 133.5] [Reference Citation Analysis (0)] |
| 6. | Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, Schrag D, Greene C, O'Neil BH, Atkins JN, Berry S, Polite BN, O'Reilly EM, Goldberg RM, Hochster HS, Schilsky RL, Bertagnolli MM, El-Khoueiry AB, Watson P, Benson AB 3rd, Mulkerin DL, Mayer RJ, Blanke C. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA. 2017;317:2392-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 681] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 7. | Shin AE, Giancotti FG, Rustgi AK. Metastatic colorectal cancer: mechanisms and emerging therapeutics. Trends Pharmacol Sci. 2023;44:222-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 286] [Reference Citation Analysis (0)] |
| 8. | Grapsa D, Syrigos K, Saif MW. Bevacizumab in combination with fluoropyrimidine-irinotecan- or fluoropyrimidine-oxaliplatin-based chemotherapy for first-line and maintenance treatment of metastatic colorectal cancer. Expert Rev Anticancer Ther. 2015;15:1267-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Ogura A, Konishi T, Cunningham C, Garcia-Aguilar J, Iversen H, Toda S, Lee IK, Lee HX, Uehara K, Lee P, Putter H, van de Velde CJH, Beets GL, Rutten HJT, Kusters M; Lateral Node Study Consortium. Neoadjuvant (Chemo)radiotherapy With Total Mesorectal Excision Only Is Not Sufficient to Prevent Lateral Local Recurrence in Enlarged Nodes: Results of the Multicenter Lateral Node Study of Patients With Low cT3/4 Rectal Cancer. J Clin Oncol. 2019;37:33-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 370] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 10. | González-Perera I, Gutiérrez-Nicolás F, Nazco-Casariego GJ, Ramos-Díaz R, Hernández-San Gil R, Pérez-Pérez JA, González García J, González De La Fuente GA. 5-fluorouracil toxicity in the treatment of colon cancer associated with the genetic polymorphism 2846 A>G (rs67376798). J Oncol Pharm Pract. 2017;23:396-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Trullas A, Delgado J, Genazzani A, Mueller-Berghaus J, Migali C, Müller-Egert S, Zander H, Enzmann H, Pignatti F. The EMA assessment of pembrolizumab as monotherapy for the first-line treatment of adult patients with metastatic microsatellite instability-high or mismatch repair deficient colorectal cancer. ESMO Open. 2021;6:100145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, Goldberg MV, Cao ZA, Ledeine JM, Maglinte GA, Kopetz S, André T. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1775] [Cited by in RCA: 2088] [Article Influence: 261.0] [Reference Citation Analysis (0)] |
| 13. | Lenz HJ, Van Cutsem E, Luisa Limon M, Wong KYM, Hendlisz A, Aglietta M, García-Alfonso P, Neyns B, Luppi G, Cardin DB, Dragovich T, Shah U, Abdullaev S, Gricar J, Ledeine JM, Overman MJ, Lonardi S. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J Clin Oncol. 2022;40:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 416] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 14. | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Colon Cancer Version 3.2025. [cited 20 April 2025]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1428. |
| 15. | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Rectal Cancer V.2.2025. [cited 20 April 2025]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1461. |
| 16. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7241] [Article Influence: 724.1] [Reference Citation Analysis (0)] |
| 17. | Giannakis M, Mu XJ, Shukla SA, Qian ZR, Cohen O, Nishihara R, Bahl S, Cao Y, Amin-Mansour A, Yamauchi M, Sukawa Y, Stewart C, Rosenberg M, Mima K, Inamura K, Nosho K, Nowak JA, Lawrence MS, Giovannucci EL, Chan AT, Ng K, Meyerhardt JA, Van Allen EM, Getz G, Gabriel SB, Lander ES, Wu CJ, Fuchs CS, Ogino S, Garraway LA. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell Rep. 2016;17:1206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 199] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 18. | Hegde PS, Karanikas V, Evers S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin Cancer Res. 2016;22:1865-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 685] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 19. | Marmorino F, Boccaccino A, Germani MM, Falcone A, Cremolini C. Immune Checkpoint Inhibitors in pMMR Metastatic Colorectal Cancer: A Tough Challenge. Cancers (Basel). 2020;12:2317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | Duffy AG, Greten TF. Immunological off-target effects of standard treatments in gastrointestinal cancers. Ann Oncol. 2014;25:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 1896] [Article Influence: 474.0] [Reference Citation Analysis (1)] |
| 22. | Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, Chung IJ, Yamaguchi K, Kato K, Sym SJ, Kadowaki S, Tsuji K, Chen JS, Bai LY, Oh SY, Choda Y, Yasui H, Takeuchi K, Hirashima Y, Hagihara S, Boku N. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 486] [Article Influence: 162.0] [Reference Citation Analysis (0)] |
