Published online May 24, 2025. doi: 10.5306/wjco.v16.i5.104154
Revised: March 26, 2025
Accepted: April 17, 2025
Published online: May 24, 2025
Processing time: 158 Days and 10.9 Hours
Hepatocellular carcinoma (HCC) is at the forefront of the global spectrum of cancer incidence and mortality, with conventional therapies like tyrosine kinase inhibitors limited by resistance. Recent studies have highlighted the promising anticancer effects of nitidine chloride (NC) against HCC. SAC3 domain containing 1 (SAC3D1) is critical for centrosome replication and spindle formation. However, research on SAC3D1 in HCC and NC remains limited.
To investigate the mechanisms underlying SAC3D1’s role in HCC progression and evaluated its potential as a therapeutic target of NC.
RNA sequencing (RNA-seq) identified SAC3D1 expression changes in HCC cells after NC treatment. Molecular docking was further employed to validate the direct binding between NC and SAC3D1. Additionally, HCC multicenter data (The Cancer Genome Atlas_GTEx, ArrayExpress), pathway analysis, Pearson correlation analysis and SAC3D1 in vitro knockdown experiments were integrated to explore the molecular mechanisms underlying SAC3D1's involvement in HCC progression.
RNA-seq showed that NC treatment significantly downregulated SAC3D1 expression [log2(fold change) = - 0.95, P < 0.05], with molecular docking revealing that NC directly bound to SAC3D1 proteins (binding energy: -9.7 kcal/mol). Enrichment analysis showed that most pathways were closely related to the cell cycle. Pearson correlation analysis indicated that SAC3D1 and cell cycle genes were significantly positively correlated(correlation coefficient ≥ 0.3, P < 0.05). SAC3D1 knockdown inhibited HCC cell invasion, migration, and proliferation by arresting cells in the S and G2/M phases. Flow cytometry confirmed that after SAC3D1 knockdown, the early and total apoptosis percentage of HCC cells decreased, while the late apoptosis percentage increased.
As a potential target of NC, SAC3D1 may inhibit HCC progression through cell cycle regulation following its downregulation by NC.
Core Tip: The role of SAC3 domain containing 1 (SAC3D1) in hepatocellular carcinoma (HCC) remains unclear. Through bioinformatic analysis, molecular docking techniques, and in vitro experiments, this study demonstrates for the first time that SAC3D1 serves as a novel target for nitidine chloride (NC), which previous studies have shown to have anti-HCC effects. Downregulation of SAC3D1 by NC may inhibit HCC progression by regulating the cell cycle, providing opportunities for novel therapeutic approaches in HCC treatment.
- Citation: Huang QL, Zhou SS, Li JD, Xiong DD, He RQ, Huang ZG, Wang L, Tan TM, Dang YW, Mo WJ, Feng ZB, Chen G, Yang ZD. Role of cell cycle-related gene SAC3 domain containing 1 as a potential target of nitidine chloride in hepatocellular carcinoma progression. World J Clin Oncol 2025; 16(5): 104154
- URL: https://www.wjgnet.com/2218-4333/full/v16/i5/104154.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i5.104154
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related deaths globally[1]. Approximately 70%–80% of HCC cases present at advanced stages, where targeted therapy, chemotherapy and immunotherapy become the main clinical treatments[2,3]. However, rapid drug resistance, unsatisfactory clinical remission rates and substantial side effects restrict these clinical efficacy, so the five-year survival percentage of HCC is still less than 20%[4,5]. Therefore, it is urgent to find a longer-term treatment plan and new therapeutic targets for HCC cases.
Traditional Chinese medicine (TCM) can improve HCC patients’ immunity and quality of life by mitigating adverse reactions to radiotherapy and chemotherapy through multi-target and multi-pathway mechanisms, thereby compensating for the limitations of Western medicine[6-8]. Nitidine chloride (NC) is an alkaloid extracted from the dried roots of Zanthoxylum nitidum, a characteristic herbal TCM remedy from Guangxi Zhuang Autonomous Region. We previously confirmed that the growth of HCC transplanted tumors can be significantly inhibited by NC[9]. Previous studies have also focused on the regulation of HCC by NC through circRNA-mediated networks. Building upon these findings, the current research delved deeper into the evidence at the mRNA level, aiming to construct a comprehensive, multi-layered regulatory mechanism.
SAC3 domain containing 1 (SAC3D1) is widely distributed in centrosome replication and spindle formation, which are critical components of the cell cycle process. Abnormalities in these structures can lead to cell cycle dysregulation and tumorigenesis[10-12]. However, the contribution of SAC3D1 to HCC remains inadequately investigated. RNA sequencing (RNA-seq) analysis of HCC cells treated with NC has revealed a significant downregulation of SAC3D1, suggesting that SAC3D1 may be a potential target of NC.
Still, RNA-seq alone has limitations in directly identifying drug targets. To address these limitations, molecular docking offers a complementary approach by simulating interactions between small molecules and proteins[13], allowing the validation of NC’s direct binding to the protein product encoded by SAC3D1.
Although SAC3D1 has not been reported in the field of TCM, the HERB database reveals intriguing clues that suggest its potential relevance[14]. Notably, SAC3D1 is closely associated with colchicine, a bioactive component of many TCM components. Multiple studies have confirmed that colchicine inhibits HCC growth cells by triggering cell cycle arrest and apoptosis[15,16], implying that the downregulation of SAC3D1 by NC may also inhibit HCC progression by regulating cell cycle processes. Therefore, this study subsequently integrated enrichment analysis, SAC3D1 correlation analysis with cell cycle-related genes, and in vitro knockdown experiments to validate the above hypothesis.
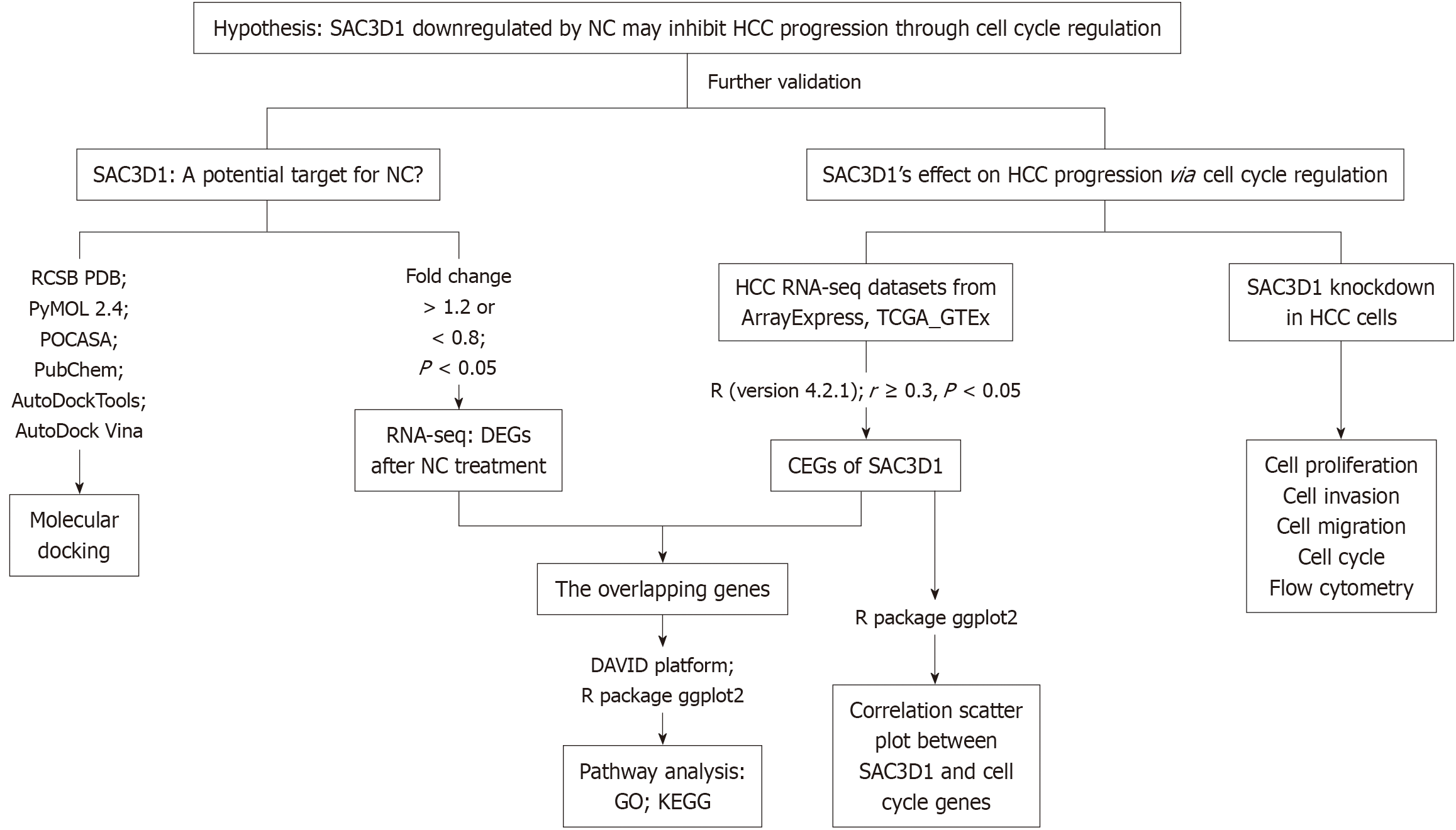
In summary, this study integrated RNA-seq data of HCC cells after NC treatment, molecular docking, HCC multicenter data, and SAC3D1 in vitro knockdown experiments to explore the role of SAC3D1 in HCC progression and its significance for the clinical treatment of HCC (Figure 1).
RNA-seq was conducted on HCC cells treated with NC, as described in our previous study, which demonstrated the inhibitory effects of NC on HCC. A detailed description of the experimental methods can be found in our prior publication[9]. The resulting RNA-seq data were analyzed in the current study to identify key target genes involved in the response to NC. Genes with significant differential expression after NC treatment were identified using R software (version 4.2.1) with dplyr, library, ggrepel and patchwork packages. Genes with a fold change (FC) > 1.2 or FC < 0.8 and adjusted P value < 0.05 were regarded as statistically.
To directly verify whether SAC3D1 is the drug target of NC, SAC3D1 was molecularly docked with NC in this study[17]. The crystal structure of SAC3D1 [Protein Data Bank (PDB) ID: AF-A6NKF1-F1] was retrieved from the RCSB PDB database. Solvent molecules and eutectic ligands were removed using PyMOL (version 2.4). The active site of SAC3D1 was predicted using POCASA (version 1.1). The drug structure of NC was downloaded from the PubChem database. The SAC3D1 protein and NC structure were processed using AutoDockTools, and molecular docking was performed using AutoDock Vina (version 1.5.7). The optimal conformational binding energy in the docking results was inversely proportional to the stability of the interaction between NC and the active site of SAC3D1. The molecular docking models were visualized for further analysis. Docking results were assessed based on the binding energy of the optimal conformation, with lower energy indicating greater stability between NC and the active site of SAC3D1.
HCC tissue samples were obtained from The Cancer Genome Atlas (TCGA) and ArrayExpress databases. To identify HCC-related mRNA expression datasets, the following search terms were used: (1) Hepatocellular carcinoma; (2) HCC; (3) LIHC; and (4) Liver hepatocellular carcinoma. To expand the sample size of normal liver tissues, RNA-seq dataset from the GTEx and TCGA platforms were integrated. Batch effects were removed using the SVA and limma R packages. The inclusion criteria for dataset selection were: (1) The species was Homo sapiens; (2) Primary liver cancer tissue; (3) The dataset contained at least three HCC and three normal liver tissue samples; and (4) The dataset contained SAC3D1 mRNA expression data. The exclusion criteria were: (1) The study subjects were animals or plants; (2) Non-primary HCC or Recurrent HCC; (3) HCC cell lines; (4) HCC animal models; (5) Repeated samples and datasets; and (6) The dataset did not include SAC3D1 mRNA expression data. Using RNA-seq datasets from ArrayExpress, GTEx and TCGA, SAC3D1 commonly expressed genes (CEGs) were identified based on Pearson correlation analysis (correlation coefficient ≥ 0.3, P < 0.05). Statistical analyses and data visualization were carried out with the ggplot2 R package (version 4.2.1). To investigate the specific pathways through which NC exerts its effects on HCC via SAC3D1, an intersection analysis was conducted between SAC3D1 CEGs and differentially expressed genes (DEGs) downregulated by NC. The intersection genes set was uploaded to the DAVID platform (https: //davidbioinformatics.nih.gov/) for functional annotation. Data visualization was performed using the ggplot2 R package (version 4.2.1).
Using the ggplot2 R package (version 4.2.1) and the datasets identified from the TCGA, ArrayExpress and GTEx, this study calculated correlations between SAC3D1 and classical cell cycle-related genes reported in previous literature, including cyclins (CCN) (CCNB1, CCNA2, CCNE1) and cyclin-dependent kinases (CDK) (CDK1, CDK4)[18].
SMMC7721 cells were grown in Dulbecco's Modified Eagle's Medium (DMEM) with 100 mL/L fetal bovine serum (FBS) (Gibco) at 37 °C in a 50 mL/L CO2 atmosphere.
To study the functional role of the SAC3D1, a knockdown plasmid pSpCas9(BB)-2A-Puro (PX459) V2.0 (ADDgene; 62988) was constructed using the PX459 backbone. The construction process followed an optimized protocol (https://media.addgene.org/data/plasmids/62/62988/62988-attachment_i-jdFt6Gm-ft.pdf) to minimize off-target effects. To enhance gene knockout efficiency and ensure targeting specificity, two plasmids were generated, each carrying a specific guide RNA (gRNA) targeting distinct critical regions of SAC3D1. The selected gRNA sequences were as follows: (1) SAC3D1_gRNA_E1_U (5’-CACCGAACCCGGCACCACCTCCAAG-3’); (2) SAC3D1_gRNA_E1_D (5’-AAACCTTGGAGGTGGTGCCGGGTT-3’); (3) SAC3D1_gRNA_E2_U (5’-CACCGTCGAGGACGTACCCTGGAGG-3’); and (4) SAC3D1_gRNA_E2_D (5’-AAACCCTCCAGGGTACGTCCTCGA-3’). SAC3D1_gRNA_E1_U stands for the upstream of gRNA on exon 1 of SAC3D1. D stands for the downstream, and E2 stands for exon 2. Each gRNA was designed to specifically target the designated regions of exon 1 and exon 2 of SAC3D1.
HCC cells were plated at a concentration of 5 × 105 cells per well in a 6-well plate. After 24 hours, transfection was carried out with Lipofectamine 3000 (Thermo Fisher Scientific) as per the provided instructions. Specifically, 1 μg of each plasmid was mixed with 4 μL of P3000 reagent in 100 μL of Opti-MEM (Gilbco) without serum and vortexed lightly. In a separate tube, 100 μL of Opti-MEM was combined with 6 μL of Lipofectamine 3000 and left for 5 minutes at room temperature. Following gentle mixing, the two solutions were combined and added dropwise to the cells. The cells were maintained for 24 hours after transfection, followed by a 48-hour puromycin selection in complete medium with 2 μg/mL puromycin. Subsequently, the medium was switched to puromycin-free complete medium, and cells were further cultured.
The efficiency of gene knockout was assessed using real-time reverse transcriptase-polymerase chain reaction (RT-qPCR). The Beta-actin gene was treated as a reference gene. The mRNA sequence between the two gRNAs binding sites was used for designing primers targeting SAC3D1. The sequences of the primers were outlined below: (1) Forward primer (5’-TTCGCTCGTGCCTTTAGCA-3’); and (2) Reverse primer (5’-GTGCTTCCCTGAGTCCATCC-3’). These primers were designed to detect mRNA expression levels, providing a quantitative measure of the knockout effect.
A total of 2000 SAC3D1 knockdown cells per well were cultured in a 96-well plate. After 24 hours, cell proliferation was quantified by the Cell Counting Kit-8 assay, and the cell growth curve was generated.
Cell migration was evaluated using a 24-well transwell chamber assay. In the upper chamber, 100 μL of cell-containing medium (5 × 104 cells) was loaded, while 500 μL of DMEM with 100 mL/L FBS was placed in the lower chamber. Following a 24-hour incubation at 37 °C, non-migratory cells were washed off the upper membrane surface. The membrane underwent two phosphate-buffered saline (PBS) washes, followed by a 30-minute fixation in methanol. After air-drying, the cells on the lower surface were exposed to 1 mL/L crystal violet for 30 minutes. Excess dye was removed by washing with PBS. The stained membranes were allowed to dry, and migrated cells were observed under a microscope.
Cell invasion was assessed using a 24-well transwell chamber assay. Matrigel diluted at 1:8 in serum-free DMEM (60 μL) was transferred to the upper chamber and maintained under 37 °C conditions for 1 hour to allow gel formation. The chamber was washed twice with DMEM after liquid aspiration. Subsequently, 100 μL of serum-free DMEM containing 5 × 104 cells was added to the upper chamber, and 500 μL of DMEM with 200 mL/L FBS was applied to the lower chamber. After 24 hours at 37 °C, the non-invaded cells on the upper membrane were cleared. subsequent procedure was performed as described for the migration assay.
Following centrifugation of 5 × 105 cells for 5 minutes at 1200 r/minute, the supernatant was removed, and the cell pellet was re-dissolved in 1 mL of precooled 750 mL/L ethanol. Following 2.5 hours of fixation at 4 °C, the cells underwent centrifugation for 5 min at 1200 r/minute, and the supernatant was carefully removed. The pellet was washed once with 1 mL of PBS, followed by centrifugation and removal of the supernatant. After incubation with propidium iodide (PI)/RNase staining solution in the dark at room temperature for 15 minutes, the cells were analyzed by flow cytometry within 30 minutes.
Apoptosis was assessed using flow cytometry. A total of 1 × 105 cells were harvested and centrifuged at 1200 r/minute for 5 minutes. After discarding the supernatant, the pellet was resuspended in 50 μL 1× Binding Buffer. The following staining protocols were applied depending on the experimental group: (1) Negative control: No dye added; (2) Single positive control 1: 5 μL of Annexin V-APC was added; (3) Single positive control 2: 10 μL of 7-aminoactinomycin D was added; and (4) Sample group: 5 μL of Annexin V-FITC and 10 μL of PI were added. The samples were gently mixed and incubated in the dark at 25 °C for 15 minutes. After incubation 200 μL of 1× Binding Buffer was added to each tube. Flow cytometry analysis was completed within 1 hour post-staining.
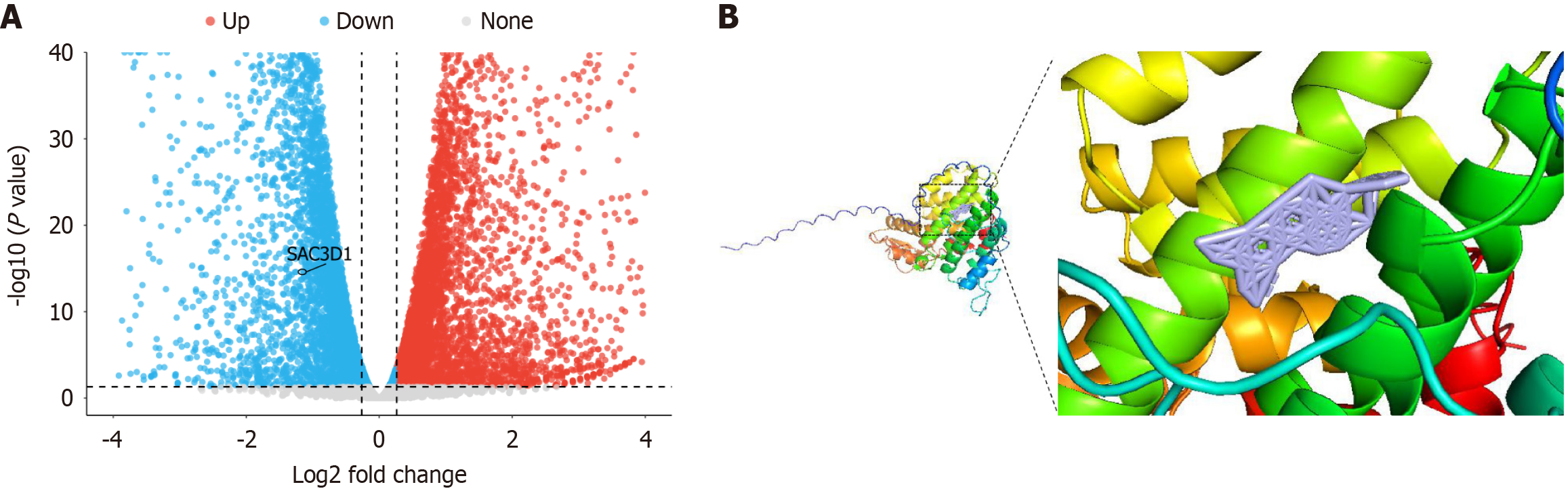
The DEGs after NC treatment were identified through RNA-seq, and it was found that SAC3D1 mRNA expression level was significantly decreased following NC treatment (Figure 2A, log2FC = - 0.95, P < 0.05). Molecular docking results indicated that the optimal binding energy between NC and SAC3D1 was -9.7 kcal/mol. Docking analysis showed no hydrogen bond formation between NC and SAC3D1, suggesting that they had strong binding activity, and that SAC3D1 may be a potential target of NC (Figure 2B).
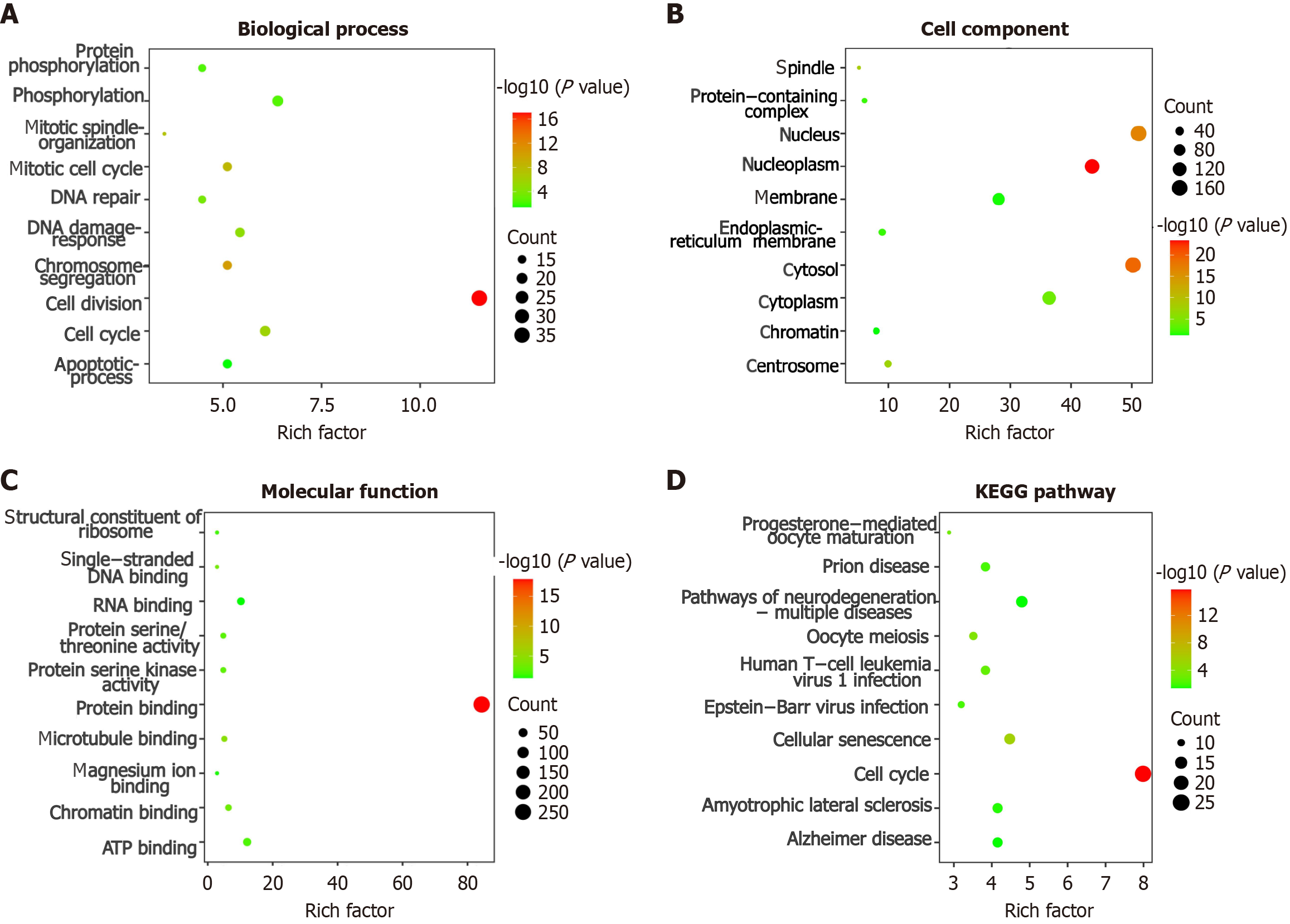
Based on the aforementioned screening criteria and data integration, three datasets E_MTAB_4171, E_MTAB_8887, and TCGA_GTEx_liver were ultimately selected. We screened 491 CEGs using the R package. Enrichment analysis of the intersecting genes between SAC3D1 CEGs and 11917 downregulated DEGs following NC interaction showed that the top three pathways of Biological Process (BP) are enriched in “cell division”, “cell cycle” and “phosphorylation” (Figure 3A). The top three pathways of cell components are enriched in “nucleoplasm”, “nucleus” and “cytosol”(Figure 3B). The top three molecular functions pathways are enriched in “protein binding”, “adenosine triphosphate binding” and “RNA binding” (Figure 3C). The top three pathways of Kyoto Encyclopedia of Genes and Genomes are enriched in “cell cycle”, “pathways of neurodegeneration-multiple diseases” and “cellular senescence” (Figure 3D).
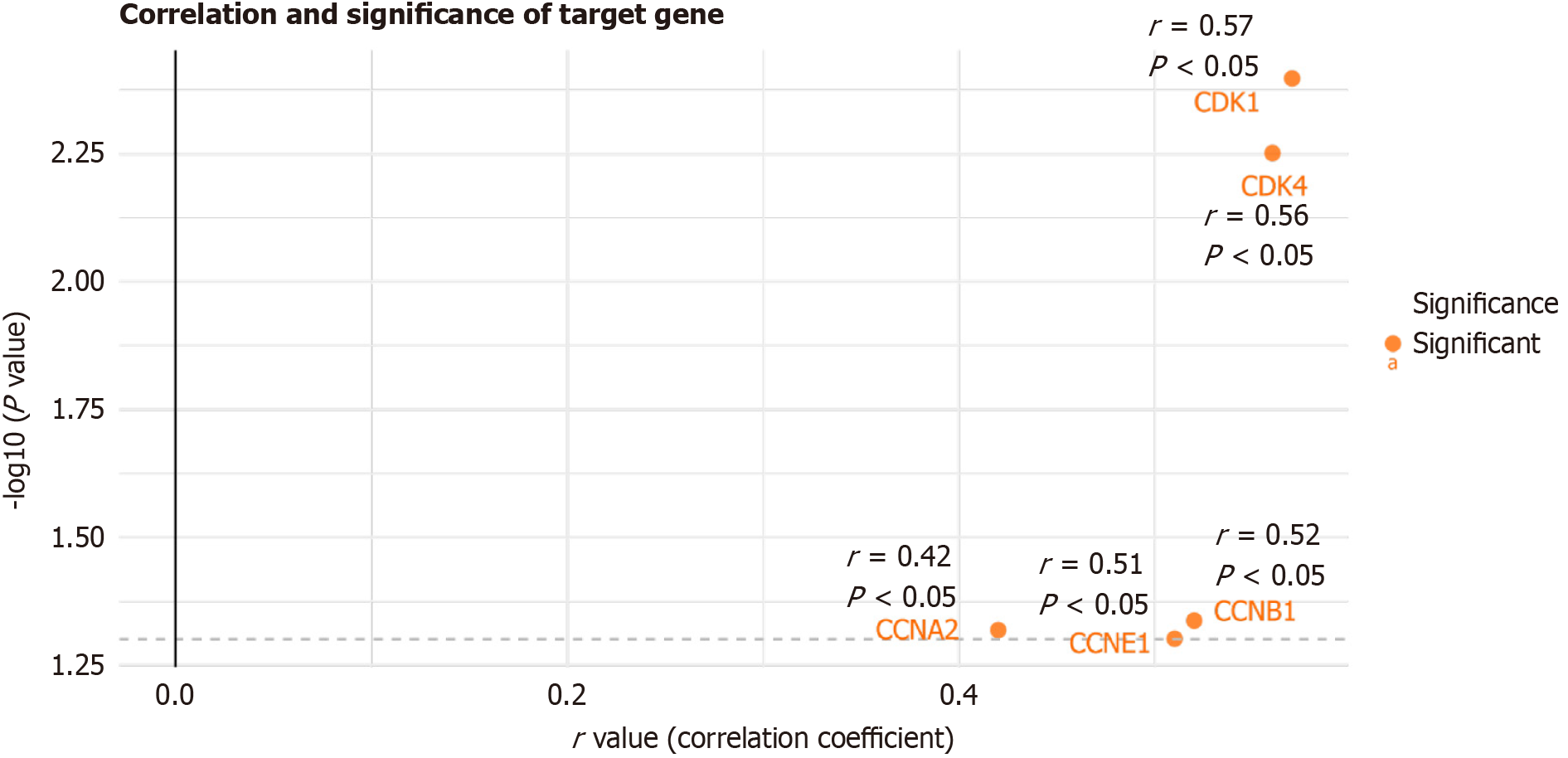
SAC3D1 showed a significant positive correlation with classical cell cycle-related genes (Figure 4, P < 0.05).
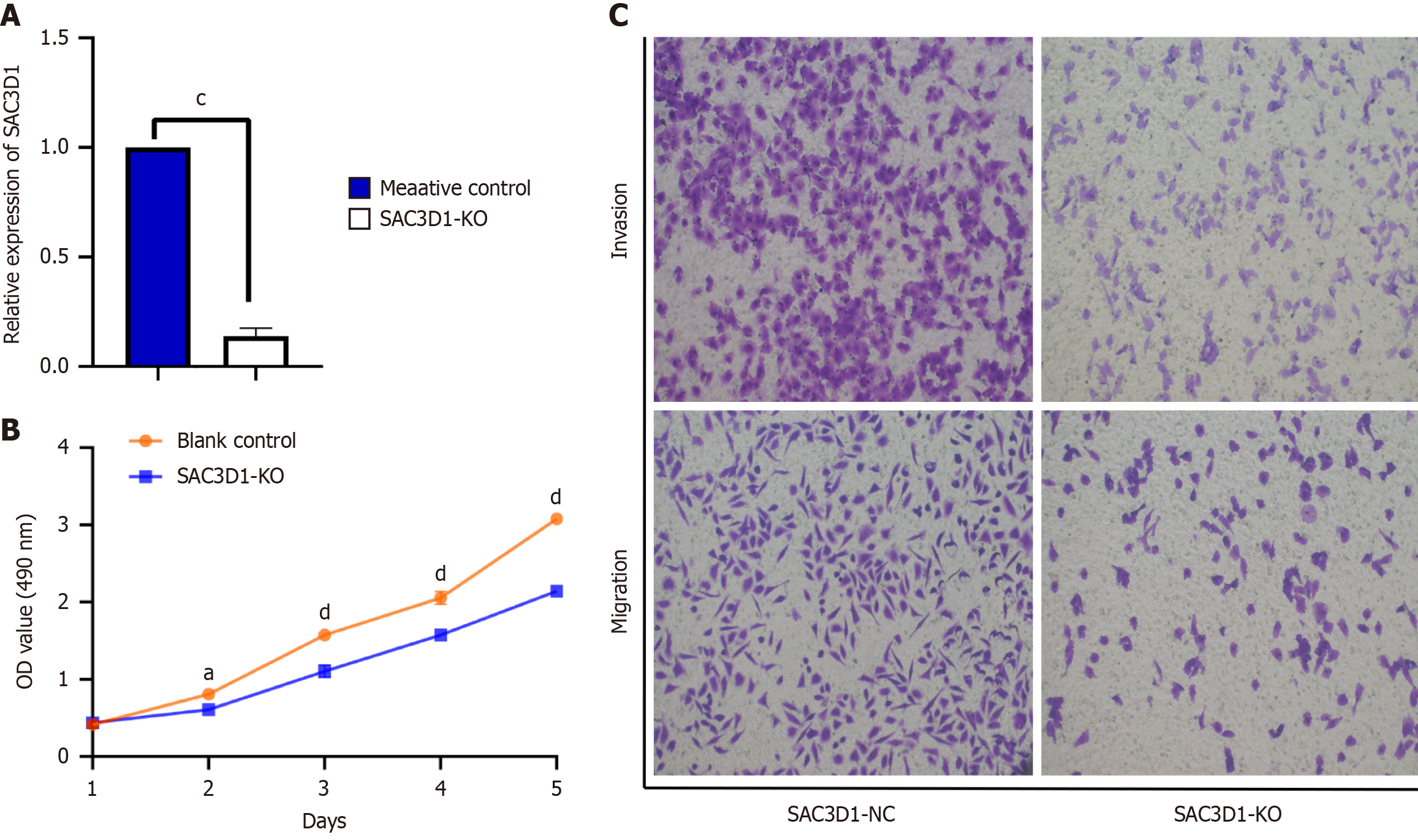
The results were as follows: (1) A SAC3D1 knockdown HCC cell model was established through lentivirus infection; and (2) RT-qPCR was used to detect knockdown efficiency. The experimental results showed that compared with the control group, the SAC3D1 mRNA expression level of HCC cells infected with SAC3D1 silencing plasmid lentivirus was significantly down-regulated (P < 0.05, Figure 5A), which indicated that the SAC3D1 knock-down cell model was successfully constructed and the subsequent functional experiments could be carried out. The specific functional experiment results are as follows.
Impact of knocking down SAC3D1 on the proliferation of HCC cells: Compared with the control group, the number of HCC cells decreased obviously after knocking down SAC3D1. The proliferative capacity of HCC cells progressively declined over time following SAC3D1 knockdown (Figure 5B, P < 0.05).
Impact of knocking down SAC3D1 on migration and invasion ability of HCC cells: Compared with the control group, the number of migrating HCC cells decreased following SAC3D1 knockdown (Figure 5C, P < 0.05). Moreover, the number of HCC cells that passed through the Matrigel significantly reduced after SAC3D1 knockdown, indicating a decrease in invasive ability (Figure 5C, P < 0.05). These results indicated that SAC3D1 knockdown may regulate tumor growth by inhibiting the migration and invasion ability of HCC cells.
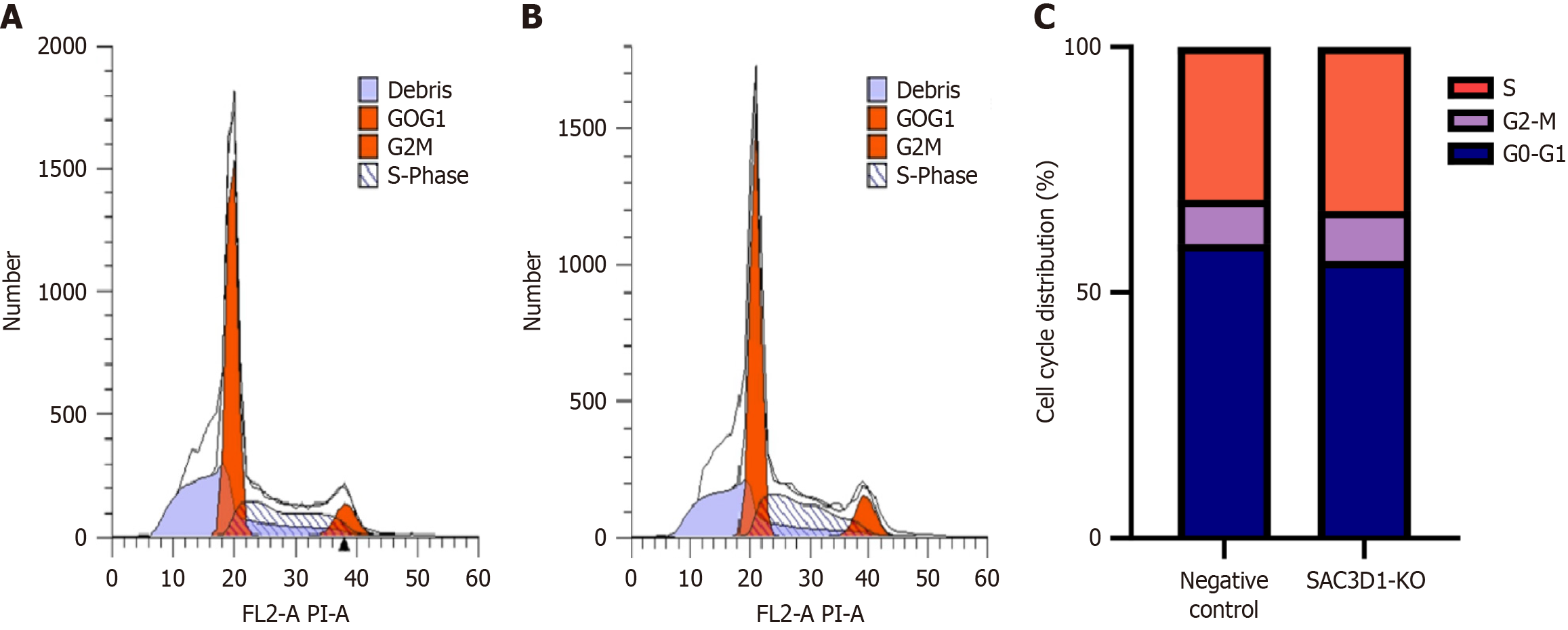
Impact of knocking down SAC3D1 on cell cycle of HCC cells: In addition, approximately 10.12% of SAC3D1 knockout cells were distributed in G2/M cycle, which was higher than 8.97% observed in the control group. About 33.39% of SAC3D1 knockout cells were distributed in S phase, which was higher than 31.16% in control group, indicating that SAC3D1 knockdown inhibited cell proliferation by arresting HCC cells in the S and G2/M phases (Figure 6, P < 0.05).
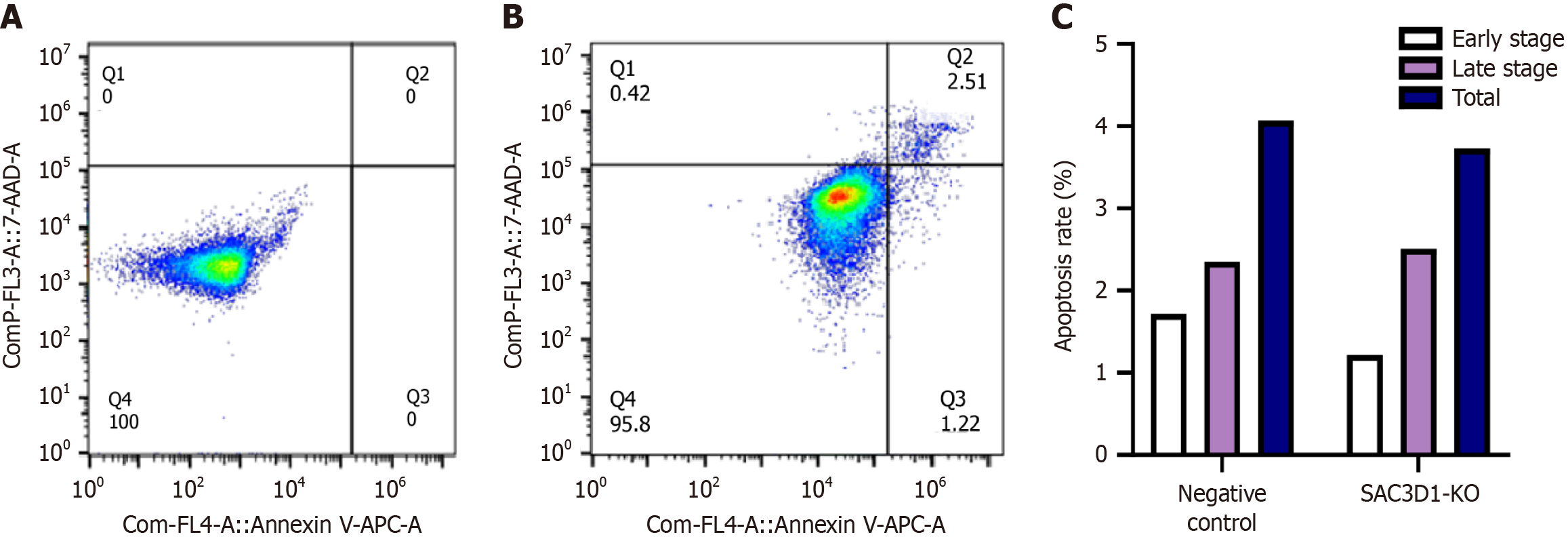
Impact of knocking down SAC3D1 on apoptosis of HCC cells: Flow cytometry was used to detect apoptosis in HCC cells. After SAC3D1 knockdown, the percentages of early and total apoptosis decreased, while the percentage of late apoptosis increased (Figure 7).
Systemic therapies, including tyrosine kinase inhibitors and immune checkpoint inhibitors, offer hope for patients with advanced HCC. However, challenges such as high resistance rates and therapeutic heterogeneity continue to limit the efficacy of traditional treatments, leaving the survival rate of advanced HCC patients still disappointingly low[19]. Notably, our previous studies have demonstrated that NC exhibits significant anticancer effects against HCC, providing a promising new therapeutic approach[9]. However, the potential molecular targets and underlying biological functions of NC remain to be further investigated.
Although limited studies have explored the role of SAC3D1 in promoting HCC, its underlying mechanisms remain poorly understood[4,20,21]. SAC3D1 is closely associated with spindle and centrosome functions, both of which play critical roles in cell cycle regulation. However, no studies to date have investigated whether SAC3D1 directly promotes HCC progression through cell cycle regulation, nor its potential connection to TCM treatment. This study is the first to explore the role of the SAC3D1 in cell cycle regulation in HCC and evaluate its potential contribution to NC intervention.
RNA-seq data showed that SAC3D1 was significantly down-regulated in HCC cells after NC treatment. While RNA-seq provided insights into SAC3D1 expression changes, it cannot establish a causal relationship between these changes and the direct action of NC. In contrast, molecular docking demonstrated a direct interaction between NC and SAC3D1 protein. The combination of these approaches provided complementary evidence, further strengthening the conclusion that SAC3D1 was a potential target gene for NC to exert its anti-HCC effect, and the downregulation of SAC3D1 expression may suppress HCC progression.
Enrichment analysis revealed that most pathways were closely related to cell cycle-related pathways. SAC3D1 showed a significant positive correlation with the expression of classical cell cycle genes, further suggesting its critical role in cell cycle regulation. These suggested that SAC3D1 downregulation may inhibit HCC progression by regulating the cell cycle.
To validate the above hypothesis, we conducted in vitro knockdown experiments in HCC cell lines, followed by assays on cell proliferation, invasion, migration, and apoptosis. The results demonstrated that SAC3D1 knockdown significantly suppressed the proliferation, migration, and invasion capabilities of HCC cells, while also promoting late-stage apoptosis. Late apoptosis represents an irreversible stage of cell death where tumor cells lose their proliferative capacity entirely. This increase in late apoptosis strongly suggests an effective suppression of tumor growth. Interestingly, early apoptosis was found to be higher in the control group, possibly indicating that HCC cells evade apoptosis by reversing early apoptotic processes, which enhances their survival and proliferative potential[22]. Notably, the involvement of the SAC3D1 in apoptosis regulation remains unexplored. Our findings represent a novel contribution to the understanding of SAC3D1’s role in HCC progression, particularly its potential as a modulator of apoptosis.
Cell cycle assays revealed that SAC3D1 knockdown induced G2/M and S phase arrest, suggesting that SAC3D1 contributes to cell cycle progression. Consistent with prior studies, both S phase and G2/M phase arrest have been implicated in suppressing HCC progression[16,23].
Given the close association of SAC3D1 with cell cycle-related BP, as supported by pathway and correlation analyses, and further confirmed by cell cycle experiments showing that SAC3D1 knockdown induces cell cycle arrest, this study hypothesizes that silencing SAC3D1 may inhibit HCC progression by regulating the cell cycle.
In conclusion, this study demonstrated that SAC3D1 was a potential target of NC. SAC3D1 knockdown significantly suppressed the proliferation, migration, and invasion of tumor cells, while inducing S phase and G2/M phase arrest and late apoptosis to inhibit tumor growth.
However, the current study has some limitations. Future work will incorporate additional experiments, including surface plasmon resonance and isothermal titration calorimetry, to further validate the interaction between SAC3D1 and NC. Additionally, RT-qPCR and Western blotting will be employed to assess the changes in cell cycle and apoptosis-related genes.
SAC3D1 promotes HCC progression. However, as a potential target of NC, its downregulation can affect cell cycle progression, thereby inhibiting HCC growth, which provides a new molecular and theoretical basis for the NC treatment of clinical HCC patients.
The authors would like to thank Guangxi Zhuang Autonomous Region Clinical Medicine Research Center for Molecular Pathology and Intelligent Pathology Precision Diagnosis and Guangxi key Laboratory of Enhanced Recovery after Surgery for Gastrointestinal Cancer for the technical support.
| 1. | Sankar K, Gong J, Osipov A, Miles SA, Kosari K, Nissen NN, Hendifar AE, Koltsova EK, Yang JD. Recent advances in the management of hepatocellular carcinoma. Clin Mol Hepatol. 2024;30:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 62] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 2. | Fan Y, Xue H, Zheng H. Systemic Therapy for Hepatocellular Carcinoma: Current Updates and Outlook. J Hepatocell Carcinoma. 2022;9:233-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Yang X, Yang C, Zhang S, Geng H, Zhu AX, Bernards R, Qin W, Fan J, Wang C, Gao Q. Precision treatment in advanced hepatocellular carcinoma. Cancer Cell. 2024;42:180-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 142] [Reference Citation Analysis (0)] |
| 4. | Wang H, Shi X. SAC3D1 activates Wnt/βcatenin signalling in hepatocellular carcinoma. Mol Med Rep. 2022;26:317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Ding J, Zhao W. The Application of Liquid Biopsy Techniques in High-Risk Population for Hepatocellular Carcinoma. Cancer Manag Res. 2022;14:2735-2748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 6. | Weng W, Goel A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin Cancer Biol. 2022;80:73-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 186] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 7. | Li K, Xiao K, Zhu S, Wang Y, Wang W. Chinese Herbal Medicine for Primary Liver Cancer Therapy: Perspectives and Challenges. Front Pharmacol. 2022;13:889799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 8. | Meng X, Liu J, Zheng Q, Li S, Xiao H, Huang J, Ma L, Liu Y, Tang J. Gold-Crowned Bismuth-Based Nanocomposites for Sonodynamic, Photothermal, and Chemotherapeutic Cancer Therapy. ACS Appl Mater Interfaces. 2023;15:58041-58053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 9. | Xiong DD, Feng ZB, Lai ZF, Qin Y, Liu LM, Fu HX, He RQ, Wu HY, Dang YW, Chen G, Luo DZ. High throughput circRNA sequencing analysis reveals novel insights into the mechanism of nitidine chloride against hepatocellular carcinoma. Cell Death Dis. 2019;10:658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 10. | Liu AG, Zhong JC, Chen G, He RQ, He YQ, Ma J, Yang LH, Wu XJ, Huang JT, Li JJ, Mo WJ, Qin XG. Upregulated expression of SAC3D1 is associated with progression in gastric cancer. Int J Oncol. 2020;57:122-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Han ME, Kim JY, Kim GH, Park SY, Kim YH, Oh SO. SAC3D1: a novel prognostic marker in hepatocellular carcinoma. Sci Rep. 2018;8:15608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Khuda SE, Yoshida M, Xing Y, Shimasaki T, Takeya M, Kuwahara K, Sakaguchi N. The Sac3 homologue shd1 is involved in mitotic progression in mammalian cells. J Biol Chem. 2004;279:46182-46190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Aguiar C, Camps I. Molecular Docking in Drug Discovery: Techniques, Applications, and Advancements. Curr Med Chem. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Fang S, Dong L, Liu L, Guo J, Zhao L, Zhang J, Bu D, Liu X, Huo P, Cao W, Dong Q, Wu J, Zeng X, Wu Y, Zhao Y. HERB: a high-throughput experiment- and reference-guided database of traditional Chinese medicine. Nucleic Acids Res. 2021;49:D1197-D1206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 380] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 15. | Yang M, Su Y, Wang Z, Du D, Wei S, Liao Z, Zhang Q, Zhao L, Zhang X, Han L, Jiang J, Zhan M, Sun L, Yuan S, Zhou Z. C118P, a novel microtubule inhibitor with anti-angiogenic and vascular disrupting activities, exerts anti-tumor effects against hepatocellular carcinoma. Biochem Pharmacol. 2021;190:114641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Abdelhameid MK, Taher ES, Hara MA, Ramadan M, Mohamed KO. Discovery of novel octahydroquinazoline scaffolds endowed with dual inhibition of tubulin polymerization/Eg5 against HCC: Apoptotic and radio-chemotherapeutic studies. Bioorg Chem. 2024;148:107449. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Khan H, Azad I, Arif Z, Parveen S, Kumar S, Rais J, Ansari JA, Nasibullah M, Kumar S, Arshad M. Structure based docking and biological evaluation towards exploring potential anti-cancerous and apoptotic activity of 6-Gingerol against human prostate carcinoma cells. BMC Complement Med Ther. 2024;24:8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Suski JM, Braun M, Strmiska V, Sicinski P. Targeting cell-cycle machinery in cancer. Cancer Cell. 2021;39:759-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 329] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 19. | Wang Y, Deng B. Hepatocellular carcinoma: molecular mechanism, targeted therapy, and biomarkers. Cancer Metastasis Rev. 2023;42:629-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 161] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 20. | Liu Y, He M, Ke X, Chen Y, Zhu J, Tan Z, Chen J. Centrosome amplification-related signature correlated with immune microenvironment and treatment response predicts prognosis and improves diagnosis of hepatocellular carcinoma by integrating machine learning and single-cell analyses. Hepatol Int. 2024;18:108-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 21. | Zhang Z, Zhang Y, Hu G, Wu Q, Zhou Y, Luo F. Conduction and validation of a novel mitotic spindle assembly related signature in hepatocellular carcinoma: prognostic prediction, tumor immune microenvironment and drug susceptibility. Front Genet. 2024;15:1412303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Ai Y, Meng Y, Yan B, Zhou Q, Wang X. The biochemical pathways of apoptotic, necroptotic, pyroptotic, and ferroptotic cell death. Mol Cell. 2024;84:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 155] [Reference Citation Analysis (0)] |
| 23. | Zheng Z, Zhao Y, Yu H, Wang T, Li J, Xu L, Ding C, He L, Wu L, Dong Z. Suppressing MTERF3 inhibits proliferation of human hepatocellular carcinoma via ROS-mediated p38 MAPK activation. Commun Biol. 2024;7:18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |