INTRODUCTION
Overview of cancer and the importance of novel therapeutics
The concept of oncology dates back to ancient Greek medicine, where early observations laid the groundwork for modern cancer research[1]. To date, more than 200 types of cancer have been identified[2]. According to cancer statistics for 2025, 39.9% of men and 39% of women are expected to be diagnosed with the most common invasive types of cancer[3]. While lung and breast cancers top the chart, the incidence of other cancers, such as prostate, colorectal, genital, and skin cancers, is also rising rapidly[1,2]. Currently, available cancer treatment options include chemotherapy, selective immunotherapy, and surgery[4]. However, these conventional methods have repeatedly shown multi-drug resistance. Understanding the mechanisms of drug resistance is crucial for advancing cancer therapy[5]. Consequently, there is an urgent need to explore alternative therapeutic strategies that selectively target cancer cells while minimizing harm to healthy tissues[6]. It is imperative to validate novel targeted therapies along with innovative drug delivery systems (e.g., receptor/ligand-binding targets, aptamers, siRNA, peptides), which induce cancer cell death and help alleviate disease progression[6,7].
Cell death is a primary hallmark of cancer, and its connection to cancer is both intricate and crucial. Cells utilize various pathways to control their death, which is essential for maintaining healthy tissues and preventing uncontrolled cell growth—a key characteristic of cancer. Cell death can be accidental, resulting from unexpected damage or injury, or it can be regulated, occurring purposefully through genetic interventions or pharmacological agents[8].
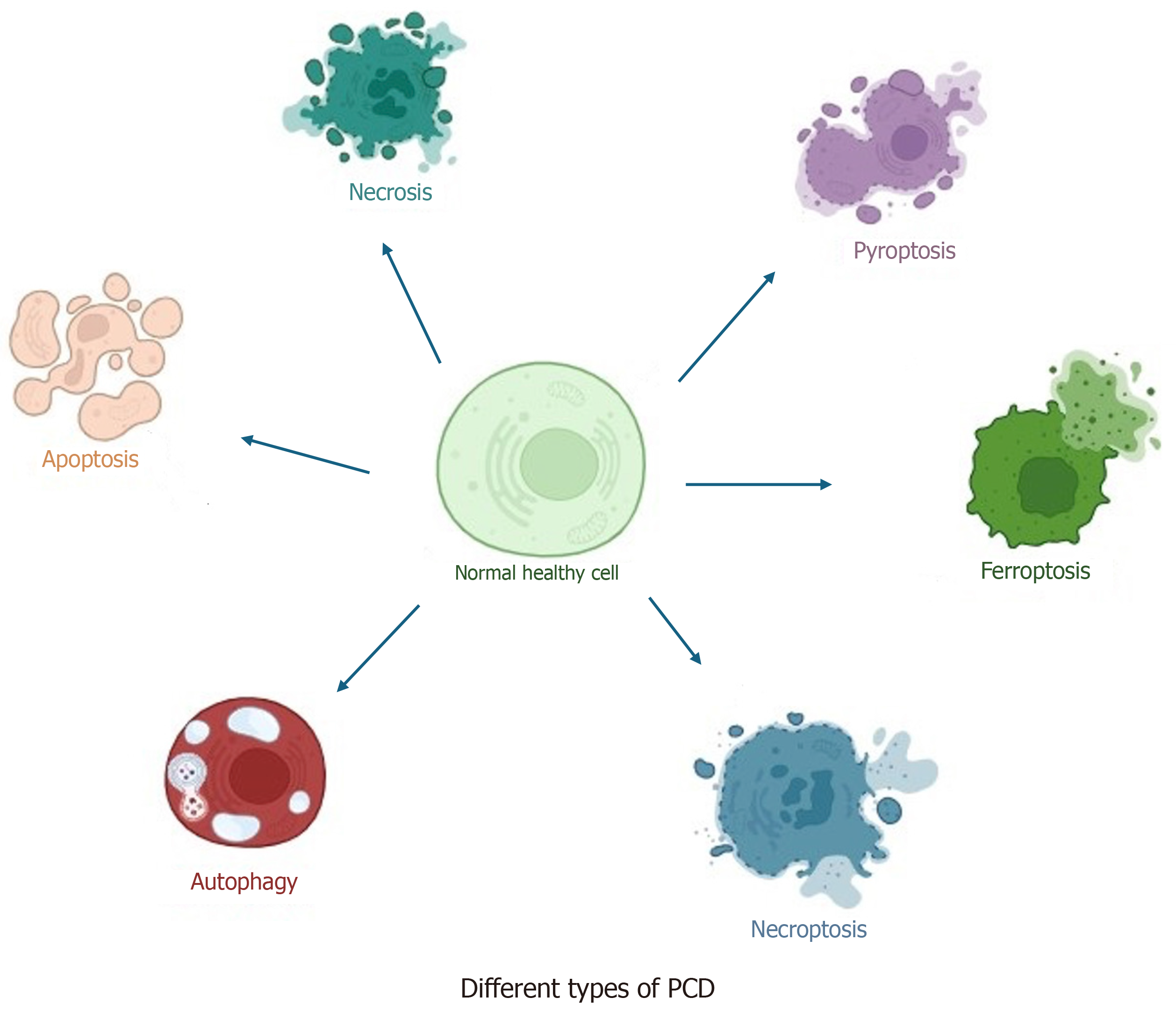
Over the past decade, researchers have gained deep insights into regulated cell death (RCD), also known as programmed cell death (PCD), with a therapeutic focus on cancers such as colorectal, lung, breast, and bladder cancers, where resistance and dedifferentiation are commonly observed[9,10]. RCD encompasses various mechanisms, including apoptosis, autophagy, and more recently identified subroutines that depend on distinct metabolic pathways such as necroptosis, pyroptosis, NETosis, PANoptosis, and ferroptosis[11-14] (Figure 1).
Figure 1 Various forms of programmed cell death.
Programmed cell death is a crucially controlled form of cell death, where different pathways regulate the process. Programmed cell death (PCD) is necessary to maintain cell homeostasis, control growth, and support immunity. PCD primarily occurs in two forms: Apoptotic and non-apoptotic. Non-apoptotic forms of cell death have been studied only in the past decade, including ferroptosis, necroptosis, and pyroptosis.
While significant progress has been made in studying these novel cell death mechanisms, this review focuses on unveiling the potential of ferroptosis-induced mitochondrial cell death. It further explores the therapeutic relevance of the interplay between ferroptosis, autophagy, and mitochondrial dysfunction in cancer.
Ferroptosis is a type of cell death that depends on iron and is characterized by the excessive accumulation of lipid peroxides. This process can serve as a defense mechanism against cancer by eliminating cells that evade other forms of cell death, such as apoptosis[15,16]. However, some cancer cells may develop resistance to ferroptosis, thereby promoting tumor growth and treatment resistance. Inducing ferroptosis represents a promising strategy for treating cancers, particularly those resistant to conventional therapies. A detailed discussion is provided in the ferroptosis section.
Autophagy is a lysosome-mediated form of cell death, primarily responsible for maintaining homeostasis and lipid metabolism by degrading damaged proteins or organelles. It plays a dual role in tumor progression, acting as both a tumor suppressor and a promoter under different conditions. Unc-51-like kinase 1 serves as a key initiator of autophagy[12,14]. Additionally, autophagy can be induced through ferroptosis in a process known as ferritinophagy[17]. The dual role, molecular mechanisms, and interconnection with ferroptosis are discussed in detail in the section on autophagy.
Lastly, the role of mitochondria in ferroptosis is crucial. Mitochondria serve as a significant site for iron utilization and reactive oxygen species (ROS) production, both of which are key factors in ferroptosis. Dysfunctional mitochondria can lead to elevated ROS levels, promoting lipid peroxidation and triggering ferroptosis. Glutamine, through the tricarboxylic acid cycle in mitochondria, also contributes to cysteine depletion via glutaminolysis, thereby inducing ferroptosis[18].
The crosstalk among ferroptosis, autophagy, and mitochondrial cell death plays a significant role in RCD or PCD. Regulating these mechanisms through chemotherapy and immunotherapy could bring about a paradigm shift in cancer treatment.
METHODOLOGY AND REVIEW CRITERIA
This review highlights recent developments in utilizing ferroptosis as a therapeutic mechanism for treating aggressive cancers that are resistant to conventional therapies. The search strategy included keywords such as ferroptosis in cancer, the interplay between autophagy and ferroptosis, mitochondrial dynamics and their connection with ferroptosis, the mechanism of ferroptosis through oxidative stress in mitochondria, pathways regulating cell death through ferroptosis, and recent advancements in ferroptosis, autophagy, and mitochondrial dynamics for cancer treatment.
The databases referred to for compiling this review included Google Scholar, PubMed, the Directory of Open Access Journals, and Science Direct, among others.
FERROPTOSIS AND ITS THERAPEUTIC POTENTIAL IN CANCER
Ferroptosis as a process has been widely studied in various human diseases, including those affecting the lungs, liver, heart, breast, kidneys, nervous system, reproductive organs, and skin. However, in the past decade, it has gained significant attention as a novel therapeutic opportunity in cancer research. Despite this, the concept has not yet been translated into clinical studies[19]. This presents an opportunity to explore small molecules, nanoparticles, macromolecules, and other innovative therapeutic agents that target iron-dependent cell death via lipid peroxidation. Such approaches may enable selective targeting of cancer cells while sparing normal cells. Additionally, this strategy holds potential for overcoming drug resistance and enhancing the efficacy of conventional treatments such as immunotherapy, radiotherapy, and chemotherapy[20,21].
The term ferroptosis was first coined in 2012 by the laboratory of Brent R. Stockwell. However, the mechanistic foundation dates back to the 1980s with the discovery of System Xc-, an amino acid antiporter responsible for the exchange of extracellular cystine and intracellular glutamate across the plasma membrane, which functions through glutathione (GSH)[22]. Although the mechanism was known earlier, the terminology was formally introduced only in 2012 by Stockwell and colleagues[15]. During their screening of small molecules, the Stockwell and Dixon laboratories discovered erastin, a lethal compound that induces ferroptosis by triggering RAS mutations and dysfunction in the RAS/MAPK/MEK pathways, resulting in iron-dependent, non-apoptotic cell death[15].
Ferroptosis is now recognized as a distinct form of regulated cell death characterized by the iron-dependent accumulation of lethal lipid peroxides. It is biochemically, morphologically, and genetically different from other forms of non-apoptotic cell death, such as apoptosis, pyroptosis, necroptosis, and autophagy[23,24].
The hallmark of ferroptotic cell death lies in the mitochondria, which undergo shrinkage, membrane disruption, and a loss of cristae features not observed in other types of regulated cell death[15,23]. Interestingly, mesenchymal and dedifferentiated cancer cells that resist conventional cell death pathways appear to be vulnerable to ferroptosis, making it a promising therapeutic target. The ferroptotic process is initiated by intracellular iron accumulation, free radical generation via the Fenton reaction[25], and disruption of antioxidant defenses, culminating in lipid peroxidation.
Dixon et al[15] conducted pivotal research demonstrating that the small molecules Erastin and RSL3, known for their selective lethality against RAS-driven cancers, induce iron-dependent cell death through a mechanism termed ferroptosis. Their findings revealed that the accumulation of ROS plays a critical role in this process, with intracellular iron acting as a central contributor.
In their experiments using HT-1080 cells, treatment with 10 μmol/L erastin led to a significant increase in both cytosolic and lipid ROS. This was followed by cell detachment and a marked increase in cell death. To further explore the role of iron, the researchers treated cells with the iron chelator deferoxamine (100 μmol/L), which resulted in a substantial suppression of ROS accumulation and reduced cell death, underscoring the essential role of intracellular iron in ferroptosis. Interestingly, the addition of exogenous iron further intensified erastin’s cytotoxic effects, reinforcing the idea that elevated iron levels potentiate ferroptosis-mediated cell death. Overall, these findings highlight the intricate connection between iron metabolism and regulated cell death pathways, positioning ferroptosis as a promising therapeutic target in RAS-driven tumors. This research paves the way for strategies that exploit iron dependency to enhance cancer treatment outcomes[15]. These results confirm the iron dependency of ferroptosis-mediated cell death via ROS accumulation.
It is also noteworthy that the buildup of ROS is significantly driven by lipid peroxides, which play a crucial role in inducing cell death by damaging lipid membranes rich in polyunsaturated fatty acids (PUFAs). The incorporation of PUFAs into cellular membranes is facilitated by the endoplasmic reticulum, emphasizing the role of intracellular structures in this mechanism. Lipid peroxidation, a central component of ferroptosis, occurs in two main steps. Initially, free ferrous iron reacts with hydrogen peroxide (H2O2) via the Fenton reaction to produce highly reactive hydroxyl radicals. These radicals then initiate a cascade of oxidative reactions, beginning with the abstraction of hydrogen atoms from the bis-allylic position of PUFAs. This is followed by the addition of oxygen to the PUFA molecules, leading to extensive lipid peroxidation, loss of membrane integrity, and ultimately cell death[26,27].
This intricate interplay among iron, ROS, and lipid peroxidation emphasizes the therapeutic potential of targeting these pathways in efforts to mitigate oxidative stress and combat treatment-resistant cancers.
Yan et al[23] demonstrated that glutathione peroxidase 4 (GPX4) plays a crucial role in regulating ferroptosis. GPX4 catalyzes the reduction of lipid peroxides by converting phospholipid hydroperoxides into non-toxic phospholipid alcohols. For GPX4 to function effectively, intracellular GSH is essential. GSH is synthesized within cells through the uptake of cysteine in exchange for glutamate via the system xCT antiporter. Consequently, inactivation of GPX4 leads to the accumulation of lipid peroxides and triggers ferroptosis-mediated cell death. This highlights the central role of the GPX4 pathway, along with lipid peroxide metabolism and iron regulation, in ferroptosis-driven cell death, particularly in cancers where resistance to traditional therapies is observed[23,28].
In the context of breast cancer, the sensitivity to gefitinib (tyrosine kinase inhibitor) is enhanced when GPX4 is inhibited, thereby promoting ferroptosis. Additionally, silencing GPX4 results in elevated malondialdehyde and ROS production, further supporting the role of GPX4 in regulating and promoting ferroptosis-mediated cancer cell death[29]. Other targets, such as divalent metal transporter 1 (DMT1), have also been explored in triple-negative breast cancer (TNBC). Inhibiting DMT1 leads to increased mitochondrial ROS levels and GSH elevation, which disrupts the function of DMT1 and promotes ferroptosis-induced cell death[29].
In prostate cancer, particularly retinoblastoma transcriptional corepressor 1 (RB1)-deficient types known for their aggressive nature and resistance to conventional therapies, ferroptosis has emerged as a promising therapeutic approach. Loss of RB1 activates E2F transcription factors, which in turn upregulate Acyl-CoA synthetase long chain family member 4. Recent studies have shown that inducing ferroptosis in RB1-deficient prostate cancer cells can significantly suppress tumor growth and metastasis. For example, the use of GPX4 inhibitors, such as JKE-1674, has demonstrated potent antitumor effects in preclinical models[29,30]. These findings suggest that targeting ferroptosis could serve as a viable strategy for treating aggressive, therapy-resistant prostate cancers.
Although ferroptosis-based cancer therapies are still in their early stages, emerging research focused on modulating the tumor microenvironment and leveraging various molecular targets and pathways to induce ferroptosis may accelerate its translation into clinical applications.
ROLE OF AUTOPHAGY IN CANCER
The term “autophagy” was coined by Christian de Duve in 1963[31]. Macroautophagy—now commonly referred to simply as autophagy—is a self-degradative process often described as the cellular mechanism for clearing unwanted components or "garbage" inside the cell. Three primary types of autophagy are widely recognized: Macroautophagy, microautophagy, and chaperone-mediated autophagy, with macroautophagy being the most extensively studied and prevalent form[32].
Among these, macroautophagy has attracted considerable research interest, particularly for its potential therapeutic applications in cancer. Autophagy is a degradative and catabolic pathway in which damaged or aged cellular components and organelles are sequestered into double-membrane vesicles called autophagosomes. These autophagosomes then fuse with lysosomes, where the contents are broken down by hydrolytic enzymes[33,34]. This process not only clears cellular debris but also provides energy and molecular building blocks to maintain cellular homeostasis.
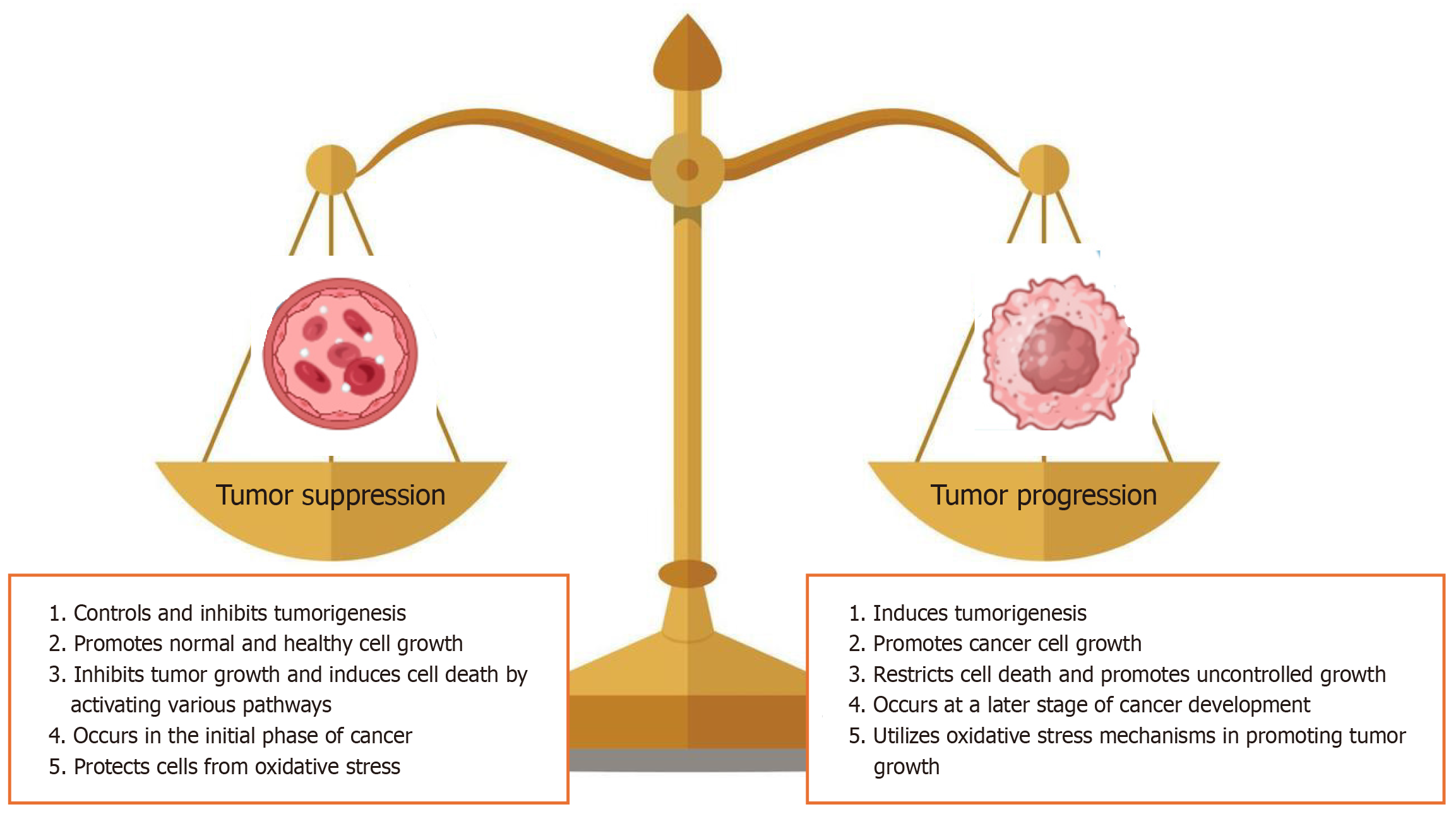
Autophagy plays a critical role in protecting normal cells and preventing tumorigenesis by eliminating dysfunctional proteins and organelles. However, its role in cancer is context-dependent. While autophagy generally maintains cell homeostasis, its inhibition can lead to the accumulation of ROS and genomic instability, thereby promoting tumor formation. Conversely, when oxidative stress stimulates autophagy, the energy and nutrients generated may support the survival of cancer cells. Figure 2 illustrates the key factors underlying the dual role of autophagy in cancer biology[35-37]. The energy produced through autophagy is essential for cell survival under stress conditions[31].
Figure 2 Dual role of autophagy in tumorigenesis.
At an early stage of cancer, autophagy controls the tumor progression and causes cell death. By contrast, autophagy promotes tumor growth under stress conditions where reactive oxygen species and oxidative stress increase.
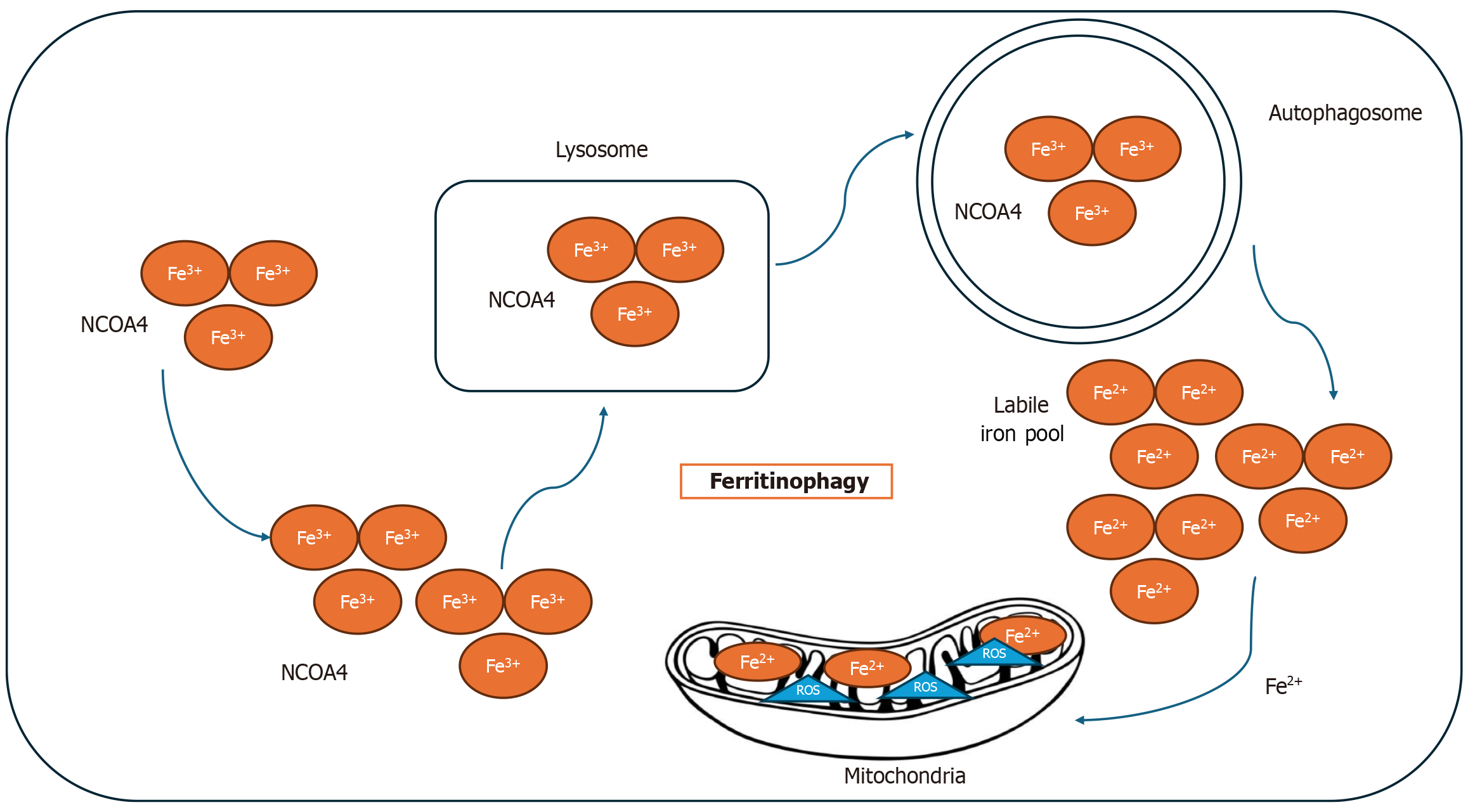
Autophagy is also implicated in cellular development and the regulation of aging. A specialized form of autophagy, known as ferritinophagy, is closely linked to ferroptosis. In ferritinophagy, the nuclear receptor coactivator 4 binds to ferritin and facilitates its degradation within autophagosomes, thereby releasing free iron and contributing to ferroptosis[17]. Figure 3 represents the connection between autophagy and ferroptosis.
Figure 3 Mechanism of ferritinophagy.
Ferritin is transported to the autophagolysosome with the help of nuclear receptor coactivator 4 (NCOA4), leading to the degradation of ferritin and the release of free iron. The increase of free iron intracellularly leads to the production of reactive oxygen species in labile iron pool. As a result, the cells become sensitive to ferroptosis. Fe2+: Ferrous iron; Fe3+: Ferric iron.
The interplay between autophagy and ferroptosis represents a significant area of interest in cancer research. Autophagy can regulate ferroptosis by degrading ferritin, thereby increasing intracellular free iron levels and promoting lipid peroxidation. Depending on the cellular context, this relationship may either inhibit tumor growth or contribute to therapy resistance. A deeper understanding of the autophagy-ferroptosis axis is crucial for designing targeted cancer therapies that leverage these interconnected pathways[38,39].
The pathways known to regulate autophagy during tumor progression include the beclin 1 interactome, the RAS/RAF/MAPK pathway, the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (mTOR) pathway, and tumor protein p53 signaling[33]. The loss of beclin 1 has been associated with a reversal of autophagy's protective role, leading instead to tumorigenesis. Mutations in beclin 1 and the consequent loss of autophagy’s survival functions are reported in approximately 95% of cancers. The BECN1 gene is frequently mutated in several cancer types—including colorectal, gastric, breast, and prostate cancers—likely due to its genomic proximity to the breast cancer susceptibility gene breast cancer gene 1[40].
The process of autophagy involves several key steps: Initiation, nucleation, elongation, and maturation, all of which are regulated by a complex network of proteins. The mTOR, a serine/threonine protein kinase, plays a central role in cell proliferation, stress response, and cancer progression. mTOR forms two major complexes: mTOR complex 1 (mTORC1) and mTORC2. Notably, mTORC1 negatively regulates autophagy. When mTORC1 is activated, it phosphorylates and inhibits autophagy-related genes, suppressing autophagy. Conversely, under stress conditions, inhibition of mTORC1 Leads to autophagy activation.
5′ AMP-activated protein kinase (AMPK) acts as an upstream regulator of mTORC1. Inhibition of mTORC1 and simultaneous activation of AMPK together promote autophagy. When mTORC1 is inhibited, the ULK complex becomes activated and dephosphorylated. This active ULK complex then modulates the class III PI3K complex, which comprises vacuolar protein sorting-associated protein 34 (Vps34), Vps15, autophagy-related 14 (Atg14), and beclin 1. Beclin 1, the mammalian ortholog of yeast Atg6/Vps30, is essential for autophagosome formation in conjunction with the class III PI3K complex (PIK3C3 or Vps34)[36]. This complex facilitates the formation of the phagophore assembly site.
Beclin 1 recruits several proteins involved in the elongation and maturation of the autophagosome. Elongation is driven by the autophagy-related proteins Atg5, Atg12, and ATg-like 1, which form a complex that recruits microtubule-associated protein 1 light chain 3B (LC3B). LC3B is critical for phagophore membrane expansion. Pro-LC3 is first cleaved by Atg4B to form the cytosolic isoform LC3-I. LC3-I is then conjugated with phosphatidylethanolamine via the actions of Atg3 and Atg7 to produce LC3-II, which integrates into both the inner and outer membranes of the autophagosome, allowing it to bind and sequester cargo for degradation.
Finally, maturation occurs as the autophagosome fuses with lysosomes, resulting in the degradation of engulfed organelles and proteins. This highly regulated process is essential for maintaining cellular homeostasis and plays a dual role in cancer, functioning as both a tumor suppressor and, under certain conditions, a mechanism supporting tumor survival[35-37].
ROLE OF MITOCHONDRIA IN FERROPTOSIS MEDIATED CELL DEATH IN CANCER
Mitochondrial dynamics—fission and fusion—play a vital role in maintaining organelle biogenesis and the overall mitochondrial population. In addition to these key functions, mitochondria paradoxically participate in a form of programmed cell death known as apoptosis[41-45].
Mitochondria-mediated apoptosis involves two primary signaling pathways: The extrinsic and intrinsic pathways[43,44]. The extrinsic pathway is initiated outside the cell when ligands bind to cell surface receptors such as Fas, tumor necrosis factor (TNF), TNF-related apoptosis-inducing ligand, and death receptor 3. This binding activates caspases 8 and 10 through a death-inducing signaling complex, which subsequently signals caspases 3 and 7 in the intrinsic pathway, leading to the release of cytochrome c from the mitochondria.
Mitochondria play a critical role in the intrinsic pathway of apoptosis. This pathway is triggered by various forms of cellular stress, such as DNA damage, hypoxia, and the deprivation of survival factors. Cytochrome c, an essential component of the electron transport chain, combines with apoptotic peptidase-activating factor 1 to form a complex known as the apoptosome. The apoptosome then activates caspase 9, initiating a cascade of events that ultimately leads to cell death[46,47].
The regulation of caspase activation and apoptosis by mitochondria is termed mitochondrial outer membrane permeabilization (MOMP). When MOMP occurs, the cell is committed to death. MOMP is driven by the B-cell lymphoma 2 (Bcl-2) family of proteins, such as Bcl-2-associated X protein (BAX) and Bcl-2 homologous antagonist/killer (BAK), which act as pro-apoptotic effectors[27]. BAX and BAK shuttle between the mitochondria and the cytoplasm. Small molecules that activate BAX have demonstrated potent antitumor activity. Upon activation, BAX and BAK form lipid pores in the outer mitochondrial membrane, causing the inner and outer membranes to fuse, resulting in membrane permeabilization.
Overall, the intrinsic and extrinsic pathways of apoptosis converge at the mitochondria, where critical steps such as cytochrome c release and caspase activation occur. The intrinsic pathway's regulation by mitochondrial proteins like BAX and BAK underscores the importance of mitochondrial integrity in cell death. Targeting these pathways and molecules holds potential for developing antitumor therapies that promote apoptosis in cancer cells.
Mitochondrial dynamics refers to the continuous changes in the shape, size, and distribution of mitochondria within cells, as well as the functional alterations that accompany these morphological changes. The processes of fission and fusion are crucial for maintaining mitochondrial integrity and function. Mitochondrial fission, primarily regulated by dynamin-related protein 1 (Drp1), involves the division of a single mitochondrion into two distinct organelles. By contrast, mitochondrial fusion is mediated by fusion proteins, including mitofusin 1 (MFN1) and MFN2 and optic atrophy 1 (OPA1), which enable the merging of mitochondria into elongated structures[48]. These dynamic processes play a significant role in cellular energy metabolism, apoptosis, and the overall health of cells.
In the context of breast cancer, particularly TNBC, mitochondrial dynamics are critically altered. TNBC is known for its aggressive behavior and resistance to standard chemotherapeutic agents such as tamoxifen, doxorubicin, and paclitaxel. Research has shown that in drug-resistant TNBC cells, mitochondria often exhibit damage and a fragmented network, indicating disrupted mitochondrial dynamics. This fragmentation is associated with impaired mitochondrial function, which can lead to increased oxidative stress and contribute to the development of drug resistance.
Recent advancements in biotechnology have introduced innovative therapeutic strategies aimed at overcoming drug resistance in TNBC. One promising approach is mitochondrial transplantation, which involves transferring healthy mitochondria into cancer cells exhibiting mitochondrial dysfunction. This technique has demonstrated potential antitumor activity by restoring the bioenergetics of the recipient cells and enhancing their sensitivity to chemotherapy.
Mitochondrial transplantation involves isolating healthy mitochondria from donor cells and delivering them intratumorally to cancerous tissues. This process has shown remarkable efficacy in reversing the drug resistance observed in TNBC. Upon transplantation, the healthy mitochondria integrate into the host cells, promoting the expression of fusion proteins like MFN1 and OPA1[49]. This increase in fusion proteins facilitates the reorganization of the mitochondrial network, leading to a more interconnected and functional mitochondrial system.
The restoration of mitochondrial dynamics through transplantation not only improves cellular metabolism but also enhances the apoptotic response to chemotherapy. By promoting mitochondrial fusion and restoring the normal function of mitochondria, cancer cells become more susceptible to the cytotoxic effects of chemotherapeutic agents. Studies have indicated that this strategy can significantly reduce tumor growth and size, leading to improved responses to breast cancer treatments.
Moreover, the positive impact of mitochondrial transplantation extends beyond merely reversing drug resistance. The healthy mitochondria can influence the tumor microenvironment by reducing oxidative stress and modulating apoptotic pathways. This dual action—improving mitochondrial function and altering the tumor's biochemical landscape—makes mitochondrial transplantation a compelling avenue for therapeutic intervention in TNBC.
In conclusion, mitochondrial dynamics, encompassing the processes of fission and fusion, play a pivotal role in the pathophysiology of breast cancer, particularly in the context of drug resistance seen in TNBC. The emerging biotechnology of mitochondrial transplantation offers a novel and promising strategy to combat this resistance. By restoring healthy mitochondrial function and enhancing the apoptotic response, this innovative therapy has the potential to improve treatment outcomes for patients with TNBC. Continued research in this area may not only deepen our understanding of mitochondrial biology in cancer but also pave the way for new therapeutic approaches that harness the power of mitochondria to combat one of the most challenging forms of cancer. This sounds like a promising target for treating TNBC through animal models and clinical use in humans, particularly in treating drug-resistant cancers[50-53].
INTEGRATING ROLES OF FERROPTOSIS, AUTOPHAGY, AND MITOCHONDRIAL FUNCTIONS
Ferroptosis is a non-apoptotic form of regulated cell death characterized by iron-dependent lipid peroxidation. Although its occurrence is independent of mitochondrial DNA or the presence of functional mitochondria, these organelles play a crucial role in regulating ferroptosis. Mitochondria are a major source of ROS, which are essential in driving ferroptosis. ROS generation in mitochondria primarily stems from oxidative phosphorylation, a process that increases oxidative stress and triggers lipid peroxidation, a key hallmark of ferroptosis. Moreover, mitochondria help regulate iron homeostasis through mitochondrial ferritin, which sequesters iron, preventing excess free iron from catalyzing the Fenton reaction. This reaction produces highly reactive hydroxyl radicals that drive lipid peroxidation and ferroptosis. Consequently, the accumulation of iron within mitochondria, particularly during apoptosis, intensifies ferroptotic activity via enhanced lipid peroxidation[46].
Recent research has highlighted the interplay between ferroptosis, autophagy, and mitochondrial function, particularly in cancer therapy. Autophagy, a process of cellular self-digestion, plays a dual role in both promoting and inhibiting cancer progression. In the context of breast cancer, inhibition of autophagy can enhance the efficacy of cancer therapies, particularly those that rely on inducing cell death. For instance, mitochondrial division inhibitor 1 (mdivi-1) is a selective inhibitor of the mitochondrial fission protein Drp1, which decreases mitophagy, the removal of damaged mitochondria. Studies have shown that inhibiting mitophagy with mdivi-1 enhances the effects of silibinin, a plant-based compound, in promoting apoptosis in breast cancer cells. This finding suggests that targeting mitochondrial dynamics can enhance autophagy-dependent cancer treatments, including those that leverage ferroptosis[34,35]. Similarly, autophagy inhibitors such as sunitinib and erlotinib, which target vacuolar protein sorting-associated protein 34 (Vps34), have demonstrated effectiveness in breast cancer treatment by interfering with cancer cell survival mechanisms. These inhibitors block the cancer cells' ability to recycle cellular components, enhancing the effects of cell death pathways, including ferroptosis[35].
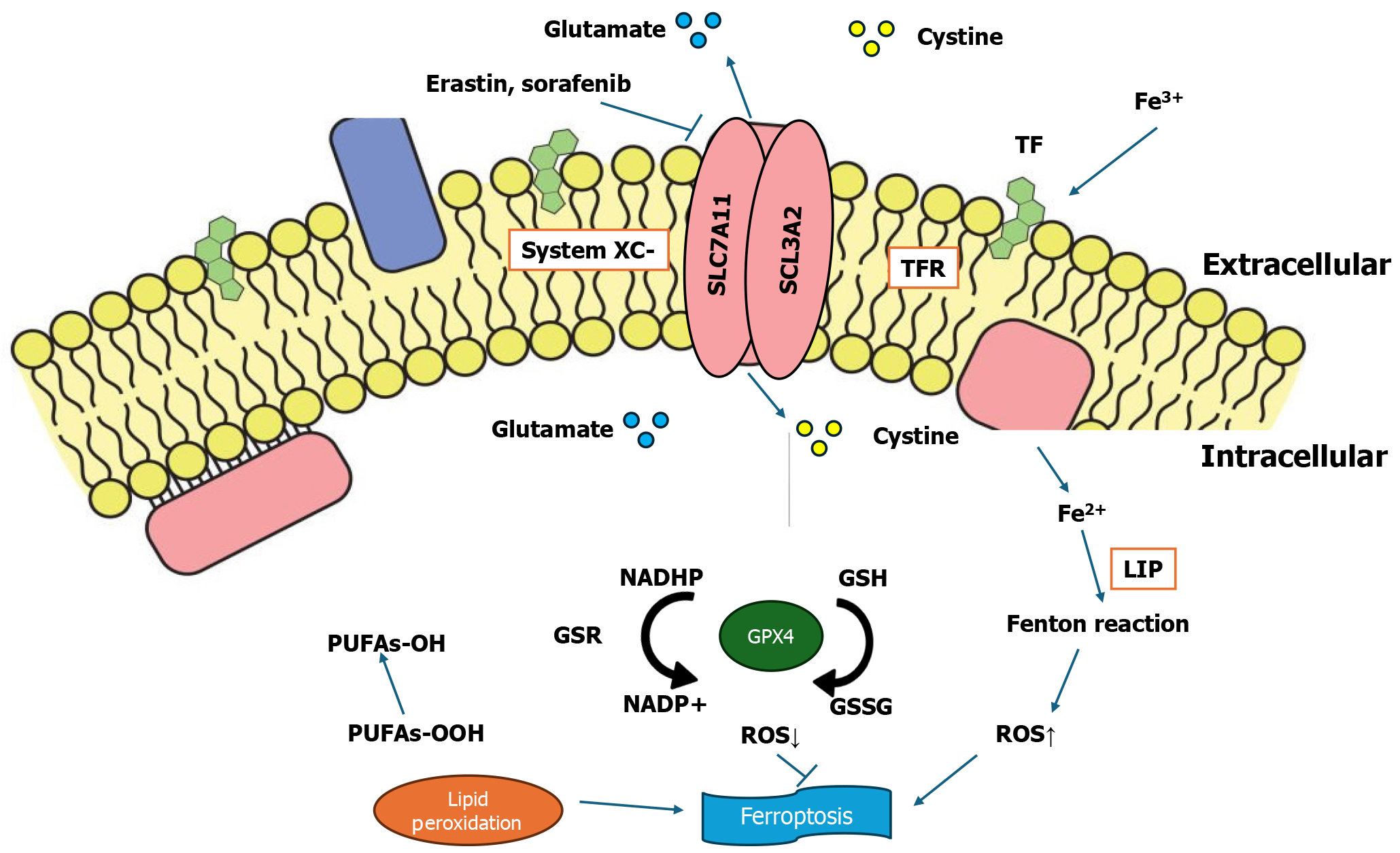
Chemotherapeutic agents like sorafenib and cisplatin are also known to induce ferroptosis, offering additional therapeutic options for cancer treatment. Sorafenib, a multi-kinase inhibitor used primarily in liver and kidney cancers, functions by inhibiting the SLC7A11/GPX4 axis. This inhibition reduces the cancer cells' ability to neutralize lipid peroxides, driving them toward ferroptosis. The pro-ferroptotic activity of sorafenib enhances its anticancer efficacy, making it a promising candidate for combination therapies. Cisplatin, a widely used chemotherapy agent, induces ferroptosis by depleting GSH and inactivating GPX4, a key enzyme in preventing lipid peroxidation. By pushing cancer cells toward both ferroptosis and apoptosis, cisplatin can target drug-resistant cancer cells, especially in aggressive cancers like TNBC[54]. Figure 4 explains the ferroptosis pathway and its effect in the presence of drugs that regulate ferroptosis.
Figure 4 Ferroptosis regulatory pathway, along with the effects of drugs on ferroptosis.
System Xc- is a heterodimer of a light chain and heavy chain subunit, solute carrier family 7 member 11 (SLC7A11) and SLC3A2, respectively. These function in importing cysteine and exporting glutamate. The accumulation of a labile iron pool, reactive oxygen species, and lipid peroxidation plays a crucial role in causing ferroptosis. Glutathione phosphate (GPX4) is responsible for lipid peroxidation. Drugs such as erastin and sorafenib may inhibit the role of System Xc-. RAS-selective lethal 3 inhibits the activity of GPX4.
Other compounds, such as siramesine and lapatinib, have also shown potential in promoting ferroptosis by increasing iron levels and ROS generation, thus triggering cancer cell death. Siramesine, an experimental sigma-2 receptor agonist, and lapatinib, a tyrosine kinase inhibitor used in HER2-positive breast cancer, both elevate intracellular ROS levels, pushing cells into ferroptosis. This mechanism highlights the therapeutic potential of drugs that elevate oxidative stress within cancer cells while sparing normal cells, which can better manage oxidative damage[55-57].
Overall, the intersection of ferroptosis, autophagy, and mitochondrial function offers a novel landscape for cancer research, particularly in breast cancer. Although autophagy and mitochondrial cell death pathways have been extensively studied, ferroptosis has only recently gained attention as a unique form of regulated cell death, opening new avenues for therapeutic strategies. The intricate interplay between these processes highlights how targeting multiple pathways simultaneously can enhance cancer treatments by overcoming drug resistance and selectively inducing cancer cell death. As research into these interconnected mechanisms continues to grow, it may provide innovative therapeutic approaches that improve outcomes for breast cancer patients, especially those with aggressive forms like TNBC. By understanding and manipulating the synergy between ferroptosis, autophagy, and mitochondrial dynamics, researchers can develop more effective, targeted treatments that reduce cancer progression and improve patient prognosis[58].
CONCLUSION
In conclusion, the treatment of resistant cancers remains a significant challenge due to their high heterogeneity and poor prognosis, despite advancements in various treatment modalities like chemotherapy, immunotherapy, and targeted therapies. Ferroptosis, a novel form of regulated cell death, offers a promising therapeutic avenue in cancer treatment by targeting iron-dependent lipid peroxidation in cancer cells. Additionally, understanding the complex interplay between ferroptosis, autophagy, and mitochondrial cell death can provide insights into overcoming drug resistance and improving the effectiveness of conventional treatments. Although still in its early stages, breakthroughs in research on ferroptosis and related cellular mechanisms may lead to novel therapies that can better target cancer cells while sparing normal cells. The continued exploration of these pathways and mechanisms could potentially revolutionize breast cancer treatment, particularly for challenging subtypes like TNBC.