| 23. | Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, El Hajbi F, Di Bartolomeo M, Braghiroli MI, Holtved E, Ostoich SA, Kim HR, Ueno M, Mansoor W, Yang WC, Liu T, Bridgewater J, Makino T, Xynos I, Liu X, Lei M, Kondo K, Patel A, Gricar J, Chau I, Kitagawa Y; CheckMate 648 Trial Investigators. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med. 2022;386:449-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 642] [Article Influence: 214.0] [Reference Citation Analysis (2)] |
| 24. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13349] [Article Influence: 834.3] [Reference Citation Analysis (0)] |
| 25. | Higgins JPT, Green S, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons, 2019. |
| 26. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4738] [Cited by in RCA: 4953] [Article Influence: 275.2] [Reference Citation Analysis (0)] |
| 27. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46504] [Article Influence: 2113.8] [Reference Citation Analysis (3)] |
| 28. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40519] [Article Influence: 1447.1] [Reference Citation Analysis (2)] |
| 29. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10586] [Cited by in RCA: 12182] [Article Influence: 406.1] [Reference Citation Analysis (0)] |
| 30. | Lenz HJ, Parikh A, Spigel DR, Cohn AL, Yoshino T, Kochenderfer M, Elez E, Shao SH, Deming D, Holdridge R, Larson T, Chen E, Mahipal A, Ucar A, Cullen D, Baskin-Bey E, Kang T, Hammell AB, Yao J, Tabernero J. Modified FOLFOX6 plus bevacizumab with and without nivolumab for first-line treatment of metastatic colorectal cancer: phase 2 results from the CheckMate 9X8 randomized clinical trial. J Immunother Cancer. 2024;12:e008409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 31. | Antoniotti C, Rossini D, Pietrantonio F, Catteau A, Salvatore L, Lonardi S, Boquet I, Tamberi S, Marmorino F, Moretto R, Ambrosini M, Tamburini E, Tortora G, Passardi A, Bergamo F, Kassambara A, Sbarrato T, Morano F, Ritorto G, Borelli B, Boccaccino A, Conca V, Giordano M, Ugolini C, Fieschi J, Papadopulos A, Massoué C, Aprile G, Antonuzzo L, Gelsomino F, Martinelli E, Pella N, Masi G, Fontanini G, Boni L, Galon J, Cremolini C; GONO Foundation Investigators. Upfront FOLFOXIRI plus bevacizumab with or without atezolizumab in the treatment of patients with metastatic colorectal cancer (AtezoTRIBE): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2022;23:876-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 161] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 32. | Antoniotti C, Rossini D, Pietrantonio F, Salvatore L, Lonardi S, Tamberi S, Marmorino F, Moretto R, Prisciandaro M, Tamburini E, Tortora G, Passardi A, Bergamo F, Raimondi A, Ritorto G, Borelli B, Conca V, Ugolini C, Aprile G, Antonuzzo L, Gelsomino F, Martinelli E, Pella N, Masi G, Boni L, Galon J, Cremolini C. Upfront Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan Plus Bevacizumab With or Without Atezolizumab for Patients With Metastatic Colorectal Cancer: Updated and Overall Survival Results of the ATEZOTRIBE Study. J Clin Oncol. 2024;42:2637-2644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 33. | Wang ZX, Peng J, Liang X, Cheng Y, Deng Y, Chen K, Zhang M, Zhang J, Wang W, Cao B, Jin Y, Sun M, Lin Y, Luo S, Li Z, Yang L, Ke Y, Yu H, Li J, Wang Q, Zhu J, Wang F, Xu RH. First-line serplulimab in metastatic colorectal cancer: Phase 2 results of a randomized, double-blind, phase 2/3 trial. Med. 2024;5:1150-1163.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 34. | Redman JM, Tsai YT, Weinberg BA, Donahue RN, Gandhy S, Gatti-Mays ME, Abdul Sater H, Bilusic M, Cordes LM, Steinberg SM, Marte JL, Jochems C, Kim SS, Marshall JL, McMahon S, Redmond E, Schlom J, Gulley JL, Strauss J. A Randomized Phase II Trial of mFOLFOX6 + Bevacizumab Alone or with AdCEA Vaccine + Avelumab Immunotherapy for Untreated Metastatic Colorectal Cancer. Oncologist. 2022;27:198-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 35. | Ree AH, Šaltytė Benth J, Hamre HM, Kersten C, Hofsli E, Guren MG, Sorbye H, Johansen C, Negård A, Bjørnetrø T, Nilsen HL, Berg JP, Flatmark K, Meltzer S. First-line oxaliplatin-based chemotherapy and nivolumab for metastatic microsatellite-stable colorectal cancer-the randomised METIMMOX trial. Br J Cancer. 2024;130:1921-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 36. | Segal NH, Tie J, Kopetz S, Ducreux M, Chen E, Dienstmann R, Hollebecque A, Reilley MJ, Elez E, Cosaert J, Cain J, Soo-Hoo Y, Hewson N, Cooper ZA, Kumar R, Tabernero J. COLUMBIA-1: a randomised study of durvalumab plus oleclumab in combination with chemotherapy and bevacizumab in metastatic microsatellite-stable colorectal cancer. Br J Cancer. 2024;131:1005-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 37. | Grothey A, Fakih M, Tabernero J. Management of BRAF-mutant metastatic colorectal cancer: a review of treatment options and evidence-based guidelines. Ann Oncol. 2021;32:959-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 135] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 38. | Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2286] [Cited by in RCA: 2429] [Article Influence: 269.9] [Reference Citation Analysis (31)] |
| 39. | Ganesh K. Optimizing immunotherapy for colorectal cancer. Nat Rev Gastroenterol Hepatol. 2022;19:93-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 40. | Yuan J, Li J, Gao C, Jiang C, Xiang Z, Wu J. Immunotherapies catering to the unmet medical need of cold colorectal cancer. Front Immunol. 2022;13:1022190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Ros J, Balconi F, Baraibar I, Saoudi Gonzalez N, Salva F, Tabernero J, Elez E. Advances in immune checkpoint inhibitor combination strategies for microsatellite stable colorectal cancer. Front Oncol. 2023;13:1112276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 42. | Diaz LA Jr, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fourchardiere C, Rivera F, Elez E, Le DT, Yoshino T, Zhong WY, Fogelman D, Marinello P, Andre T; KEYNOTE-177 Investigators. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022;23:659-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 511] [Article Influence: 170.3] [Reference Citation Analysis (0)] |
| 43. | Hirano H, Takashima A, Hamaguchi T, Shida D, Kanemitsu Y; Colorectal Cancer Study Group (CCSG) of the Japan Clinical Oncology Group (JCOG). Current status and perspectives of immune checkpoint inhibitors for colorectal cancer. Jpn J Clin Oncol. 2021;51:10-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 44. | Taieb J, Karoui M. FOxTROT: Are We Ready to Dance? J Clin Oncol. 2023;41:1514-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 45. | Buchler T. Microsatellite Instability and Metastatic Colorectal Cancer - A Clinical Perspective. Front Oncol. 2022;12:888181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 46. | Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3297] [Cited by in RCA: 4769] [Article Influence: 397.4] [Reference Citation Analysis (1)] |
| 47. | Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052-3061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 963] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 48. | Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, Mendiboure J, Pignon JP, Jooste V, van Endert P, Ducreux M, Zitvogel L, Piard F, Kroemer G. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29:482-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 919] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 49. | Moretto R, Corallo S, Belfiore A, Rossini D, Boccaccino A, Lonardi S, Centonze G, Morano F, Germani MM, Loupakis F, Morelli L, Urbani L, Brich S, Marmorino F, Prisciandaro M, Aprile G, Fassan M, Cillo U, Cattaneo L, Fontanini G, De Braud F, Falcone A, Milione M, Pietrantonio F, Cremolini C. Prognostic impact of immune-microenvironment in colorectal liver metastases resected after triplets plus a biologic agent: A pooled analysis of five prospective trials. Eur J Cancer. 2020;135:78-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70:6171-6180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 530] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 51. | Maajani K, Khodadost M, Fattahi A, Shahrestanaki E, Pirouzi A, Khalili F, Fattahi H. Survival Rate of Colorectal Cancer in Iran: A Systematic Review and Meta-Analysis. Asian Pac J Cancer Prev. 2019;20:13-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 52. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3799] [Cited by in RCA: 4947] [Article Influence: 618.4] [Reference Citation Analysis (0)] |
| 53. | Barzi A, Azad NS, Yang Y, Tsao-wei D, Rehman R, Fakih M, Iqbal S, El-khoueiry AB, Millstein J, Jayachandran P, Zhang W, Lenz H. Phase I/II study of regorafenib (rego) and pembrolizumab (pembro) in refractory microsatellite stable colorectal cancer (MSSCRC). J Clin Oncol. 2022;40:15-15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 54. | Huyghe N, Benidovskaya E, Stevens P, Van den Eynde M. Biomarkers of Response and Resistance to Immunotherapy in Microsatellite Stable Colorectal Cancer: Toward a New Personalized Medicine. Cancers (Basel). 2022;14:2241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 55. | Huang Z, Li C, Huang Y, Liang W, Tao H. Efficacy and safety of PD-1/L1 inhibitors as first-line therapy for metastatic colorectal cancer: a meta-analysis. Front Immunol. 2024;15:1425596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |